PCAT6 May Be a Whistler and Checkpoint Target for Precision Therapy in Human Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Discovery of PCAT6
3. Expression and Subcellular Localization of PCAT6
3.1. The Abnormal Expression of PCAT6 in Cancers
3.2. The Subcellular Localization of PCAT6 in Cancer Cell Lines
4. PCAT6 Promotes Cancer Progression by ceRNA Mechanisms
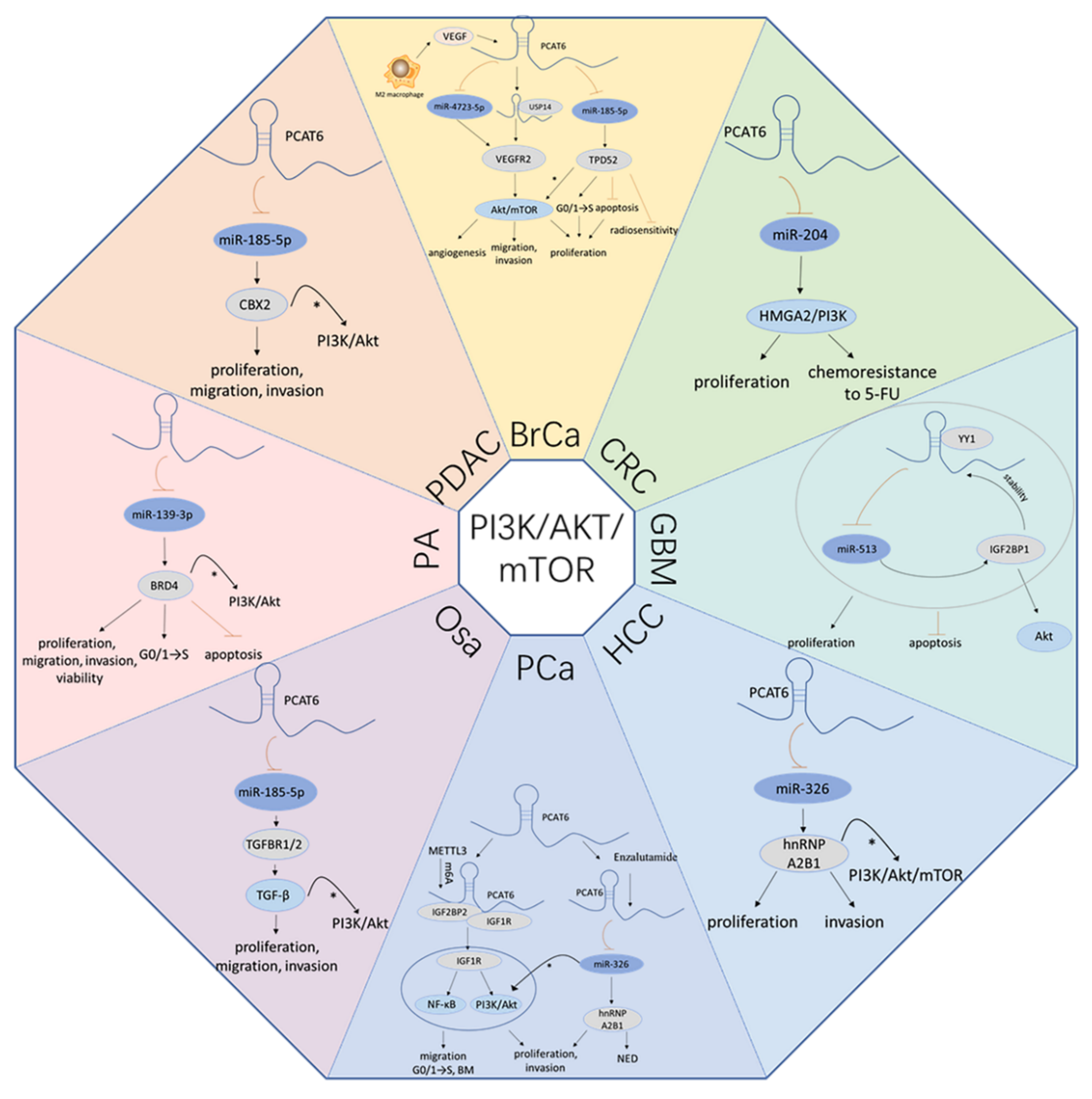
4.1. PCAT6 Boosts the PI3K/Akt/mTOR Signaling Pathway
4.2. PCAT6 Promotes the Wnt/β-Catenin Signaling Pathway
4.3. PCAT6 Facilitates the EMT Process
4.4. PCAT6 Leads to Radioresistance and Chemoresistance
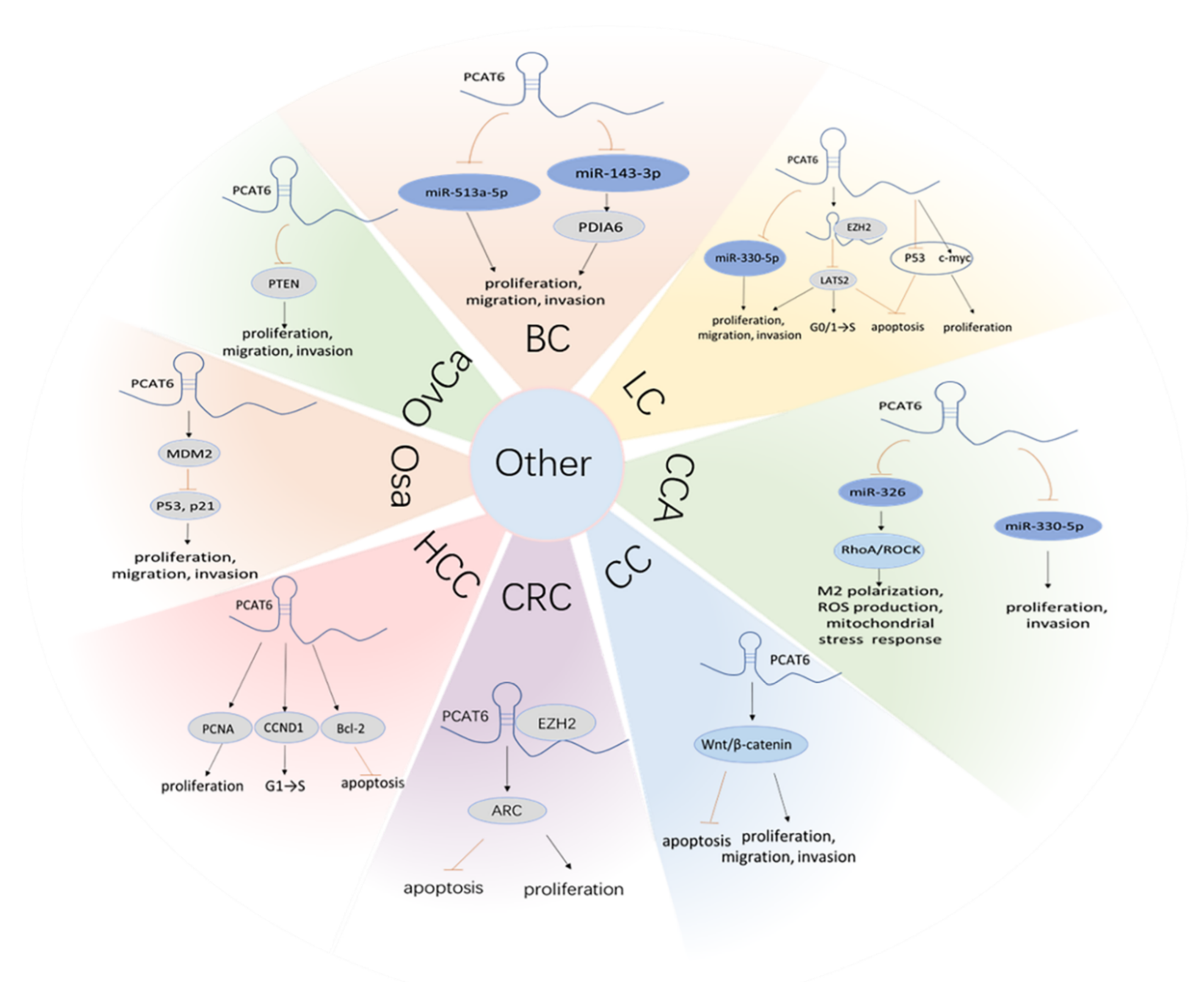
4.5. Other ceRNA Mechanisms of PCAT6
5. The Mechanisms of PCAT6 above ceRNA
6. The Diagnostic and Prognostic Value of PCAT6 Overexpression
7. Discussion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akhade, V.; Pal, D.; Kanduri, C. Long noncoding RNA: Genome organization and mechanism of action. Adv. Exp. Med. Biol. 2017, 1008, 47–74. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, J.; Wei, M.; Liu, B.; Dong, K. Emerging role of long noncoding RNA-encoded micropeptides in cancer. Cancer Cell Int. 2020, 20, 506. [Google Scholar] [CrossRef]
- Boland, R.C. Non-coding RNA: It’s not junk. Dig. Dis. Sci. 2017, 62, 1107–1109. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Wong, N.; Huang, C.; Islam, R.; Yip, S. Long non-coding RNAs in hematological malignancies: Translating basic techniques into diagnostic and therapeutic strategies. J. Hematol. Oncol. 2018, 11, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ransohoff, J.; Wei, Y.; Khavari, P. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Adams, B.; Parsons, C.; Walker, L.; Zhang, W.; Slack, F. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017, 127, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Mo, Y.; Peng, M.; Tang, T.; Zhong, Y.; Deng, X.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol. Cancer 2020, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dang, W.; Zhang, S.; Yue, W.; Yang, L.; Zhai, X.; Yan, Q.; Lu, J. The role of exosomal noncoding RNAs in cancer. Mol. Cancer 2019, 18, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huarte, M. The emerging role of lncRNAs in cancer. Nat. Med. 2015, 21, 1253–1261. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, Z.; Ling, Y.; Luo, W. LncRNA-H19 acts as a ceRNA to regulate HE4 expression by sponging miR-140 in human umbilical vein endothelial cells under hyperglycemia with or without α-Mangostin. Biomed. Pharmacother. 2019, 118, 109256. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhao, Y.; Li, Q.; Zhou, J.; Mao, Y. Long non-coding RNA TUSC8 inhibits breast cancer growth and metastasis via miR-190b-5p/MYLIP axis. Aging 2020, 12, 2974–2991. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, R.; Wu, Y.; Liu, Y.; Su, W.; Xiong, W.; Zeng, Z. PVT1 Promotes Cancer Progression via MicroRNAs. Front. Oncol. 2019, 9, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Qin, Y.; Wang, R.; Yang, L.; Zeng, H.; Zhu, P.; Li, Q.; Qiu, Y.; Chen, S.; Liu, Y.; et al. A novel Lnc408 maintains breast cancer stem cell stemness by recruiting SP3 to suppress CBY1 transcription and increasing nuclear β-catenin levels. Cell Death Dis. 2021, 12, 437. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dang, Y.; Li, D.; Lu, G.; Chan, W.; Leung, P.; Zhao, S.; Qin, Y.; Chen, Z. Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res. 2020, 48, 4480–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prensner, J.; Zhao, S.; Erho, N.; Schipper, M.; Iyer, M.; Dhanasekaran, S.; Magi-Galluzzi, C.; Mehra, R.; Sahu, A.; Siddiqui, J.; et al. RNA biomarkers associated with metastatic progression in prostate cancer: A multi-institutional high-throughput analysis of SChLAP1. Lancet. Oncol. 2014, 15, 1469–1480. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Wang, Z.; Chen, Q.; Li, Y.; He, H.; Hsieh, J.; Xue, S.; Wu, Z.; Liu, B.; Tang, H.; et al. Prognostic Value of a Long Non-coding RNA Signature in Localized Clear Cell Renal Cell Carcinoma. Eur. Urol. 2018, 74, 756–763. [Google Scholar] [CrossRef]
- Tong, J.; Ma, X.; Yu, H.; Yang, J. SNHG15: A promising cancer-related long noncoding RNA. Cancer Manag. Res. 2019, 11, 5961–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhan, A.; Soleimani, M.; Mandal, S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Zhang, L.; Fan, K.; Cheng, Z.; Sun, Q.; Wang, J. Knockdown of Long Noncoding RNA PCAT6 inhibits proliferation and invasion in lung cancer cells. Oncol. Res. 2016, 24, 161–170. [Google Scholar] [CrossRef]
- Xia, W.; Chen, C.; Zhang, M.; Zhu, L. LncRNA PCAT6 aggravates the progression of bladder cancer cells by targeting miR-513a-5p. Eur. Rev. Med Pharmacol. Sci. 2020, 24, 9908–9914. [Google Scholar] [CrossRef]
- Ørom, U.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010, 143, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Fagerberg, L.; Hallström, B.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Du, D.; Yi, S.; Li, X. LncRNA PCAT6: A potential biomarker for diagnosis and prognosis of bladder cancer. Ann. Diagn. Pathol. 2020, 49, 151642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Luo, G. Long non-coding RNA PCAT6 regulates bladder cancer progression via the microRNA-143-3p/PDIA6 axis. Exp. Ther. Med. 2021, 22, 947. [Google Scholar] [CrossRef]
- Dong, F.; Ruan, S.; Wang, J.; Xia, Y.; Le, K.; Xiao, X.; Hu, T.; Wang, Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wu, P.; Liu, M.; Chen, B.; Cong, L. Knockdown of lncRNA PCAT6 enhances radiosensitivity in triple-negative breast cancer cells by regulating miR-185-5p/TPD52 axis. OncoTargets Ther. 2020, 13, 3025–3037. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Tang, Q.; Tu, Y.; Yan, D.; Wei, Q. Long noncoding RNA PCAT6 regulates cell growth and metastasis via Wnt/β-catenin pathway and is a prognosis marker in cervical cancer. Eur. Rev. Med Pharmacol. Sci. 2019, 23, 1947–1956. [Google Scholar] [CrossRef]
- Ma, Z.; Gu, G.; Pan, W.; Chen, X. LncRNA PCAT6 Accelerates the Progression and Chemoresistance of Cervical Cancer Through Up-Regulating ZEB1 by Sponging miR-543. OncoTargets Ther. 2020, 13, 1159–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Su, G.; Huang, X.; Zou, A.; Wu, J.; Yang, Y.; Zhu, Y.; Liang, S.; Li, D.; Ma, F.; et al. Long noncoding RNA PCAT6 inhibits colon cancer cell apoptosis by regulating anti-apoptotic protein ARC expression via EZH2. Cell Cycle 2019, 18, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zou, Q.; He, H.; Liang, Y.; Lei, M.; Zhou, Q.; Fan, D.; Shen, L. Long non-coding RNA PCAT6 targets miR-204 to modulate the chemoresistance of colorectal cancer cells to 5-fluorouracil-based treatment through HMGA2 signaling. Cancer Med. 2019, 8, 2484–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, F.; Zhang, N.; Fang, W.; He, X.; Zheng, Y.; Gu, D. PCAT6 mediates cellular biological functions in gastrointestinal stromal tumor via upregulation of PRDX5 and activation of Wnt pathway. Mol. Carcinog. 2020, 59, 661–669. [Google Scholar] [CrossRef]
- Dong, D.; Lun, Y.; Sun, B.; Sun, H.; Wang, Q.; Yuan, G.; Quan, J. Silencing of long non-coding RNA PCAT6 restrains gastric cancer cell proliferation and epithelial-mesenchymal transition by targeting microRNA-15a. Gen. Physiol. Biophys. 2020, 39, 1–12. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, J.; Jin, Y.; Yu, H. PCAT6 participates in the development of gastric cancer through endogenously competition with microRNA-30. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 5206–5213. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, P.; Li, B.; Xu, D.; Wang, K. LncRNA PCAT6 Regulated by YY1 Accelerates the Progression of Glioblastoma via miR-513/IGF2BP1. Neurochem. Res. 2020, 45, 2894–2902. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Qian, Q.; Wang, X.; Chang, Y.; Ju, S.; Xu, Y.; Zhang, C.; Qin, N.; Ding, H.; et al. Gene amplification derived a cancer-testis long noncoding RNA PCAT6 regulates cell proliferation and migration in hepatocellular carcinoma. Cancer Med. 2019, 8, 3017–3025. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Lin, J.; Zhang, Y.; Dai, G.; Li, A.; Liu, X. LncRNA PCAT6 predicts poor prognosis in hepatocellular carcinoma and promotes proliferation through the regulation of cell cycle arrest and apoptosis. Cell Biochem. Funct. 2020, 38, 895–904. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, J.; Hao, W.; Zeng, H.; Zhang, Z.; Shao, G. lncRNA PCAT6 facilitates cell proliferation and invasion via regulating the miR-326/hnRNPA2B1 axis in liver cancer. Oncol. Lett. 2021, 21, 471. [Google Scholar] [CrossRef]
- Cui, L.; Xu, H.; Yang, W.; Yu, L. lncRNA PCAT6 promotes non-small cell lung cancer cell proliferation, migration and invasion through regulating miR-330-5p. OncoTargets Ther. 2018, 11, 7715–7724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Liu, Z.; Liu, Z.; Feng, X.; Hua, F.; Hu, X.; Wang, B.; Lu, K.; Nie, F. Long noncoding RNA PCAT6 functions as an oncogene by binding to EZH2 and suppressing LATS2 in non-small-cell lung cancer. EBioMedicine 2018, 37, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Zhang, L.; Fan, K.; Wang, J. Diagnostic significance of circulating long noncoding RNA PCAT6 in patients with non-small cell lung cancer. OncoTargets Ther. 2017, 10, 5695–5702. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Yan, Z.; Deng, M. Sevoflurane Inhibits Proliferation, Invasion, but Enhances Apoptosis of Lung Cancer Cells by Wnt/β-catenin Signaling via Regulating lncRNA PCAT6/miR-326 Axis. Open Life Sci. 2020, 15, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, B.; Wei, D.; Zhou, X.; Chen, H. Long non-coding RNA KDM5B anti-sense RNA 1 enhances tumor progression in non-small cell lung cancer. J. Clin. Lab. Anal. 2020, 34, e22897. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Feng, Q.; Li, L.; Xiong, Y.; Liu, S.; Liu, J.; Wu, Q. Long-Noncoding RNA PCAT6 Aggravates Osteosarcoma Tumourigenesis via the MiR-143-3p/ZEB1 Axis. OncoTargets Ther. 2020, 13, 8705–8714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, L.; Xu, F.; Li, P.; Li, P.; Hu, F. LncRNA PCAT6 promotes tumor progression in osteosarcoma via activation of TGF-β pathway by sponging miR-185-5p. Biochem. Biophys. Res. Commun. 2020, 521, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, Y.; Zheng, J. Long non-coding RNA PCAT6 promotes the development of osteosarcoma by increasing MDM2 expression. Oncol. Rep. 2020, 44, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lv, Y.; Yao, H.; Zhang, H.; Zhou, Y.; Liu, S. LncRNA PCAT6 promotes occurrence and development of ovarian cancer by inhibiting PTEN. Eur. Rev. Med Pharmacol. Sci. 2019, 23, 8230–8238. [Google Scholar] [CrossRef]
- Tan, X.; Shao, Y.; Teng, Y.; Liu, S.; Li, W.; Xue, L.; Cao, Y.; Sun, C.; Zhang, J.; Han, J.; et al. The Cancer-Testis Long Non-coding RNA PCAT6 Facilitates the Malignant Phenotype of Ovarian Cancer by Sponging miR-143-3p. Front. Cell Dev. Biol. 2021, 9, 593677. [Google Scholar] [CrossRef]
- Xin, Y.; He, X.; Zhao, W.; Zhan, M.; Li, Y.; Xiao, J.; He, K.; Lu, L. LncRNA PCAT6 increased cholangiocarcinoma cell proliferation and invasion via modulating miR-330-5p. Am. J. Transl. Res. 2019, 11, 6185–6195. [Google Scholar]
- Zhao, P.; Cheng, J.; Li, B.; Nie, D.; Wang, H.; Li, C.; Gui, S.; Zhang, Y. LncRNA PCAT6 regulates the progression of pituitary adenomas by regulating the miR-139-3p/BRD4 axis. Cancer Cell Int. 2021, 21, 14. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Guan, C.; Hu, Z.; Zhao, Y.; Li, W.; Jiang, X. LncRNA PCAT6 promotes the proliferation, migration and invasion of pancreatic ductal adenocarcinoma via regulating miR-185-5p/CBX2 axis. Pathol. Res. Pract. 2020, 216, 153074. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, H.; Yuan, T.; Luo, J.; Zhou, W.; Jiang, Q.; Wu, D. Enzalutamide-Induced Upregulation of PCAT6 Promotes Prostate Cancer Neuroendocrine Differentiation by Regulating miR-326/HNRNPA2B1 Axis. Front. Oncol. 2021, 11, 650054. [Google Scholar] [CrossRef]
- Lang, C.; Yin, C.; Lin, K.; Li, Y.; Yang, Q.; Wu, Z.; Du, H.; Ren, D.; Dai, Y.; Peng, X. m A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin. Transl. Med. 2021, 11, e426. [Google Scholar] [CrossRef]
- Tu, J.; Wu, F.; Chen, L.; Zheng, L.; Yang, Y.; Ying, X.; Song, J.; Chen, C.; Hu, X.; Zhao, Z.; et al. viaLong Non-Coding RNA PCAT6 Induces M2 Polarization of Macrophages in Cholangiocarcinoma Modulating miR-326 and RhoA-ROCK Signaling Pathway. Front. Oncol. 2020, 10, 605877. [Google Scholar] [CrossRef] [PubMed]
- Acha-Sagredo, A.; Uko, B.; Pantazi, P.; Bediaga, N.; Moschandrea, C.; Rainbow, L.; Marcus, M.; Davies, M.; Field, J.; Liloglou, T. Long non-coding RNA dysregulation is a frequent event in non-small cell lung carcinoma pathogenesis. Br. J. Cancer 2020, 122, 1050–1058. [Google Scholar] [CrossRef]
- Bai, Y.; Qu, Y.; Wu, Z.; Ren, Y.; Cheng, Z.; Lu, Y.; Hu, J.; Lou, J.; Zhao, J.; Chen, C.; et al. Absolute quantification and analysis of extracellular vesicle lncRNAs from the peripheral blood of patients with lung cancer based on multi-colour fluorescence chip-based digital PCR. Biosens. Bioelectron. 2019, 142, 111523. [Google Scholar] [CrossRef]
- Siddique, H.; Al-Ghafari, A.; Choudhry, H.; AlTurki, S.; Alshaibi, H.; Al Doghaither, H.; Alsufiani, H. Long noncoding RNAs as prognostic markers for colorectal cancer in Saudi patients. Genet. Test. Mol. Biomark. 2019, 23, 509–514. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, Q.; da Rocha, S.; Flynn, R.; Bharadwaj, M.; Calabrese, J.; Magnuson, T.; Heard, E.; Chang, H. Systematic discovery of Xist RNA binding proteins. Cell 2015, 161, 404–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhu, Y.; Li, R.; Huang, J.; You, K.; Yuan, Y.; Zhuang, S. αLncRNA Lnc-APUE is Repressed by HNF4 and Promotes G1/S Phase Transition and Tumor Growth by Regulating MiR-20b/E2F1 Axis. Adv. Sci. 2021, 8, 2003094. [Google Scholar] [CrossRef]
- Yin, G.; Peng, Y.; Lin, Y.; Wang, P.; Li, Z.; Wang, R.; Lin, H. MSTRG.24008.1Long Non-coding RNA Regulates the Regeneration of the Sciatic Nerve via the miR-331-3p-NLRP3/MAL Axis. Front. Cell Dev. Biol. 2021, 9, 641603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, F.; Zhou, Y.; Mao, F.; Lin, Y.; Shen, S.; Li, Y.; Zhang, S.; Sun, Q. Long noncoding RNA AFAP1-AS1 promotes tumor progression and invasion by regulating the miR-2110/Sp1 axis in triple-negative breast cancer. Cell Death Dis. 2021, 12, 627. [Google Scholar] [CrossRef]
- Yu, T.; Li, G.; Wang, C.; Gong, G.; Wang, L.; Li, C.; Chen, Y.; Wang, X. MIR210HG regulates glycolysis, cell proliferation, and metastasis of pancreatic cancer cells through miR-125b-5p/HK2/PKM2 axis. RNA Biol. 2021, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Deng, Y.; Zhang, W.; Zhu, B.; Yan, H.; Lou, J.; Zhang, P.; Cui, Q.; Tang, H.; Sun, H.; et al. LncRNA Bmp1 promotes the healing of intestinal mucosal lesions via the miR-128-3p/PHF6/PI3K/AKT pathway. Cell Death Dis. 2021, 12, 595. [Google Scholar] [CrossRef]
- Da Rocha, S.; Boeva, V.; Escamilla-Del-Arenal, M.; Ancelin, K.; Granier, C.; Matias, N.; Sanulli, S.; Chow, J.; Schulz, E.; Picard, C.; et al. Jarid2 Is Implicated in the Initial Xist-Induced Targeting of PRC2 to the Inactive X Chromosome. Mol. Cell 2014, 53, 301–316. [Google Scholar] [CrossRef] [Green Version]
- Marín-Béjar, O.; Marchese, F.; Athie, A.; Sánchez, Y.; González, J.; Segura, V.; Huang, L.; Moreno, I.; Navarro, A.; Monzó, M.; et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biol. 2013, 14, R104. [Google Scholar] [CrossRef] [Green Version]
- Venkatraman, A.; He, X.; Thorvaldsen, J.; Sugimura, R.; Perry, J.; Tao, F.; Zhao, M.; Christenson, M.; Sanchez, R.; Yu, J.; et al. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature 2013, 500, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhang, Z.; Mao, C.; Zhou, Y.; Yu, L.; Yin, Y.; Wu, S.; Mou, X.; Zhu, Y. ANRIL inhibits p15(INK4b) through the TGFβ1 signaling pathway in human esophageal squamous cell carcinoma. Cell. Immunol. 2014, 289, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hann, S. HOTAIR: An Oncogenic Long Non-Coding RNA in Human Cancer. Cell. Physiol. Biochem. 2018, 47, 893–913. [Google Scholar] [CrossRef]
- Faghihi, M.; Modarresi, F.; Khalil, A.; Wood, D.; Sahagan, B.; Morgan, T.; Finch, C.; St Laurent, G.; Kenny, P.; Wahlestedt, C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008, 14, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Wang, X.; Fu, C.; Wang, X.; Zou, J.; Hua, H.; Bi, Z. Long noncoding RNA FGFR3-AS1 promotes osteosarcoma growth through regulating its natural antisense transcript FGFR3. Mol. Biol. Rep. 2016, 43, 427–436. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, S.; Shi, Y. LncRNA PCAT6 activated by SP1 facilitates the progression of breast cancer by the miR-326/LRRC8E axis. Anti-Cancer Drugs 2021. [Google Scholar] [CrossRef]
- Wang, K.; Chang, H. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, S.; Zhou, X.; Chen, Z.; Wang, M.; Zheng, X.; Xie, M. LncRNA BCAR4, targeting to miR-665/STAT3 signaling, maintains cancer stem cells stemness and promotes tumorigenicity in colorectal cancer. Cancer Cell Int. 2019, 19, 72. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, X.; Yan, P.; Liu, Y.; Jiang, X. LINC00261 Suppresses Cisplatin Resistance of Esophageal Squamous Cell Carcinoma Through miR-545-3p/MT1M Axis. Front. Cell Dev. Biol. 2021, 9, 687788. [Google Scholar] [CrossRef]
- Yang, X.; Wu, G.; Yang, F.; He, L.; Xie, X.; Li, L.; Yang, L.; Ma, Y.; Zhang, Q.; Chen, J.; et al. Elevated LINC00909 Promotes Tumor Progression of Ovarian Cancer via Regulating the miR-23b-3p/MRC2 Axis. Oxidative Med. Cell. Longev. 2021, 2021, 5574130. [Google Scholar] [CrossRef]
- Sun, H.; Wang, T.; Zhang, W.; Dong, H.; Gu, W.; Huang, L.; Yan, Y.; Zhu, C.; Chen, Z. LncRNATUG1 Facilitates Th2 Cell Differentiation by Targeting the miR-29c/B7-H3 Axis on Macrophages. Front. Immunol. 2021, 12, 631450. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Li, J.; Li, X.; Wang, X.; Xiao, Y.; Sun, Z.; Yu, Q. Downregulating lncRNA NEAT1 induces proliferation and represses apoptosis of ovarian granulosa cells in polycystic ovary syndrome via microRNA-381/IGF1 axis. J. Biomed. Sci. 2021, 28, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Wu, H.; Ni, P.; Gu, Z.; Qiao, Y.; Chen, N.; Sun, F.; Fan, Q. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010, 38, 5366–5383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.; Tay, Y. Noncoding RNA: RNA regulatory networks in cancer. Int. J. Mol. Sci. 2018, 19, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupinacci, S.; Perri, A.; Toteda, G.; Vizza, D.; Lofaro, D.; Pontrelli, P.; Stallone, G.; Divella, C.; Tessari, G.; La Russa, A.; et al. Rapamycin promotes autophagy cell death of Kaposi’s sarcoma cells through P75NTR activation. Exp. Dermatol. 2021, 00, 1–11. [Google Scholar] [CrossRef]
- Liang, W.; Shi, J.; Xia, H.; Wei, X. A Novel ruthenium-fluvastatin complex downregulates SNCG expression to modulate breast carcinoma cell proliferation and apoptosis via activating the PI3K/Akt/mTOR/VEGF/MMP9 pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5537737. [Google Scholar] [CrossRef]
- Shu, X.; Zhan, P.; Sun, L.; Yu, L.; Liu, J.; Sun, L.; Yang, Z.; Ran, Y.; Sun, Y. BCAT1 Activates PI3K/AKT/mTOR pathway and contributes to the angiogenesis and tumorigenicity of gastric cancer. Front. Cell Dev. Biol. 2021, 9, 659260. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Hou, J.; Li, T.; Du, X.; Zhao, X.; Xu, W.; Xu, W.; Chang, J. Tumor protein D52 (TPD52) inhibits growth and metastasis in renal cell carcinoma cells through the PI3K/Akt signaling pathway. Oncol. Res. 2017, 25, 773–779. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Chen, Z.; Chen, J.; Meng, Y.; Feng, B.; Sun, L.; Dou, L.; Li, J.; Cui, Q.; et al. Long noncoding RNA lncSHGL recruits hnRNPA1 to suppress hepatic gluconeogenesis and lipogenesis. Diabetes 2018, 67, 581–593. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Ran, L.; Liu, Y.; Zhong, S.; Zhou, P.; Liao, M.; Fang, W. Knockdown of hnRNP A2/B1 inhibits cell proliferation, invasion and cell cycle triggering apoptosis in cervical cancer via PI3K/AKT signaling pathway. Oncol. Rep. 2018, 39, 939–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Liao, Y.; Xu, X.; Yi, Q.; He, L.; Tang, L. hnRNP A1 promotes keratinocyte cell survival post UVB radiation through PI3K/Akt/mTOR pathway. Exp. Cell Res. 2018, 362, 394–399. [Google Scholar] [CrossRef]
- Choi, H.; Lee, H.; Jang, Y.; Kim, C.; Ryu, C. Heterogeneous nuclear ribonucleoprotein A2/B1 regulates the self-renewal and pluripotency of human embryonic stem cells via the control of the G1/S transition. Stem Cells 2013, 31, 2647–2658. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, H.; Liu, D.; Zhu, X.; Huang, N.; Wei, Y.; Li, L. Heat shock protein 90 inhibitor ameliorates pancreatic fibrosis by degradation of transforming growth factor-β receptor. Cell. Signal. 2021, 84, 110001. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.; Savira, F.; Hua, Y.; Xiong, X.; Huang, L.; Reid, C.; Flynn, B.; Kaye, D.; Liew, D.; Wang, B. Attenuating PI3K/Akt- mTOR pathway reduces dihydrosphingosine 1 phosphate mediated collagen synthesis and hypertrophy in primary cardiac cells. Int. J. Biochem. Cell Biol. 2021, 134, 105952. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Z.; Jiang, J.; Piao, Y.; Li, L.; Xu, C.; Piao, H.; Li, L.; Yan, G. DEK-targeting aptamer DTA-64 attenuates bronchial EMT-mediated airway remodelling by suppressing TGF-β1/Smad, MAPK and PI3K signalling pathway in asthma. J. Cell. Mol. Med. 2020, 24, 13739–13750. [Google Scholar] [CrossRef]
- Hong, X.; Wang, J.; Li, S.; Zhao, Z.; Feng, Z. MicroRNA-375-3p in endothelial progenitor cells-derived extracellular vesicles relieves myocardial injury in septic rats via BRD4-mediated PI3K/AKT signaling pathway. Int. Immunopharmacol. 2021, 96, 107740. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Kong, F.; Wu, S.; Liu, Q.; Yang, C.; Wu, X.; Zhang, W. microRNA-612 suppresses the malignant development of non-small-cell lung cancer by directly targeting bromodomain-containing protein 4. OncoTargets Ther. 2019, 12, 4167–4179. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Yang, Z.; Wang, L.; Zhang, Y.; Shu, Y.; Jiang, L.; Hu, Y.; Lv, W.; Dong, P.; Liu, Y. Downregulation of BRD4 inhibits gallbladder cancer proliferation and metastasis and induces apoptosis via PI3K/AKT pathway. Int. J. Oncol. 2017, 51, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lin, Q.; Song, C.; Ma, R.; Li, X. Circ_0007841 promotes the progression of multiple myeloma through targeting miR-338-3p/BRD4 signaling cascade. Cancer Cell Int. 2020, 20, 383. [Google Scholar] [CrossRef]
- Zheng, S.; Lv, P.; Su, J.; Miao, K.; Xu, H.; Li, M. Overexpression of CBX2 in breast cancer promotes tumor progression through the PI3K/AKT signaling pathway. Am. J. Transl. Res. 2019, 11, 1668–1682. [Google Scholar] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Z.; Zielske, S.; Ibrahim, K.; Cackowski, F. WNT and β-catenin signaling in the bone metastasis of prostate cancer. Life 2021, 11, 1099. [Google Scholar] [CrossRef]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT signaling in tumors: The way to evade drugs and immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef]
- Farahmand, L.; Darvishi, B.; Majidzadeh-A, A.; Madjid Ansari, A. Naturally occurring compounds acting as potent anti-metastatic agents and their suppressing effects on Hedgehog and WNT/β-catenin signalling pathways. Cell Prolif. 2017, 50, e12299. [Google Scholar] [CrossRef]
- Wang, G.; Ma, C.; Shi, X.; Guo, W.; Niu, J. miR-107 enhances the sensitivity of breast cancer cells to paclitaxel. Open Med. 2019, 14, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Katsaros, D.; Biglia, N.; Wang, Z.; Pagano, I.; Tius, M.; Tiirikainen, M.; Rosser, C.; Yang, H.; Yu, H. Vitamin D receptor upregulates lncRNA TOPORS-AS1 which inhibits the Wnt/β-catenin pathway and associates with favorable prognosis of ovarian cancer. Sci. Rep. 2021, 11, 7484. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Liu, F.; Gao, L.; Han, R.; Chen, C.; Ding, X.; Li, S.; Lu, K.; Yang, L.; et al. Heterogeneous nuclear ribonucleoprotein A2/B1 is a negative regulator of human breast cancer metastasis by maintaining the balance of multiple genes and pathways. EBioMedicine 2020, 51, 102583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Quan, Y.; Lv, J.; Gong, S.; Dong, D. BRD4 promotes glioma cell stemness via enhancing miR-142-5p-mediated activation of Wnt/β-catenin signaling. Environ. Toxicol. 2020, 35, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shi, L.; Xu, Y.; Xu, T.; Fan, R.; Cao, M.; Xu, W.; Song, J. BRD4 promotes the stemness of gastric cancer cells via attenuating miR-216a-3p-mediated inhibition of Wnt/β-catenin signaling. Eur. J. Pharmacol. 2019, 852, 189–197. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, Z.; Wang, X.; Zhang, Y. BRD4 induces osteogenic differentiation of BMSCs via the Wnt/β-catenin signaling pathway. Tissue Cell 2021, 72, 101555. [Google Scholar] [CrossRef]
- Dou, Y.; Chen, F.; Lu, Y.; Qiu, H.; Zhang, H. Effects of Wnt/β-catenin signal pathway regulated by miR-342-5p targeting CBX2 on proliferation, metastasis and invasion of ovarian cancer cells. Cancer Manag. Res. 2020, 12, 3783–3794. [Google Scholar] [CrossRef]
- Garcia-Moreno, S.; Lin, Y.; Futtner, C.; Salamone, I.; Capel, B.; Maatouk, D. CBX2 is required to stabilize the testis pathway by repressing Wnt signaling. PLoS Genet. 2019, 15, e1007895. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Li, R.; Zhang, Y.; Zhou, L.; Dai, Y. Knockdown of peroxiredoxin 5 inhibits the growth of osteoarthritic chondrocytes via upregulating Wnt/β-catenin signaling. Free Radic. Biol. Med. 2014, 76, 251–260. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Weinberg, R. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaffer, C.; San Juan, B.; Lim, E.; Weinberg, R. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef]
- Lambert, A.; Pattabiraman, D.; Weinberg, R. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xiao, Z.; Zheng, J.; Wu, J.; Hu, X.; Yang, X.; Shen, Q. ZEB1 Represses neural differentiation and cooperates with CTBP2 to dynamically regulate cell migration during neocortex development. Cell Rep. 2019, 27, 2335-2353.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Yu, T.; Han, Y.; Jiang, H.; Wang, C.; You, T.; Zhao, X.; Shan, H.; Yang, R.; Yang, L.; et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Cancer 2018, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wei, H.; Guo, H.; Li, Y.; Feng, Y.; Bian, Q.; Wang, Y. LncRNA MALAT1, an lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM in lung bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L87–L98. [Google Scholar] [CrossRef]
- Dong, N.; Tang, X.; Yuan, X.; Song, H.; Li, J. [TAK1 promotes epithelial-mesenchymal transition of lens epithelial cells]. Chin. J. Ophthalmol. 2016, 52, 278–284. [Google Scholar] [CrossRef]
- Gardner, A.; Fisher, A.; Richter, C.; Johnson, G.; Moisey, E.; Brodlie, M.; Ward, C.; Krippner-Heidenreich, A.; Mann, D.; Borthwick, L. The critical role of TAK1 in accentuated epithelial to mesenchymal transition in obliterative bronchiolitis after lung transplantation. Am. J. Pathol. 2012, 180, 2293–2308. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Y.; Jin, W.; Yang, Q.; Shao, Z.; Tian, X. ABT-737 reverses the acquired radioresistance of breast cancer cells by targeting Bcl-2 and Bcl-xL. J. Exp. Clin. Cancer Res. 2012, 31, 102. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Chen, S.; Yang, B.; Mao, W.; Yang, X.; Cai, J. Molecular mechanisms of lncRNAs in regulating cancer cell radiosensitivity. Biosci. Rep. 2019, 39, BSR20190590. [Google Scholar] [CrossRef]
- Zhu, C.; Li, K.; Jiang, M.; Chen, S. RBM5-AS1 promotes radioresistance in medulloblastoma through stabilization of SIRT6 protein. Acta Neuropathol. Commun. 2021, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Chen, X.; Liu, J.; Gu, H.; Fan, R.; Ge, H. Long non-coding RNA HOTAIR knockdown enhances radiosensitivity through regulating microRNA-93/ATG12 axis in colorectal cancer. Cell Death Dis. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jing, Y.; Wei, F.; Tang, Y.; Yang, L.; Luo, J.; Yang, P.; Ni, Q.; Pang, J.; Liao, Q.; et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 2018, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Assaraf, Y.; Brozovic, A.; Gonçalves, A.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.; Xavier, C.; Vasconcelos, M. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates 2019, 46, 100645. [Google Scholar] [CrossRef]
- Levin, M.; Stark, M.; Berman, B.; Assaraf, Y. Surmounting Cytarabine-resistance in acute myeloblastic leukemia cells and specimens with a synergistic combination of hydroxyurea and azidothymidine. Cell Death Dis. 2019, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Yeldag, G.; Rice, A.; Del Río Hernández, A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef] [Green Version]
- Wambecke, A.; Ahmad, M.; Morice, P.; Lambert, B.; Weiswald, L.; Vernon, M.; Vigneron, N.; Abeilard, E.; Brotin, E.; Figeac, M.; et al. The lncRNA ‘UCA1’ modulates the response to chemotherapy of ovarian cancer through direct binding to miR-27a-5p and control of UBE2N levels. Mol. Oncol. 2021. [Google Scholar] [CrossRef]
- Chen, S.; Yang, M.; Wang, C.; Ouyang, Y.; Chen, X.; Bai, J.; Hu, Y.; Song, M.; Zhang, S.; Zhang, Q. Forkhead box D1 promotes EMT and chemoresistance by upregulating lncRNA CYTOR in oral squamous cell carcinoma. Cancer Lett. 2021, 503, 43–53. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Z.; Guan, J.; Liu, X.; Li, J.; Chen, Y.; Lin, L.; Kou, J.; Lv, J.; Zhang, L.; et al. Long Noncoding RNA TINCR-Mediated Regulation of Acetyl-CoA Metabolism Promotes Nasopharyngeal Carcinoma Progression and Chemoresistance. Cancer Res. 2020, 80, 5174–5188. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J. TCF-4 Regulated lncRNA-XIST Promotes M2 polarization of macrophages and is associated with lung cancer. OncoTargets Ther. 2019, 12, 8055–8062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Zhou, Y.; Ma, T.; Chen, S.; Shi, N.; Zou, Y.; Hou, B.; Zhang, C. Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. J. Cell. Mol. Med. 2020, 24, 5028–5038. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Shi, X.; Ye, G.; Xu, Y.; Xu, J.; Lu, J.; Lu, W. Up-regulated long non-coding RNA DUXAP8 promotes cell growth through repressing Krüppel-like factor 2 expression in human hepatocellular carcinoma. OncoTargets Ther. 2019, 12, 7429–7436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2021, 25, S1044-579X(21)00188-7. [Google Scholar] [CrossRef]
- Tewari, D.; Bawari, S.; Sharma, S.; DeLiberto, L.; Bishayee, A. Targeting the crosstalk between canonical Wnt/β-catenin and inflammatory signaling cascades: A novel strategy for cancer prevention and therapy. Pharmacol. Ther. 2021, 227, 107876. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yeow, W.; Ertel, A.; Coleman, I.; Clegg, N.; Thangavel, C.; Morrissey, C.; Zhang, X.; Comstock, C.; Witkiewicz, A.; et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J. Clin. Investig. 2010, 120, 4478–4492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, H.; Fleming, N.; Roth, I.; Mehta, S.; Wiles, A.; Williams, G.; Vennin, C.; Arsic, N.; Parkin, A.; Pajic, M.; et al. ∆133p53 isoform promotes tumour invasion and metastasis via interleukin-6 activation of JAK-STAT and RhoA-ROCK signalling. Nat. Commun. 2018, 9, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Tumor Types | Expression | Sample Type | Role | Functional Role in Vitro | Functional Role in Vivo | Related Genes/Protein/Pathways | Ref. |
|---|---|---|---|---|---|---|---|
| Bladder cancer | Up | cells (RT4, T24, J82, UMUC3, 5637), patient tissue and serum | Tumor promoter | Cell proliferation and apoptosis | NA | [24] | |
| Up | Cells (T24, EJ, 253j, 5637), patient tissue | Tumor promoter | Cell proliferation, migration, and invasion | miR-513a-5p | [21] | ||
| Up | Cells (T24T, EJ, UMUC3, 5637), patient tissue | Tumor promoter | Cell proliferation, migration, and invasion | miR-143-3p, PDIA6 | [25] | ||
| Breast cancer | Up | Cells (MDA-MB-231, MDA-MB-468, MDA-MB-436, HCC-1937), patient tissue | Tumor promoter | Cell proliferation, migration, invasion, EMT process, and angiogenesis | Tumor growth, metastasis, and angiogenesis | VEGF, VEGFR2/Akt/mTOR, miR-4723-5p, USP14, E-cadherin, N-cadherin, Slug, Twist, | [26] |
| Up | Cells (MDA-MB-468, MDA-MB-231), patient tissue | Tumor promoter | Cell proliferation, apoptosis, cell cycle, and radiosensitivity | miR-185-5p, TPD52 | [27] | ||
| Cervical cancer | Up | Cells (Caski, SW756, HeLa, ME-180, SiHa, C33A), patient tissue | Tumor promoter | Cell proliferation, apoptosis, migration, and invasion | Wnt/β-catenin, β-catenin, cyclin D1, c-myc | [28] | |
| Up | Cells (SiHa, HeLa, ME180, C-33A), patient tissue | Tumor promoter | Cell proliferation, apoptosis, migration, invasion, and chemoresistance | Tumor growth | miR-543, Bcl-2, cleaved-caspase 3, ZEB1 | [29] | |
| Colorectal cancer | Up | Cells (SW628, SW480, RKO, COLO320HSR, HCT116), patient tissue | Tumor promoter | Cell proliferation and apoptosis | Tumor growth | Cleaved-caspase 3, ARC, EZH2 | [30] |
| Up | Cells (HCT116, HT-29, SW620, SW480, DLD-1, RKO, LoVo), patient tissue | Tumor promoter | Cell proliferation and chemoresistance | miR-204, HMGA2, PI3K, Akt | [31] | ||
| Gastrointestinal stromal tumor | Up | Cells (GIST-H1, GIST-882, GIST-T1, GIST-48), patient tissue | Tumor promoter | Cell proliferation, stemness, and apoptosis | Wnt/β-catenin, miR-143-3p, PRDX5 | [32] | |
| Gastric cancer | Up | Cells (BGC-823, SGC-7901, HGC-27, MKN45), patient tissue | Tumor promoter | Cell proliferation, migration, EMT, and apoptosis | miR-30, MKRN3, caspase 3, caspase 9, Bax, Bcl-2, E-cadherin, N-cadherin, Vimentin, ZEB1, Snail | [34] | |
| Up | Cells (MKN45, SGC-7901, AGS, MKN28), patient tissue | Tumor promoter | Cell proliferation, EMT, and apoptosis | Cyclin D1, p53, Bax, cleaved caspase 3, E-cadherin, N-cadherin, Vimentin, Snail, ZEB1, miR-15a, RB/E2F, Wnt/β-catenin | [33] | ||
| Glioblastoma | Up | Cells (A172, U251, U87, LN229), patient tissue | Tumor promoter | Cell proliferation and apoptosis | YY1, miR-513, IGF2BP1, Akt | [35] | |
| Hepatocellular carcinoma | Up | Patient tissue | Tumor promoter | Cell proliferation and migration | NA | [36] | |
| Up | Cells (HuH7, SMMC-7721, Hep3B, HepG2, PLC/PRF/5), patient tissue | Tumor promoter | Cell proliferation, cycle, apoptosis, and migration | PCNA, CCND1, Bcl-2 | [37] | ||
| Up | Cells (MHCC97H, HepG2, Huh7), patient tissue | Tumor promoter | Cell proliferation and invasion | Tumor growth | miR-326, hnRNPA2B1 | [38] | |
| Lung cancer | Up | Cells (H1650, HCC827, H1975, A549), patient tissue | Tumor promoter | Cell proliferation, migration, and invasion, | Tumor growth | miR-330-5p | [39] |
| Up | Cells (SK-MES-1, H1703, H520, H1299, H1975, SPCA1, A549), patient tissue | Tumor promoter | Cell proliferation, cycle, apoptosis, migration, and invasion | Tumor growth | EZH2, LATS2 | [40] | |
| Up | Cells (H292, PC-9, CL1-5, H460, H1650, A549, H446, H1975) | Tumor promoter | Cell proliferation, apoptosis, and invasion | Bcl-2, Bax, c-myc, p53 | [20] | ||
| Up | Cells (H446, H1975), patient tissue | Tumor promoter | Cell proliferation, migration, invasion, and apoptosis | c-myc, MMP9, cleaved-caspase-3, Wnt5a, β-catenin. miR-326. | [42] | ||
| Up | Cells (H1838, H522, H2228, H358, H1299, A549), | Tumor promoter | Cell proliferation, migration, invasion, cycle, apoptosis | Tumor growth | Caspase-3, Ki-67 | [43] | |
| Osteosarcoma | Up | Cells (MG-63, Saos-2, 143B, U2OS), patient tissue | Tumor promoter | Cell proliferation, migration, invasion, and cell cycle | Tumor growth | ZEB1, miR-143-3p | [44] |
| Up | Cells (Saos2, MG63, U2OS, HOS) | Tumor promoter | Cell proliferation, migration, and invasion | Tumor growth | MMP2, MMP9, p53, p21, MDM2 | [46] | |
| Up | Cells (Saos2, HOS, U2OS, 143B, KHOS/240S, MG63, SK-ES-1), patient tissue | Tumor promoter | Cell proliferation, migration, and invasion | miR-185-5p, TGF-β, p-SMAD, TGFBR1/2 | [45] | ||
| Ovarian cancer | Up | Cells (OVCAR3, PEO1, A2780, 3AO, CAOV3, SKOV3), patient tissue | Tumor promoter | Cell proliferation, migration, and invasion | PTEN | [47] | |
| Up | Patient tissue | Tumor promoter | Cell proliferation, migration, and invasion | miR-143-3p, TAK1 | [48] | ||
| Cholangiocarcinoma | Up | Patient-derived macrophages, patient tissue | Tumor promoter | M2 polarization of macrophages, cellular reactive oxygen species production, mitochondrial and metabolic dysfunction | Tumor growth | miR-326, RhoA, ROCK1, ROCK2 | [54] |
| Up | Cell (ICC-9810, CCLP1, HuCC-T1, QBC939), patient tissue | Tumor promoter | Cell proliferation and invasion | miR-330-5p | [49] | ||
| Pituitary adenomas | Up | Patient tissue | Tumor promoter | Cell proliferation, migration, invasion, viability, apoptosis, cell cycle, and EMT | Tumor growth, apoptosis, EMT | miR-139-3p, BRD4, E-cadherin, N-cadherin, Bcl-2, Bax, cleaved-caspase 3 | [50] |
| Pancreatic ductal adenocarcinoma | Up | Cell (Capan-2, AsPC-1, PANC1, BxPC-3), Patient tissue | Tumor promoter | Cell proliferation, migration, and invasion | miR-185-5p, CBX2 | [51] | |
| Prostate cancer | Up | Cell (NCI-H660), patient tissue | Tumor promoter | Cell NED, proliferation, and invasion | Tumor growth and metastasis | NSE, SYP, ChgA, miR-326, hnRNPA2B1 | [52] |
| Up | Patient tissue | Tumor promoter | Cell proliferation, cycle, migration, and invasion | Tumor growth and BM | IGF2BP2, IGF1R, PI3K/Akt, NF-κB, METTL3, ALKBH5 | [53] |
| Tumor Types | Clinicopathologic Features | Ref. |
|---|---|---|
| Bladder cancer | Larger tumor size, high tumor differentiation, advanced TNM stage, more lymph nodes metastasis, more distant metastasis | [24] |
| Pathological stage | [21] | |
| Breast cancer | More tissues metastasis, higher tumor stages | [26] |
| More lymph nodes metastasis, advanced tumor stages | [27] | |
| Cervical cancer | Advanced FIGO stage, more lymph nodes metastasis, depth of cervical invasion | [28] |
| Colorectal cancer | Tumor subtype, N classification, metastasis, poorer clinical stage | [30] |
| Larger tumor size, advanced TNM stage, lymph node metastasis | [31] | |
| Gastric cancer | Larger tumor size, advanced TNM stage, more metastasis | [34] |
| Hepatocellular carcinoma | Moderated or poorly differentiation, advanced TNM stage | [37] |
| Lung cancer | Advanced TNM stage, more metastasis | [41] |
| Advanced TNM stage, more metastasis | [39] | |
| Larger tumor size, more lymph node metastasis, advanced TNM stage | [40] | |
| Larger tumor size, more lymph node metastasis, advanced TNM stage | [20] | |
| Larger tumor size, more metastasis, advanced TNM stage | [43] | |
| Osteosarcoma | Larger tumor size, more metastasis, advanced TNM stage | [44] |
| Larger tumor size, more metastasis, advanced TNM stage | [45] | |
| Ovarian cancer | More lymph node metastasis, more distant metastasis | [47] |
| Advanced TNM stage | [48] | |
| Cholangiocarcinoma | Advanced TNM stage | [49] |
| Pancreatic ductal adenocarcinoma | Advanced TNM stage, more lymph node invasion | [51] |
| Prostate cancer | Poor differentiation, higher serum PSA, advanced gleason grade, more BM | [53] |
| Tumor Types | Expression | Diagnostic or Prognostic Value | Ref. |
|---|---|---|---|
| Bladder cancer | Up | Poor OS, and poor PFS, AUC > 0.8 | [24] |
| Up | Poor OS | [21] | |
| Cervical cancer | Up | Shorter OS and DFS | [28] |
| Colorectal cancer | Up | Worse OS | [30] |
| Up | Poor OS | [31] | |
| Gastric cancer | Up | Worse prognosis | [34] |
| Hepatocellular carcinoma | Up | Poor OS | [36] |
| Up | Poor OS and DFS | [37] | |
| Up | Shorter OS | [38] | |
| Up | Poor PFS | [132] | |
| Lung cancer | Up | Tissue PCAT6: AUC > 0.9, sensitivity 86.67–100%, specificity 78.57–96%Plasma PCAT6: a. LUAD: AUC = 0.9213, sensitivity 87.67%, specificity 97.44; b. LUSC: AUC = 0.9583, sensitivity 94.12%, specificity 100% | [41] |
| Up | Poor OS | [40] | |
| Up | Poor OS | [20] | |
| Up | Poor OS | [43] | |
| Osteosarcoma | Up | Shorter OS and PFS | [44] |
| Up | Shorter OS and PFS | [45] | |
| Ovarian cancer | Up | Poor OS, PFS and PPS | [48] |
| Pancreatic ductal adenocarcinoma | Up | Worse OS | [51] |
| Prostate cancer | Up | Shorter overall and BM-free survival, shorter DFS | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Lv, Q.; Hu, C.; Li, Z.; Wu, H.; Gao, S.; Wang, H.; Zhao, Y.; Shao, Q. PCAT6 May Be a Whistler and Checkpoint Target for Precision Therapy in Human Cancers. Cancers 2021, 13, 6101. https://doi.org/10.3390/cancers13236101
Jiang F, Lv Q, Hu C, Li Z, Wu H, Gao S, Wang H, Zhao Y, Shao Q. PCAT6 May Be a Whistler and Checkpoint Target for Precision Therapy in Human Cancers. Cancers. 2021; 13(23):6101. https://doi.org/10.3390/cancers13236101
Chicago/Turabian StyleJiang, Feng, Qiaoyi Lv, Cexun Hu, Zhanghui Li, Haojie Wu, Shujun Gao, Hui Wang, Yangjing Zhao, and Qixiang Shao. 2021. "PCAT6 May Be a Whistler and Checkpoint Target for Precision Therapy in Human Cancers" Cancers 13, no. 23: 6101. https://doi.org/10.3390/cancers13236101







