Efficacy of Postoperative Unilateral Neck Irradiation in Patients with Buccal Mucosa Squamous Carcinoma with Extranodal Extension: A Propensity Score Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient and Treatment Characteristics
2.2. Survival Analyses
2.3. Evaluation of Prognostic Factors
2.4. Prognostic Model for CLNC
2.5. Contralateral Nodal Failure
2.6. Complications
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Treatment
4.3. Study Variables
4.4. Outcomes
4.5. Propensity Score Matching
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snow, G.B.; van den Brekel, M.W.; Leemans, C.R.; Patel, P. Surgical management of cervical lymph nodes in patients with oral and oropharyngeal cancer. Recent Results Cancer Res. 1994, 134, 43–55. [Google Scholar] [CrossRef]
- Shingaki, S.; Takada, M.; Sasai, K.; Bibi, R.; Kobayashi, T.; Nomura, T.; Saito, C. Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am. J. Surg. 2003, 185, 278–284. [Google Scholar] [CrossRef]
- Lindberg, R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972, 29, 1446–1449. [Google Scholar] [CrossRef]
- Spencer, C.R.; Gay, H.A.; Haughey, B.H.; Nussenbaum, B.; Adkins, D.R.; Wildes, T.M.; DeWees, T.A.; Lewis, J.S., Jr.; Thorstad, W.L. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer 2014, 120, 3994–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.; Overgaard, M.; Grau, C. Morbidity after ipsilateral radiotherapy for oropharyngeal cancer. Radiother. Oncol. 2007, 85, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.I.; Rao, Y.J.; Hwang, M.Y.; Spencer, C.R.; Pierro, M.; DeWees, T.; Patel, P.; Sinha, P.; Gay, H.A.; Daly, M.; et al. Comparison of unilateral versus bilateral intensity-modulated radiotherapy for surgically treated squamous cell carcinoma of the palatine tonsil. Cancer 2017, 123, 4594–4607. [Google Scholar] [CrossRef]
- Jackson, S.M.; Hay, J.H.; Flores, A.D.; Weir, L.; Wong, F.L.; Schwindt, C.; Baerg, B. Cancer of the tonsil: The results of ipsilateral radiation treatment. Radiother. Oncol. 1999, 51, 123–128. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Warde, P.; Grice, B.; Goh, C.; Payne, D.; Liu, F.F.; Waldron, J.; Bayley, A.; Irish, J.; Gullane, P.; et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 332–343. [Google Scholar] [CrossRef]
- Liu, C.; Dutu, G.; Peters, L.J.; Rischin, D.; Corry, J. Tonsillar cancer: The Peter MacCallum experience with unilateral and bilateral irradiation. Head Neck 2014, 36, 317–322. [Google Scholar] [CrossRef]
- Murthy, A.K.; Hendrickson, F.R. Is contralateral neck treatment necessary in early carcinoma of the tonsil? Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 91–94. [Google Scholar] [CrossRef]
- Kagei, K.; Shirato, H.; Nishioka, T.; Arimoto, T.; Hashimoto, S.; Kaneko, M.; Ohmori, K.; Honma, A.; Inuyama, Y.; Miyasaka, K. Ipsilateral irradiation for carcinomas of tonsillar region and soft palate based on computed tomographic simulation. Radiother. Oncol 2000, 54, 117–121. [Google Scholar] [CrossRef]
- Al-Mamgani, A.; van Rooij, P.; Fransen, D.; Levendag, P. Unilateral neck irradiation for well-lateralized oropharyngeal cancer. Radiother. Oncol. 2013, 106, 69–73. [Google Scholar] [CrossRef]
- Chronowski, G.M.; Garden, A.S.; Morrison, W.H.; Frank, S.J.; Schwartz, D.L.; Shah, S.J.; Beadle, B.M.; Gunn, G.B.; Kupferman, M.E.; Ang, K.K.; et al. Unilateral radiotherapy for the treatment of tonsil cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 204–209. [Google Scholar] [CrossRef]
- Cramer, C.K.; Palta, M.; Patel, P.; Brizel, D.M. Ipsilateral Tonsil Chemoradiation: Improved Toxicity Compared to Bilateral Radiation and Effective Rates of Local-Regional Control: Definitive Management of Head-and-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 477. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Herman, M.P.; Deraniyagala, R.L.; Amdur, R.J.; Werning, J.W.; Dziegielewski, P.T.; Morris, C.G.; Mendenhall, W.M. Ipsilateral radiotherapy for squamous cell carcinoma of the tonsil. Eur. Arch. Otorhinolaryngol. 2016, 273, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.S.; Mourad, W.F.; Gamez, M.; Safdieh, J.; Lin, W.; Jacobson, A.S.; Persky, M.S.; Urken, M.L.; Culliney, B.; Li, Z.; et al. Low rates of contralateral neck failure in unilaterally treated oropharyngeal squamous cell carcinoma with prospectively defined criteria of lateralization. Head Neck 2017, 39, 1647–1654. [Google Scholar] [CrossRef]
- Gottumukkala, S.; Pham, N.L.; Sumer, B.; Myers, L.; Truelson, J.; Nedzi, L.; Khan, S.; Hughes, R.; Sher, D.J. Risk of contralateral nodal failure following ipsilateral IMRT for node-positive tonsillar cancer. Oral Oncol. 2017, 75, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, L.; Martín, M.; López, M.; Marín, A.; Gómez, A. Ipsilateral irradiation for well lateralized carcinomas of the oral cavity and oropharynx: Results on tumor control and xerostomia. Radiat. Oncol. 2009, 4, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, M.Y.; Spencer, C.R.; Patel, P.; Chin, R.; Gay, H.A.; Haughey, B.H.; Nussenbaum, B.; Adkins, D.; Lewis, J.S.; Thorstad, W.L. Unilateral Radiation Therapy in Node-Positive Patients with Lateralized Tonsillar Carcinoma: Definitive Management of Head-and-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 475. [Google Scholar] [CrossRef]
- Dan, T.D.; Raben, D.; Schneider, C.J.; Hockstein, N.G.; Witt, R.L.; Dzeda, M.; Cormier, J.F.; Raben, A. Freedom from local and regional failure of contralateral neck with ipsilateral neck radiotherapy for node-positive tonsil cancer: Updated results of an institutional clinical management approach. Oral Oncol. 2015, 51, 616–621. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, K.H.; Moon, S.H.; Lee, C.G.; Keum, K.C.; Lee, S.W.; Ahn, Y.C.; Oh, D.; Kim, Y.S.; Won, Y.K.; et al. Comparison of the Clinical Outcomes of Patients with Squamous Cell Carcinoma of the Tonsil Receiving Postoperative Ipsilateral Versus Bilateral Neck Radiotherapy: A Propensity Score Matching Analysis (KROG 11-07). Cancer Res. Treat. 2017, 49, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Vergeer, M.R.; Doornaert, P.A.; Jonkman, A.; Kaanders, J.H.; van den Ende, P.L.; de Jong, M.A.; Leemans, C.R.; Slotman, B.J.; Langendijk, J.A. Ipsilateral irradiation for oral and oropharyngeal carcinoma treated with primary surgery and postoperative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 682–688. [Google Scholar] [CrossRef]
- Lynch, J.; Lal, P.; Schick, U.; Nutting, C.M.; Newbold, K.; Harrington, K.; Bhide, S. Multiple cervical lymph node involvement and extra-capsular extension predict for contralateral nodal recurrence after ipsilateral radiotherapy for squamous cell carcinoma of the tonsil. Oral Oncol. 2014, 50, 901–906. [Google Scholar] [CrossRef]
- Contreras, J.A.; Spencer, C.; DeWees, T.; Haughey, B.; Henke, L.E.; Chin, R.I.; Paniello, R.; Rich, J.; Jackson, R.; Oppelt, P.; et al. Eliminating Postoperative Radiation to the Pathologically Node-Negative Neck: Long-Term Results of a Prospective Phase II Study. J. Clin. Oncol. 2019, 37, 2548–2555. [Google Scholar] [CrossRef]
- Myers, J.N.; Greenberg, J.S.; Mo, V.; Roberts, D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer 2001, 92, 3030–3036. [Google Scholar] [CrossRef]
- Liao, C.T.; Lee, L.Y.; Huang, S.F.; Chen, I.H.; Kang, C.J.; Lin, C.Y.; Fan, K.H.; Wang, H.M.; Ng, S.H.; Yen, T.C. Outcome analysis of patients with oral cavity cancer and extracapsular spread in neck lymph nodes. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Cooper, J.S.; Zhang, Q.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: Postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.T.; Lee, L.Y.; Hsueh, C.; Lin, C.Y.; Fan, K.H.; Wang, H.M.; Hsieh, C.H.; Ng, S.H.; Lin, C.H.; Tsao, C.K.; et al. Pathological risk factors stratification in pN3b oral cavity squamous cell carcinoma: Focus on the number of positive nodes and extranodal extension. Oral Oncol. 2018, 86, 188–194. [Google Scholar] [CrossRef]
- Lin, Y.S.; Jen, Y.M.; Wang, B.B.; Lee, J.C.; Kang, B.H. Epidemiology of oral cavity cancer in taiwan with emphasis on the role of betel nut chewing. ORL J. Otorhinolaryngol. Relat. Spec. 2005, 67, 230–236. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, L.Y.; Huang, S.F.; Kang, C.J.; Fan, K.H.; Wang, H.M.; Chen, E.Y.; Chen, I.H.; Liao, C.T.; Cheng, A.J.; et al. Treatment outcome of combined modalities for buccal cancers: Unilateral or bilateral neck radiation? Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Waldron, J.; Bratman, S.V.; Su, J.; Kim, J.; Bayley, A.; Cho, J.; Giuliani, M.; Hope, A.; Ringash, J.; et al. Re-evaluation of Ipsilateral Radiation for T1-T2N0-N2b Tonsil Carcinoma at the Princess Margaret Hospital in the Human Papillomavirus Era, 25 Years Later. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jellema, A.P.; Slotman, B.J.; Doornaert, P.; Leemans, C.R.; Langendijk, J.A. Unilateral versus bilateral irradiation in squamous cell head and neck cancer in relation to patient-rated xerostomia and sticky saliva. Radiother. Oncol. 2007, 85, 83–89. [Google Scholar] [CrossRef]
- Kwon, M.; Roh, J.L.; Lee, J.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Extranodal extension and thickness of metastatic lymph node as a significant prognostic marker of recurrence and survival in head and neck squamous cell carcinoma. J Craniomaxillofac. Surg. 2015, 43, 769–778. [Google Scholar] [CrossRef]
- Michikawa, C.; Izumo, T.; Sumino, J.; Morita, T.; Ohyama, Y.; Michi, Y.; Uzawa, N. Small size of metastatic lymph nodes with extracapsular spread greatly impacts treatment outcomes in oral squamous cell carcinoma patients. Int. J. Oral Maxillofac. Surg. 2018, 47, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Tsan, D.L.; Lin, C.Y.; Kang, C.J.; Huang, S.F.; Fan, K.H.; Liao, C.T.; Chen, I.H.; Lee, L.Y.; Wang, H.M.; Chang, J.T. The comparison between weekly and three-weekly cisplatin delivered concurrently with radiotherapy for patients with postoperative high-risk squamous cell carcinoma of the oral cavity. Radiat. Oncol 2012, 7, 215. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.T.; Wang, H.M.; Hsieh, L.L.; Chang, J.T.; Ng, S.H.; Hsueh, C.; Lee, L.Y.; Lin, C.H.; Chen, I.H.; Kang, C.J.; et al. Higher distant failure in young age tongue cancer patients. Oral Oncol. 2006, 42, 718–725. [Google Scholar] [CrossRef]
- Anderson, C.R.; Sisson, K.; Moncrieff, M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol. 2015, 51, 464–469. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Tsai, M.H.; Chiang, C.J.; Tsai, S.T.; Liu, T.W.; Lou, P.J.; Liao, C.T.; Lin, J.C.; Chang, J.T.; Tsai, M.H.; et al. Adjuvant radiotherapy after curative surgery for oral cavity squamous cell carcinoma and treatment effect of timing and duration on outcome-A Taiwan Cancer Registry national database analysis. Cancer Med. 2018, 7, 3073–3083. [Google Scholar] [CrossRef]
- Daly, M.E.; Le, Q.T.; Kozak, M.M.; Maxim, P.G.; Murphy, J.D.; Hsu, A.; Loo, B.W., Jr.; Kaplan, M.J.; Fischbein, N.J.; Chang, D.T. Intensity-modulated radiotherapy for oral cavity squamous cell carcinoma: Patterns of failure and predictors of local control. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1412–1422. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Hess, K.R.; Abbruzzese, M.C.; Lenzi, R.; Raber, M.N.; Abbruzzese, J.L. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin. Cancer Res. 1999, 5, 3403–3410. [Google Scholar] [PubMed]
- Spurgeon, S.E.; Hsieh, Y.C.; Rivadinera, A.; Beer, T.M.; Mori, M.; Garzotto, M. Classification and regression tree analysis for the prediction of aggressive prostate cancer on biopsy. J. Urol. 2006, 175, 918–922. [Google Scholar] [CrossRef]


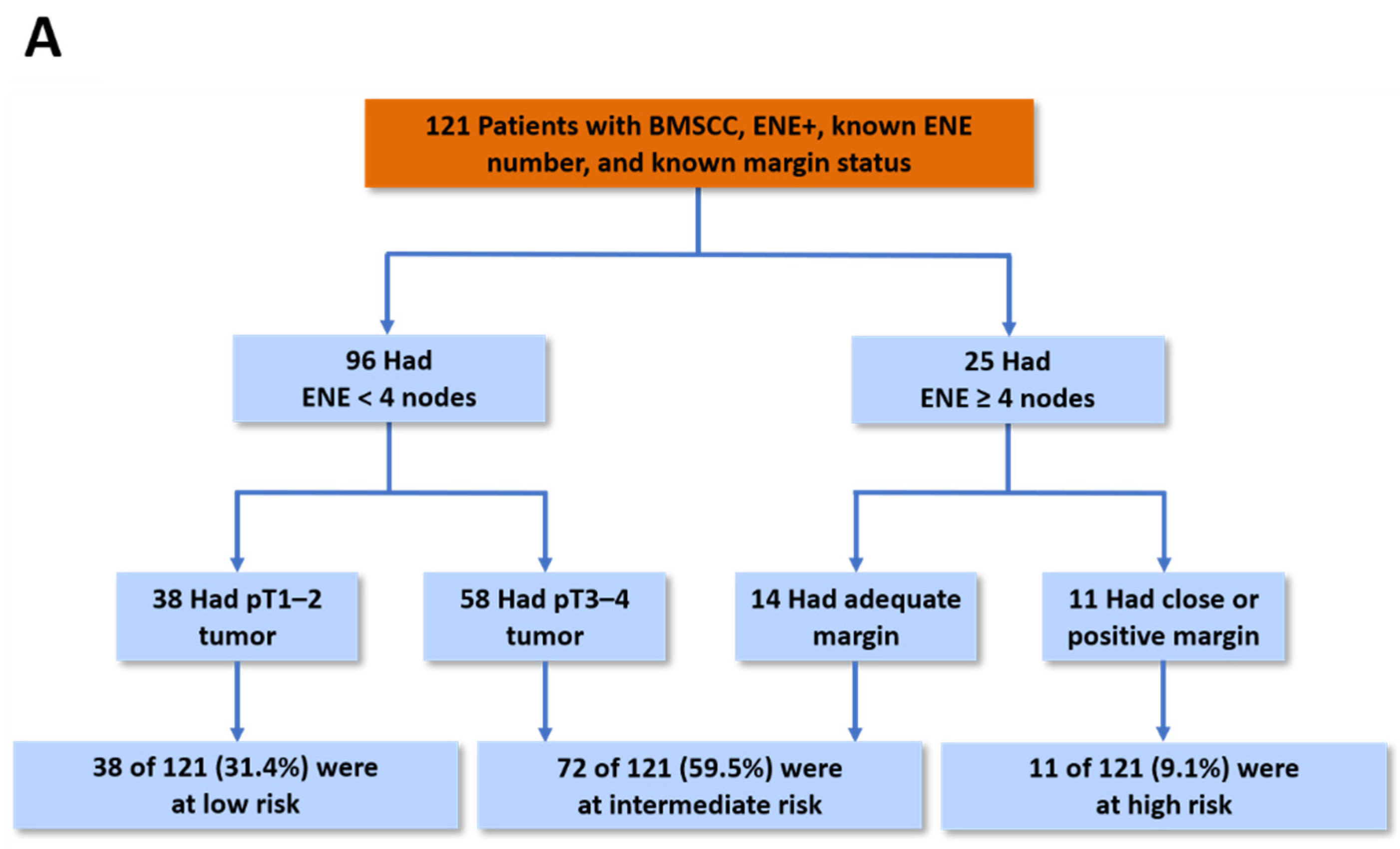

| Unmatched Groups | Propensity Score-Matched Groups | |||||
|---|---|---|---|---|---|---|
| Characteristic | Unilateral RT (n = 61) | Bilateral RT (n = 125) | p Value | Unilateral RT (n = 45) | Bilateral RT (n = 78) | p Value |
| n (%) | n (%) | n (%) | n (%) | |||
| Treatment time periods | 1999–2016 | 1997–2016 | 1999–2016 | 1997–2016 | ||
| Age, years, mean ± SD | 48.9 ± 11.66 | 50.5 ± 9.67 | 0.316 | 50.3 ± 11.05 | 50.0 ± 9.55 | 0.871 |
| <40 | 11 (18.0) | 17 (13.6) | 0.427 | 5 (11.1) | 11 (14.1) | 0.635 |
| ≥40 | 50 (82.0) | 108 (86.4) | 40 (88.9) | 67 (85.9) | ||
| Sex, male | 57 (93.4) | 122 (97.6) | 0.219 | 44 (97.8) | 75 (96.2) | 1.000 |
| Smoking status | ||||||
| No | 12 (19.7) | 28 (22.4) | 0.671 | 8 (17.8) | 13 (16.7) | 0.875 |
| Yes | 49 (80.3) | 97 (77.6) | 37 (82.2) | 65 (83.3) | ||
| Betel quid chewing | ||||||
| No | 13 (21.3) | 31 (24.8) | 0.599 | 9 (20.0) | 16 (20.5) | 0.946 |
| Yes | 48 (78.7) | 94 (75.2) | 36 (80.0) | 62 (79.5) | ||
| Alcohol drinking | ||||||
| No | 15 (24.6) | 32 (25.6) | 0.882 | 10 (22.2) | 16 (20.5) | 0.823 |
| Yes | 46 (75.4) | 93 (74.4) | 35 (77.8) | 62 (79.5) | ||
| ECOG, ≥2 | 1 (1.6) | 3 (2.4) | 1.000 | 1 (2.2) | 1 (1.3) | 1.000 |
| CCI, ≥3 | 11 (18.0) | 22 (17.6) | 0.942 | 9 (20.0) | 15 (19.2) | 0.917 |
| AJCC 8th pT classification a | ||||||
| T1–2 | 20 (32.8) | 44 (35.2) | 0.745 | 17 (37.8) | 30 (38.5) | 0.940 |
| T3–4 | 41 (67.2) | 81 (64.8) | 28 (62.2) | 48 (61.5) | ||
| AJCC 8th pN classification a | ||||||
| N2a | 12 (19.7) | 25 (20.0) | 0.958 | 8 (17.8) | 15 (19.2) | 0.842 |
| N3b | 49 (80.3) | 100 (80.0) | 37 (82.2) | 63 (80.8) | ||
| Differentiation | ||||||
| Well–moderate | 52 (85.2) | 95 (76.0) | 0.146 | 37 (82.2) | 65 (83.3) | 0.875 |
| Poor | 9 (14.8) | 30 (24.0) | 8 (17.8) | 13 (16.7) | ||
| Margin a | ||||||
| Adequate | 45 (73.8) | 86 (68.8) | 0.831 | 33 (73.3) | 56 (71.8) | 1.000 |
| Close | 13 (21.3) | 30 (24.0) | 9 (20.0) | 17 (21.8) | ||
| Positive | 3 (4.9) | 9 (7.2) | 3 (6.7) | 5 (6.4) | ||
| ENE number b, mean ± SD | 2.2 ± 1.88 | 2.2 ± 2.29 | 0.878 | 2.4 ± 1.98 | 2.1 ± 1.68 | 0.452 |
| ENE number b ≥4 | 14 (23.3) | 20 (16.4) | 0.259 | 12 (26.7) | 13 (17.1) | 0.209 |
| Lymphatic invasion, present | 13 (21.3) | 12 (9.6) | 0.028 * | 7 (15.6) | 9 (11.5) | 0.524 |
| Vascular invasion, present | 6 (9.8) | 14 (11.2) | 0.778 | 6 (13.3) | 9 (11.5) | 0.770 |
| PNI, present | 30 (49.2) | 76 (60.8) | 0.133 | 23 (51.1) | 41 (52.6) | 0.877 |
| Soft tissue invasion, present | 46 (75.4) | 110 (88.0) | 0.028 * | 34 (75.6) | 64 (82.1) | 0.389 |
| Bone invasion, present | 22 (36.1) | 39 (31.2) | 0.507 | 17 (37.8) | 26 (33.3) | 0.619 |
| Skin invasion, present | 10 (16.4) | 33 (26.4) | 0.129 | 8 (17.8) | 16 (20.5) | 0.712 |
| Chemotherapy (CDDP-based) | 53 (86.9) | 116 (93.5) | 0.130 | 41 (91.1) | 72 (93.5) | 0.625 |
| RT interval | ||||||
| <8 wk | 44 (72.1) | 101 (80.8) | 0.181 | 34 (75.6) | 64 (82.1) | 0.389 |
| ≥8 wk | 17 (27.9) | 24 (19.2) | 11 (24.4) | 14 (17.9) | ||
| Surgery to RT interval | ||||||
| <6 wk | 32 (52.5) | 79 (63.2) | 0.161 | 23 (51.1) | 52 (66.7) | 0.088 |
| ≥6 wk | 29 (47.5) | 46 (36.8) | 22 (48.9) | 26 (33.3) | ||
| Median RT dose, Gy (range) | 66 (64.0–70.0) | 66 (60.0–82.0) | 0.466 | 66 (64.0–66.8) | 66 (60.0–79.2) | 0.905 |
| RT technique | ||||||
| 2D-RT/3D-CRT | 14 (23.0) | 5 (4.0) | <0.001 * | 8 (17.8) | 5 (6.4) | 0.048 * |
| IMRT/VMAT | 47 (77.0) | 120 (96.0) | 37 (82.2) | 73 (93.6) | ||
| Variable | Hazard Ratio (95% CI) | p Value a |
|---|---|---|
| CLNC | ||
| Unilateral RT vs. Bilateral RT | 1.05 (0.33–3.28) | NS |
| IMRT/VMAT vs. 2D-RT/3D-CRT | ** | NS |
| ENE number (≥4 vs. <4) | 4.85 (1.50–15.74) | 0.008 * |
| Close/positive margin | 1.82 (0.57–5.79) | NS |
| OS | ||
| Unilateral RT vs. Bilateral RT | 1.09 (0.65–1.80) | NS |
| IMRT/VMAT vs. 2D-RT/3D-CRT | 0.77 (0.31–1.95) | NS |
| Chemotherapy (CDDP-based) | 0.55 (0.24–1.25) | NS |
| AJCC 8th T classification (pT3–4 vs. pT1–2) | 1.90 (1.01–3.60) | 0.048 * |
| ENE number (≥4 vs. <4) | 2.24 (1.30–3.85) | 0.004 * |
| Lymphatic invasion | 1.99 (1.01–3.94) | 0.047 * |
| PNI | 1.40 (0.83–2.35) | NS |
| Bone invasion | 0.92 (0.52–1.65) | NS |
| DFS | ||
| Unilateral RT vs. Bilateral RT | 1.04 (0.63–1.73) | NS |
| IMRT/VMAT vs. 2D-RT/3D-CRT | 0.69 (0.29–1.66) | NS |
| AJCC 8th T classification (pT3–4 vs. pT1–2) | 1.97 (1.05–3.71) | 0.036 * |
| ENE number (≥4 vs. <4) | 2.30 (1.34–3.94) | 0.002 * |
| Lymphatic invasion | 2.03 (1.03–4.01) | 0.041 * |
| Bone invasion | 1.06 (0.60–1.85) | NS |
| DMFS | ||
| Unilateral RT vs. Bilateral RT | 1.15 (0.58–2.30) | NS |
| IMRT/VMAT vs. 2D-RT/3D-CRT | ** | NS |
| AJCC 8th T classification (pT3–4 vs. pT1–2) | 2.41 (1.09–5.33) | 0.030 * |
| ENE number (≥4 vs. <4) | 2.35 (1.15–4.81) | 0.019 * |
| LC | ||
| Unilateral RT vs. Bilateral RT | 0.43 (0.19–1.01) | NS |
| IMRT/VMAT vs. 2D-RT/3D-CRT | 0.33 (1.00–1.09) | NS |
| Surgery to RT interval, wk (≥6) | 3.17 (1.43–7.02) | 0.004 * |
| AJCC 8th T classification (pT3–4 vs. pT1–2) | 2.18 (0.91–5.20) | NS |
| ENE number (≥4 vs. <4) | 4.04 (1.78–9.19) | 0.001 * |
| RC | ||
| Unilateral RT vs. Bilateral RT | 0.65 (0.27–1.59) | NS |
| IMRT/VMAT vs. 2D-RT/3D-CRT | ** | NS |
| AJCC 8th T classification (pT3–4 vs. pT1–2) | 4.22 (1.15–15.48) | 0.030 * |
| ENE number (≥4 vs. <4) | 4.30 (1.90–9.73) | <0.001 * |
| Bone invasion | 1.33 (0.56–3.15) | NS |
| No. | ENE Number | Pathological Margin Status a | T-Stage | RT Laterality | Site of cNF | Coincided with or Preceded by | Salvage Treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| LR | iNF | DM | |||||||
| 1 | <4 | Adequate | pT3 | Bilateral | I, II, III, RP node | ● | Surgery + PORT + CT | ||
| 2 | <4 | Adequate | pT4 | Bilateral | I, IV, VI | ● | ● | ||
| 3 | <4 | Close/positive | pT4 | Unilateral | IV | ● | |||
| 4 | <4 | Adequate | pT4 | Unilateral | IV | ● | |||
| 5 | <4 | Adequate | pT3 | Bilateral | I | ● | |||
| 6 | <4 | Adequate | pT4 | Bilateral | II | ● | |||
| 7 | ≥4 | Adequate | pT3 | Unilateral | II | Surgery + PORT | |||
| 8 | ≥4 | Close/positive | pT2 | Bilateral | I | Surgery + PORT | |||
| 9 | ≥4 | Close/positive | pT2 | Bilateral | I | ● | |||
| 10 | ≥4 | Close/positive | pT3 | Bilateral | V, RP node | ● | ● | ||
| 11 | ≥4 | Close/positive | pT4 | Bilateral | II | ● | |||
| 12 | ≥4 | Close/positive | pT4 | Unilateral | I, II, III, IV, VI | ● | ● | ||
| 13 | ≥4 | Close/positive | pT2 | Unilateral | II | ● | ● | ||
| Complication | Grade a | Bilateral RT n (%) | Unilateral RT n (%) | p Value b |
|---|---|---|---|---|
| Acute | ||||
| Xerostomia | <3 | 32/34 (94.1) | 23/23 (100.0) | 0.510 |
| ≥3 | 2/34 (5.9) | 0/23 (0.0) | ||
| Oral mucositis | <3 | 37/70 (52.9) | 23/39 (59.0) | 0.538 |
| ≥3 | 33/70 (47.1) | 16/39 (41.0) | ||
| Dermatitis | <3 | 50/54 (92.6) | 18/19 (94.7) | 1.000 |
| ≥3 | 4/54 (7.4) | 1/19 (5.3) | ||
| Late | ||||
| Xerostomia | <2 | 23/30 (76.7) | 20/28 (71.4) | 0.649 |
| ≥2 | 7/30 (23.3) | 8/28 (28.6) | ||
| Soft Tissue Fibrosis | <2 | 8/10 (80.0) | 11/13 (84.6) | 1.000 |
| ≥2 | 2/10 (20.0) | 2/13 (15.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Lin, C.-Y.; Fan, K.-H.; Hung, S.-P.; Chou, Y.-C.; Liu, C.-J.; Chou, W.-C.; Chen, Y.-C.; Huang, S.-F.; Kang, C.-J.; et al. Efficacy of Postoperative Unilateral Neck Irradiation in Patients with Buccal Mucosa Squamous Carcinoma with Extranodal Extension: A Propensity Score Analysis. Cancers 2021, 13, 5997. https://doi.org/10.3390/cancers13235997
Lin C-H, Lin C-Y, Fan K-H, Hung S-P, Chou Y-C, Liu C-J, Chou W-C, Chen Y-C, Huang S-F, Kang C-J, et al. Efficacy of Postoperative Unilateral Neck Irradiation in Patients with Buccal Mucosa Squamous Carcinoma with Extranodal Extension: A Propensity Score Analysis. Cancers. 2021; 13(23):5997. https://doi.org/10.3390/cancers13235997
Chicago/Turabian StyleLin, Chia-Hsin, Chien-Yu Lin, Kang-Hsing Fan, Sheng-Ping Hung, Yung-Chih Chou, Chia-Jen Liu, Wen-Chi Chou, Yen-Chao Chen, Shiang-Fu Huang, Chung-Jan Kang, and et al. 2021. "Efficacy of Postoperative Unilateral Neck Irradiation in Patients with Buccal Mucosa Squamous Carcinoma with Extranodal Extension: A Propensity Score Analysis" Cancers 13, no. 23: 5997. https://doi.org/10.3390/cancers13235997






