A Narrative Review of the Safety of Anti-COVID-19 Nutraceuticals for Patients with Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
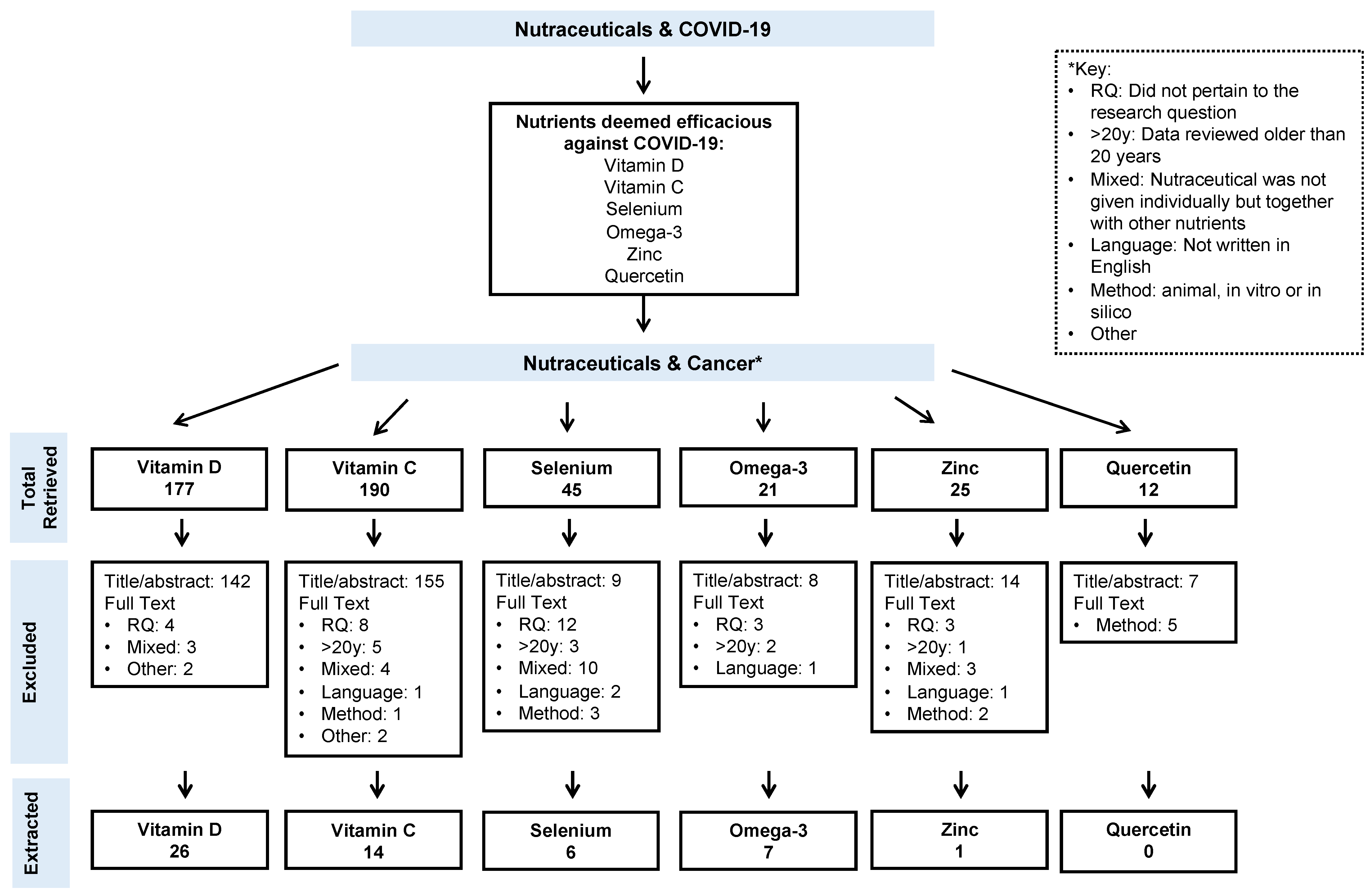
2. Methods
3. Results
3.1. Vitamin D
3.2. Vitamin C
3.3. Selenium
3.4. Omega-3 Fatty Acids
3.5. Zinc
3.6. Quercetin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part; Online; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. Timeline: WHO’s COVID-19 Response. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (accessed on 1 November 2021).
- WHO. Novel Coronavirus (2019-nCoV): Situation Report 10. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf?sfvrsn=d0b2e480_2 (accessed on 1 November 2021).
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 November 2021).
- CDC. Symptoms of COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 1 November 2021).
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Di Salvo, E.; Di Gioacchino, M.; Tonacci, A.; Casciaro, M.; Gangemi, S. Alarmins, COVID-19 and comorbidities. Ann. Med. 2021, 53, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Miyashita, H.; Mikami, T.; Chopra, N.; Yamada, T.; Chernyavsky, S.; Rizk, D.; Cruz, C. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann. Oncol. 2020, 31, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Christodoulatos, G.S.; Karampela, I.; Vallianou, N.; Apovian, C.M. Understanding the Co-Epidemic of Obesity and COVID-19: Current Evidence, Comparison with Previous Epidemics, Mechanisms, and Preventive and Therapeutic Perspectives. Curr. Obes. Rep. 2021, 10, 214–243. [Google Scholar] [CrossRef]
- Lin, M.; Dong, H.Y.; Xie, H.Z.; Li, Y.M.; Jia, L. Why do we lack a specific magic anti-COVID-19 drug? Analyses and solutions. Drug Discov. Today 2021, 26, 631–636. [Google Scholar] [CrossRef]
- Gunalan, E.; Cebioglu, I.K.; Conak, O. The Popularity of the Biologically-Based Therapies During Coronavirus Pandemic Among the Google Users in the USA, UK, Germany, Italy and France. Complement. Ther. Med. 2021, 58, 102682. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvao Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Jahaj, E.; Orfanos, S.E.; Dimopoulou, I.; Kotanidou, A. Vitamin D in infectious complications in critically ill patients with or without COVID-19. Metab. Open 2021, 11, 100106. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Rando, H.M.; Consortium, C.-R.; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems 2021, 6, e00122-21. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients 2021, 13, 1261. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478–495. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef]
- Heidari, Z.; Tajbakhsh, A.; Gheibi-Hayat, S.M.; Moattari, A.; Razban, V.; Berenjian, A.; Savardashtaki, A.; Negahdaripour, M. Probiotics/ prebiotics in viral respiratory infections: Implication for emerging pathogens. Recent Pat. Biotechnol. 2021, 15, 112–136. [Google Scholar] [CrossRef]
- Talukdar, J.; Bhadra, B.; Dattaroy, T.; Nagle, V.; Dasgupta, S. Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. Biomed. Pharmacother. 2020, 132, 110886. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Adams, D.P.; Garcia Hernandez, J.L.; Gonzalez-Bernal, J.; Gonzalez-Gross, M. Glycophosphopeptical AM3 Food Supplement: A Potential Adjuvant in the Treatment and Vaccination of SARS-CoV-2. Front. Immunol. 2021, 12, 698672. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, T.B.; Smeland, S.; Aaserud, S.; Buanes, E.A.; Skog, A.; Ursin, G.; Helland, A. COVID-19 in Cancer Patients, Risk Factors for Disease and Adverse Outcome, a Population-Based Study from Norway. Front. Oncol. 2021, 11, 652535. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Pathania, A.S.; Prathipati, P.; Abdul, B.A.; Chava, S.; Katta, S.S.; Gupta, S.C.; Gangula, P.R.; Pandey, M.K.; Durden, D.L.; Byrareddy, S.N.; et al. COVID-19 and Cancer Comorbidity: Therapeutic Opportunities and Challenges. Theranostics 2021, 11, 731–753. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, C.J.; Yiang, G.T.; Cheng, Y.L.; Tsai, A.P.; Hou, Y.T.; Ho, Y.C.; Hou, M.F.; Chu, P.Y. Molecular Regulation of Bone Metastasis Pathogenesis. Cell Physiol. Biochem. 2018, 46, 1423–1438. [Google Scholar] [CrossRef]
- Bandinelli, L.; Ornell, F.; von Diemen, L.; Kessler, F.H.P. The Sum of Fears in Cancer Patients Inside the Context of the COVID-19. Front. Psychiatry 2021, 12, 557834. [Google Scholar] [CrossRef] [PubMed]
- Hamulka, J.; Jeruszka-Bielak, M.; Gornicka, M.; Drywien, M.E.; Zielinska-Pukos, M.A. Dietary Supplements during COVID-19 Outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients 2020, 13, 54. [Google Scholar] [CrossRef]
- 15 Best Supplements to Boost Your Immune System Right Now. Available online: https://www.healthline.com/nutrition/immune-boosting-supplements (accessed on 4 October 2021).
- 20 Vitamins and Supplements to Boost Immune Health for COVID-19. Available online: https://www.medicinenet.com/covid_19_supplements/article.htm (accessed on 1 November 2021).
- Aysin, E.; Urhan, M. Dramatic Increase in Dietary Supplement Use during COVID-19. Curr. Dev. Nutr. 2021, 5, 207. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients with Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.M.; Shu, X.; Caan, B.J.; Flatt, S.W.; Holmes, M.D.; Lu, W.; Kwan, M.L.; Nechuta, S.J.; Pierce, J.P.; Chen, W.Y. Postdiagnosis supplement use and breast cancer prognosis in the after Breast Cancer Pooling Project. Breast Cancer Res. Treat. 2013, 139, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst. Rev. 2008, CD004183. [Google Scholar] [CrossRef]
- Andersen, M.R.; Sweet, E.; Hager, S.; Gaul, M.; Dowd, F.; Standish, L.J. Effects of Vitamin D Use on Health-Related Quality of Life of Breast Cancer Patients in Early Survivorship. Integr. Cancer Ther. 2019, 18, 1534735418822056. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014, CD007470. [Google Scholar] [CrossRef]
- Campbell, R.A.; Li, J.; Malone, L.; Levy, D.A. Correlative Analysis of Vitamin D and Omega-3 Fatty Acid Intake in Men on Active Surveillance for Prostate Cancer. Urology 2021, 155, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Du, M.; Blumberg, J.B.; Ho Chui, K.K.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association Among Dietary Supplement Use, Nutrient Intake, and Mortality Among U.S. Adults: A Cohort Study. Ann. Intern. Med. 2019, 170, 604–613. [Google Scholar] [CrossRef]
- Chlebowski, R.T. Vitamin D and breast cancer incidence and outcome. Anticancer Agents Med. Chem. 2013, 13, 98–106. [Google Scholar] [CrossRef]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef] [Green Version]
- Cook, L.S.; Neilson, H.K.; Lorenzetti, D.L.; Lee, R.C. A systematic literature review of vitamin D and ovarian cancer. Am. J. Obstet. Gynecol. 2010, 203, 70.e1–70.e8. [Google Scholar] [CrossRef]
- Datta, M.; Schwartz, G.G. Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: A critical review. Oncologist 2012, 17, 1171–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, C.; Yang, S.; Zhao, X.; Dong, H. Pathogenic roles of alterations in vitamin D and vitamin D receptor in gastric tumorigenesis. Oncotarget 2017, 8, 29474–29486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, W.B.; Boucher, B.J. A Review of the Potential Benefits of Increasing Vitamin D Status in Mongolian Adults through Food Fortification and Vitamin D Supplementation. Nutrients 2019, 11, 2452. [Google Scholar] [CrossRef] [Green Version]
- Harvie, M. Nutritional supplements and cancer: Potential benefits and proven harms. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e478–e486. [Google Scholar] [CrossRef] [Green Version]
- Holm, M.; Olsen, A.; Kroman, N.; Tjonneland, A. Lifestyle influences on the association between pre-diagnostic hormone replacement therapy and breast cancer prognosis—Results from The Danish ‘Diet, Cancer and Health’ prospective cohort. Maturitas 2014, 79, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulou, A.; Riza, E.; Samoli, E.; Benetou, V. Dietary Supplement Use after Cancer Diagnosis in Relation to Total Mortality, Cancer Mortality and Recurrence: A Systematic Review and Meta-Analysis. Nutr. Cancer 2021, 73, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Kimler, B.F.; Reddy, P.S.; Sharma, P.; Klemp, J.R.; Nydegger, J.L.; Yeh, H.W.; Fabian, C.J. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res. Treat. 2017, 166, 491–500. [Google Scholar] [CrossRef]
- Klapdor, S.; Richter, E.; Klapdor, R. Vitamin D status and per-oral vitamin D supplementation in patients suffering from chronic pancreatitis and pancreatic cancer disease. Anticancer Res. 2012, 32, 1991–1998. [Google Scholar] [PubMed]
- Lewis, C.; Xun, P.; He, K. Vitamin D supplementation and quality of life following diagnosis in stage II colorectal cancer patients: A 24-month prospective study. Support. Care Cancer 2016, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; Jacobs, E.T.; Baron, J.A.; Marshall, J.R.; Byers, T. Dietary supplements and cancer prevention: Balancing potential benefits against proven harms. J. Natl. Cancer Inst. 2012, 104, 732–739. [Google Scholar] [CrossRef]
- Morita, M.; Okuyama, M.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Vitamin D Supplementation Regulates Postoperative Serum Levels of PD-L1 in Patients with Digestive Tract Cancer and Improves Survivals in the Highest Quintile of PD-L1: A Post Hoc Analysis of the AMATERASU Randomized Controlled Trial. Nutrients 2021, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Mulpur, B.H.; Nabors, L.B.; Thompson, R.C.; Olson, J.J.; LaRocca, R.V.; Thompson, Z.; Egan, K.M. Complementary therapy and survival in glioblastoma. Neurooncol. Pract. 2015, 2, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Saquib, J.; Rock, C.L.; Natarajan, L.; Saquib, N.; Newman, V.A.; Patterson, R.E.; Thomson, C.A.; Al-Delaimy, W.K.; Pierce, J.P. Dietary intake, supplement use, and survival among women diagnosed with early-stage breast cancer. Nutr. Cancer 2011, 63, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Sarre, S.; Maattanen, L.; Tammela, T.L.; Auvinen, A.; Murtola, T.J. Postscreening follow-up of the Finnish Prostate Cancer Screening Trial on putative prostate cancer risk factors: Vitamin and mineral use, male pattern baldness, pubertal development and non-steroidal anti-inflammatory drug use. Scand. J. Urol. 2016, 50, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Wang, J.; Huang, X.; Cheng, Y. Longitudinal, observational study on associations between postoperative nutritional vitamin D supplementation and clinical outcomes in esophageal cancer patients undergoing esophagectomy. Sci. Rep. 2016, 6, 38962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Niu, W. Meta-analysis of randomized controlled trials on vitamin D supplement and cancer incidence and mortality. Biosci. Rep. 2019, 39, BSR20190369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zirpoli, G.R.; McCann, S.E.; Sucheston-Campbell, L.E.; Hershman, D.L.; Ciupak, G.; Davis, W.; Unger, J.M.; Moore, H.C.F.; Stewart, J.A.; Isaacs, C.; et al. Supplement Use and Chemotherapy-Induced Peripheral Neuropathy in a Cooperative Group Trial (S0221): The DELCaP Study. J. Natl. Cancer Inst. 2017, 109, djx098. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Systematic review: Primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Aliment. Pharmacol. Ther. 2008, 28, 689–703. [Google Scholar] [CrossRef]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H.; Song, J.; Castillo, A.; Weltzien, E.; Quesenberry, C.P., Jr.; Caan, B.J. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life After Cancer Epidemiology (LACE) cohort. Cancer 2012, 118, 2048–2058. [Google Scholar] [CrossRef] [Green Version]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Vitamin C intake and breast cancer mortality in a cohort of Swedish women. Br. J. Cancer 2013, 109, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Harris, H.R.; Orsini, N.; Wolk, A. Vitamin C and survival among women with breast cancer: A meta-analysis. Eur. J. Cancer 2014, 50, 1223–1231. [Google Scholar] [CrossRef]
- Jacobs, E.J.; Connell, C.J.; McCullough, M.L.; Chao, A.; Jonas, C.R.; Rodriguez, C.; Calle, E.E.; Thun, M.J. Vitamin C, vitamin E, and multivitamin supplement use and stomach cancer mortality in the Cancer Prevention Study II cohort. Cancer Epidemiol. Biomark. Prev. 2002, 11, 35–41. [Google Scholar]
- Jacobs, E.J.; Henion, A.K.; Briggs, P.J.; Connell, C.J.; McCullough, M.L.; Jonas, C.R.; Rodriguez, C.; Calle, E.E.; Thun, M.J. Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. Am. J. Epidemiol. 2002, 156, 1002–1010. [Google Scholar] [CrossRef]
- Lin, J.; Cook, N.R.; Albert, C.; Zaharris, E.; Gaziano, J.M.; Van Denburgh, M.; Buring, J.E.; Manson, J.E. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J. Natl. Cancer Inst. 2009, 101, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messerer, M.; Hakansson, N.; Wolk, A.; Akesson, A. Dietary supplement use and mortality in a cohort of Swedish men. Br. J. Nutr. 2008, 99, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer. Epidemiol. Biomark. Prev. 2011, 20, 262–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocobelli, G.; Peters, U.; Kristal, A.R.; White, E. Use of supplements of multivitamins, vitamin C, and vitamin E in relation to mortality. Am. J. Epidemiol. 2009, 170, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, K.H.; Tian, J.H.; Guan, Q.L.; Yao, N.; Cao, N.; Mi, D.H.; Wu, J.; Ma, B.; Yang, S.H. Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: A meta-analysis of randomized controlled trials. Nutr. Cancer 2010, 62, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, S.A.; Van Blarigan, E.L.; DuPre, N.; Stampfer, M.J.; Giovannucci, E.L.; Chan, J.M. Selenium supplementation and prostate cancer mortality. J. Natl. Cancer Inst. 2015, 107, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muecke, R.; Schomburg, L.; Glatzel, M.; Berndt-Skorka, R.; Baaske, D.; Reichl, B.; Buentzel, J.; Kundt, G.; Prott, F.J.; Devries, A.; et al. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Samuels, N.; Schiff, E.; Ben-Arye, E. Non-herbal nutritional supplements for symptom relief in adjuvant breast cancer: Creating a doctor-patient dialogue. BMJ Support. Palliat. Care 2014, 4, e1. [Google Scholar] [CrossRef]
- Klassen, P.; Cervantes, M.; Mazurak, V.C. N-3 fatty acids during chemotherapy: Toward a higher level of evidence for clinical application. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 82–88. [Google Scholar] [CrossRef]
- Miyata, H.; Yano, M.; Yasuda, T.; Yamasaki, M.; Murakami, K.; Makino, T.; Nishiki, K.; Sugimura, K.; Motoori, M.; Shiraishi, O.; et al. Randomized study of the clinical effects of omega-3 fatty acid-containing enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Nutrition 2017, 33, 204–210. [Google Scholar] [CrossRef]
- Shen, S.; Unger, J.M.; Crew, K.D.; Till, C.; Greenlee, H.; Gralow, J.; Dakhil, S.R.; Minasian, L.M.; Wade, J.L.; Fisch, M.J.; et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res. Treat. 2018, 172, 603–610. [Google Scholar] [CrossRef]
- Sorensen, L.S.; Rasmussen, S.L.; Calder, P.C.; Yilmaz, M.N.; Schmidt, E.B.; Thorlacius-Ussing, O. Long-term outcomes after perioperative treatment with omega-3 fatty acid supplements in colorectal cancer. BJS Open 2020, 4, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Nichetti, F.; Raimondi, A.; Pusceddu, S.; Platania, M.; Berrino, F.; de Braud, F. Diet and supplements in cancer prevention and treatment: Clinical evidences and future perspectives. Crit. Rev. Oncol. Hematol. 2018, 123, 57–73. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Improta-Caria, A.C.; Aras-Junior, R.; de Oliveira, E.M.; Soci, U.P.R.; Cassilhas, R.C. Physical exercise effects on the brain during COVID-19 pandemic: Links between mental and cardiovascular health. Neurol. Sci. 2021, 42, 1325–1334. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, H.; Zhou, Y.; Mao, F.; Lin, Y.; Pan, B.; Zhang, X.; Xu, Q.; Huang, X.; Sun, Q. Prognostic Value of PD-L1 in Breast Cancer: A Meta-Analysis. Breast J. 2017, 23, 436–443. [Google Scholar] [CrossRef]
- Gu, L.; Chen, M.; Guo, D.; Zhu, H.; Zhang, W.; Pan, J.; Zhong, X.; Li, X.; Qian, H.; Wang, X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0182692. [Google Scholar] [CrossRef] [PubMed]
- Vrankar, M.; Zwitter, M.; Kern, I.; Stanic, K. PD-L1 expression can be regarded as prognostic factor for survival of non-small cell lung cancer patients after chemoradiotherapy. Neoplasma 2018, 65, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, W.; Yan, Z.; Ma, J.; Zhu, F.; Huo, J. Prognostic value of PD-L1 expression in patients with pancreatic cancer: A PRISMA-compliant meta-analysis. Medicine 2019, 98, e14006. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef]
- de Sousa Melo, A.; de Lima Dantas, J.B.; Medrado, A.; Lima, H.R.; Martins, G.B.; Carrera, M. Nutritional supplements in the management of oral mucositis in patients with head and neck cancer: Narrative literary review. Clin. Nutr. ESPEN 2021, 43, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
| Study | Type | Participants | Cancer | Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|
| Andersen et al., 2019 [37] | Observational |
|
|
|
| (+) |
| Bjelakovic et al., 2014 [38] | Cochrane review |
|
|
|
| (+) |
| Campbell et al., 2021 [39] | Intervention |
|
|
|
| (+) |
| Chen et al., 2019 [40] | Prospective cohort study |
|
|
|
| (−) |
| Chlebowski et al., 2013 [41] | Literature review |
|
|
|
| (+) |
| Chowdhury et al., 2014 [42] | Systematic review and meta-analysis |
|
|
|
| (+) |
| Cook et al., 2010 [43] | Meta-analysis |
|
|
|
| (+) |
| Datta et al., 2012 [44] | Review |
|
|
|
| (+) |
| Du et al., 2017 [45] | Review |
|
|
|
| (+) |
| Grant et al., 2019 [46] | Review |
|
|
|
| (+) |
| Harvie et al., 2014 [47] | Review |
|
|
|
| (+) |
| Holm et al., 2014 [48] | Prospective cohort |
|
|
|
| (−) |
| Kanellopoulou et al., 2021 [49] | Meta-analysis |
|
|
|
| (+) |
| Khan et al., 2017 [50] | RCT |
|
|
|
| (+) |
| Klapdor et al., 2012 [51] | Prospective cohort |
|
|
|
| (+) |
| Lewis et al., 2016 [52] | Prospective cohort |
|
|
|
| (+) |
| Madden et al., 2018 [53] | Longitudinal cohort |
|
|
|
| (+) |
| Martinez et al., 2012 [54] | Review |
|
|
|
| (+) |
| Morita et al., 2021 [55] | Post-hoc analysis of RCT |
|
|
|
| (+/−) |
| Mulpur et al., 2015 [56] | Cohort |
|
|
|
| (+) |
| Poole et al., 2013 [35] | Cohort |
|
|
|
| (+) |
| Saquib et al., 2011 [57] | Cohort derived from RCT |
|
|
|
| (+) |
| Sarre et al., 2016 [58] | Cohort from men participating in the third round of the FinRSPC 10 randomized screening study |
|
|
|
| (+) |
| Wang et al., 2016 [59] | Longitudinal observational |
|
|
|
| (+) |
| Zhang et al., 2019 [60] | Meta-analysis of RCTs |
|
|
|
| (+) |
| Zirpoli et al., 2017 [61] | Cohort |
|
|
|
| (+) |
| Study | Type | Participants | Cancer | Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|
| Ambrosone et al., 2020 [34] | Correlative analysis from SWOG S0221 |
|
|
|
| (+) |
| Bjelakovic et al., 2008 [62] | Systematic review |
|
|
|
| (+) |
| Greenlee et al., 2012 [63] | Cohort |
|
|
|
| (+) |
| Harris et al., 2013 [64] | Cohort |
|
|
|
| (+) |
| Harris et al., 2014 [65] | Meta-analysis |
|
|
|
| (+) |
| Jacobs et al., 2002 [66] | Cohort |
|
|
|
| (+) |
| Jacobs et al., 2002 [67] | Cohort |
|
|
|
| (+) |
| Kanellopoulo et al., 2020 [49] | Meta-analysis |
|
|
|
| (+) |
| Lin et al., 2009 [68] | RCT 3 |
|
|
|
| (+) |
| Messerer et al., 2008 [69] | Cohort |
|
|
|
| (+) |
| Nechuta et al., 2011 [70] | Cohort |
|
|
|
| (+) |
| Pocobelli et al., 2009 [71] | Cohort |
|
|
|
| (+) |
| Poole et al., 2013 [35] | Cohort |
|
|
|
| (+) |
| Zirpoli et al., 2017 [61] | Cohort |
|
|
|
| (+) |
| Study | Type | Participants | Cancer | Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|
| Bjelakovic et al., 2008 [62] | Systematic review of RCTs 1 |
|
|
|
| (+) |
| Jenkins et al., 2020 [72] | Systematic review/meta-analysis of RCTs |
|
|
|
| (+) |
| Jiang L et al., 2010 [73] | Meta-analysis of RCTs |
|
|
|
| (+) |
| Kenfield et al., 2015 [74] | Prospective cohort study |
|
|
|
| (+/-) |
| Muecke R et al., 2010 [75] | RCT |
|
|
|
| (+) |
| Samuels et al., 2014 [76] | Review |
|
|
|
| (+) |
| Study | Type | Participants | Cancer | Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|
| Campbell et al., 2021 [39] | Intervention |
|
|
|
| (+) |
| Klassen et al., 2020 [77] | Review article |
|
|
|
| (+) |
| Miyata et al., 2017 [78] | RCT 2 |
|
|
|
| (+) |
| Mulpur et al., 2015 [56] | Longitudinal cohort |
|
|
|
| (+) |
| Shen et al., 2018 [79] | Exploratory analysis of RCT |
|
|
|
| (+) |
| Sorensen et al., 2020 [80] | RCT |
|
|
|
| (−) |
| Vernieri et al., 2018 [81] | Review |
|
|
|
| (+/−) |
| Study | Type | Participants | Cancer | Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|
| De Sousa Melo et al., 2021 [82] | Narrative review |
|
|
|
| (+/−) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bader-Larsen, K.S.; Larson, E.A.; Dalamaga, M.; Magkos, F. A Narrative Review of the Safety of Anti-COVID-19 Nutraceuticals for Patients with Cancer. Cancers 2021, 13, 6094. https://doi.org/10.3390/cancers13236094
Bader-Larsen KS, Larson EA, Dalamaga M, Magkos F. A Narrative Review of the Safety of Anti-COVID-19 Nutraceuticals for Patients with Cancer. Cancers. 2021; 13(23):6094. https://doi.org/10.3390/cancers13236094
Chicago/Turabian StyleBader-Larsen, Karlen Stade, Elisabeth Anne Larson, Maria Dalamaga, and Faidon Magkos. 2021. "A Narrative Review of the Safety of Anti-COVID-19 Nutraceuticals for Patients with Cancer" Cancers 13, no. 23: 6094. https://doi.org/10.3390/cancers13236094






