64Cu-ATSM Predicts Efficacy of Carbon Ion Radiotherapy Associated with Cellular Antioxidant Capacity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Materials
2.2. Irradiation
2.3. Clonogenic Survival Assays
2.4. Immunoblotting
2.5. ROS Assays
2.6. Assessment of TCA Cycle Intermediates
2.7. Assessment of Tumor Xenograft Growth
2.8. Assessment of 64Cu-ATSM Uptake
2.9. Statistical Analysis
3. Results
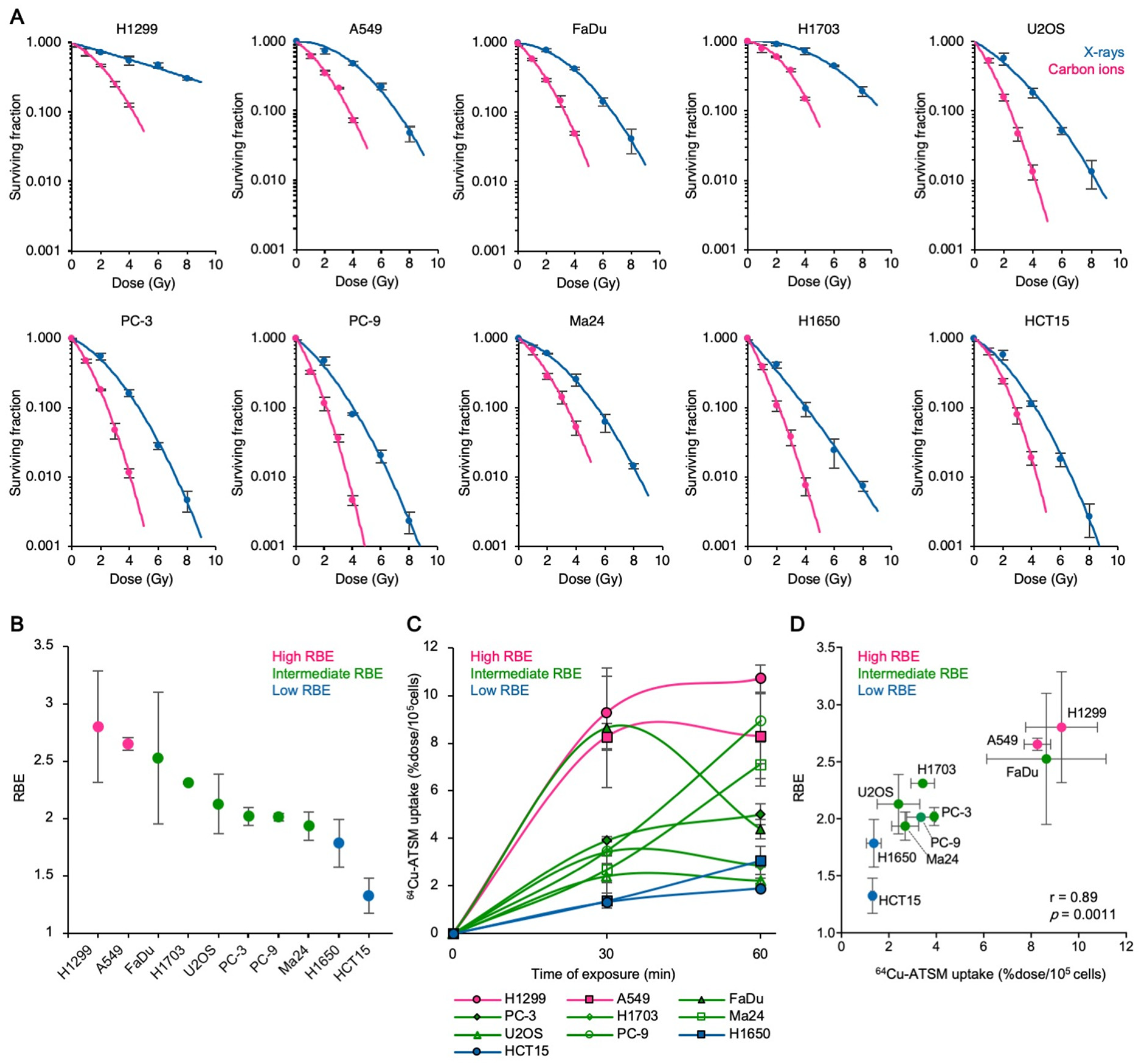
3.1. Carbon Ion RBE Correlates with 64Cu-ATSM Uptake In Vitro
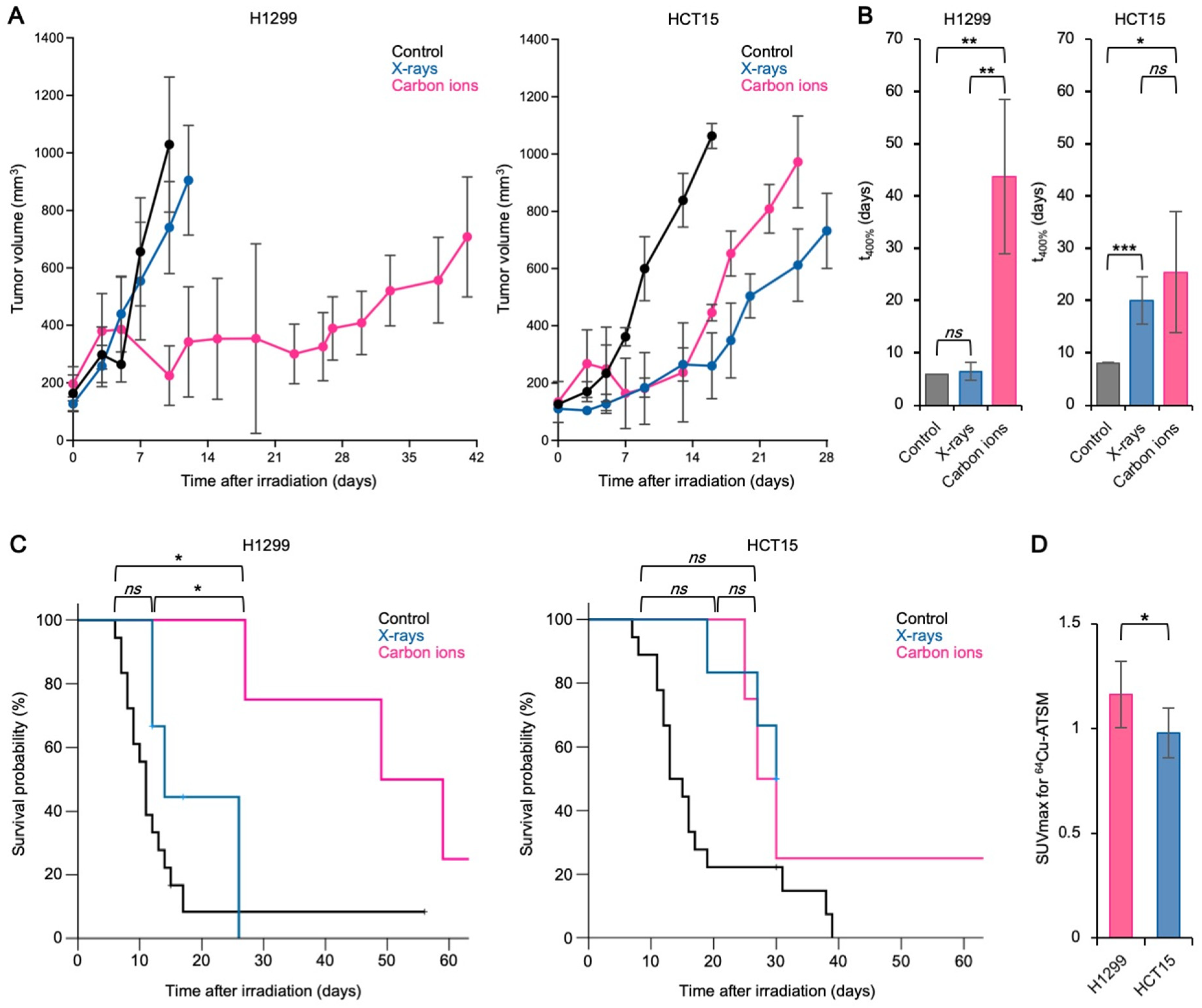
3.2. The Carbon Ion RBE Correlates with 64Cu-ATSM Uptake In Vivo
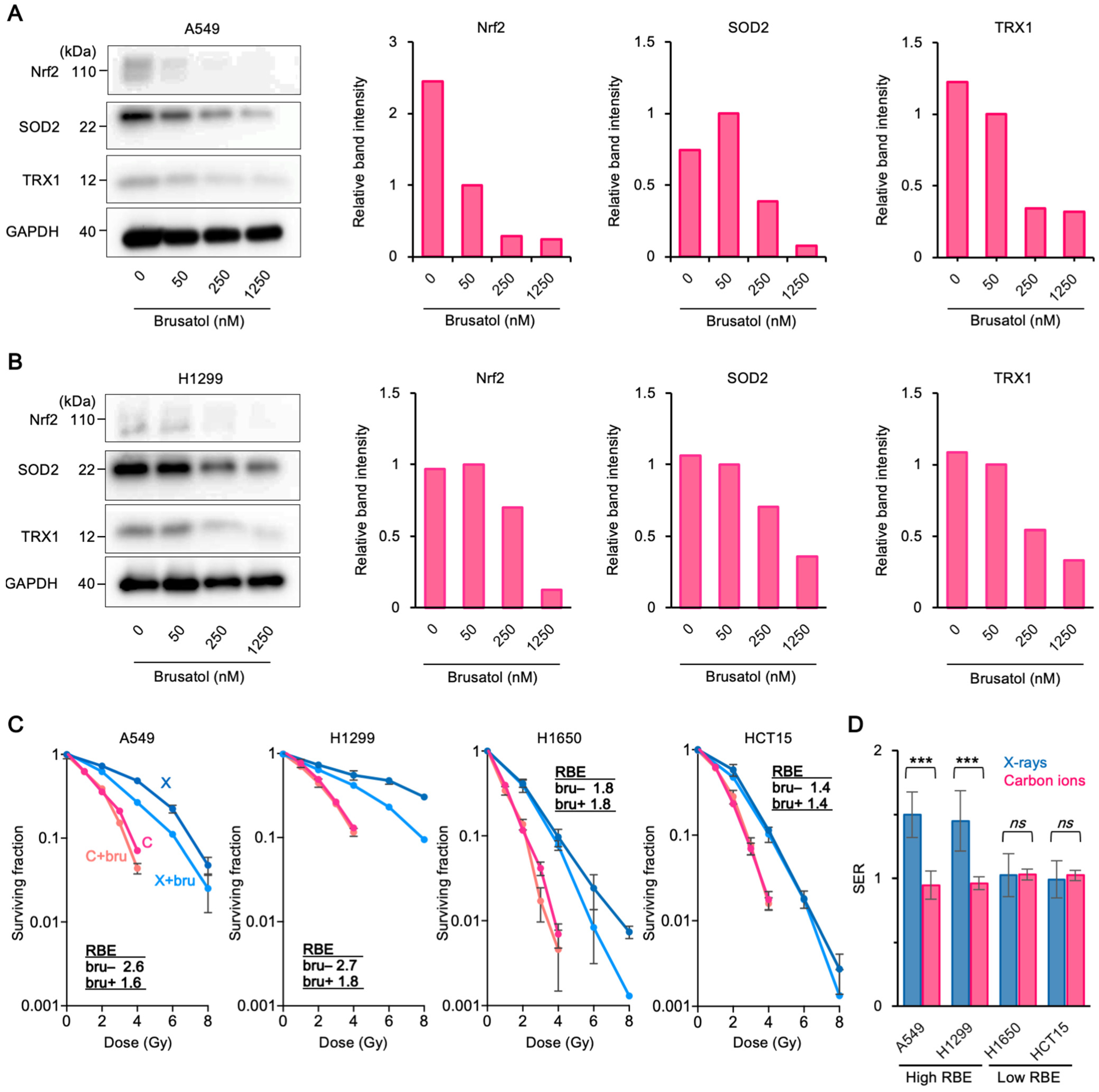
3.3. Upregulation of Antioxidant Systems Plays a Role in the High Carbon Ion RBE and 64Cu-ATSM Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Mizoe, J.E.; Hasegawa, A.; Jingu, K.; Takagi, R.; Bessyo, H.; Morikawa, T.; Tonoki, M.; Tsuji, H.; Kamada, T.; Tsujii, H.; et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother. Oncol. 2012, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, J.I.; Shirai, K.; Mizukami, T.; Abe, T.; Ebara, T.; Ohno, T.; Minato, K.; Saito, R.; Yamada, M.; Nakano, T. Hypofractionated carbon-ion radiotherapy for stage I peripheral nonsmall cell lung cancer (GUNMA0701): Prospective phase II study. Cancer Med. 2019, 8, 6644–6650. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Komatsu, S.; Abe, T.; Kubo, N.; Okano, N.; Shibuya, K.; Shirai, K.; Kawamura, H.; Saitoh, J.I.; Ebara, T.; et al. Comparison of Oncologic Outcomes between Carbon Ion Radiotherapy and Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer. Cancers 2021, 13, 176. [Google Scholar] [CrossRef]
- Yamada, S.; Takiyama, H.; Isozaki, Y.; Shinoto, M.; Makishima, H.; Yamamoto, N.; Tsuji, H. Carbon-ion Radiotherapy for Colorectal Cancer. J. Anus Rectum Colon 2021, 5, 113–120. [Google Scholar] [CrossRef]
- Sato, H.; Kasuya, G.; Ishikawa, H.; Nomoto, A.; Ono, T.; Nakajima, M.; Isozaki, Y.; Yamamoto, N.; Iwai, Y.; Nemoto, K.; et al. Long-term clinical outcomes after 12-fractionated carbon-ion radiotherapy for localized prostate cancer. Cancer Sci. 2021, 112, 3598–3606. [Google Scholar] [CrossRef]
- Ishikawa, H.; Tsuji, H.; Kamada, T.; Akakura, K.; Suzuki, H.; Shimazaki, J.; Tsujii, H. Working Group for Genitourinary Tumors. Carbon-ion radiation therapy for prostate cancer. Int. J. Urol. 2012, 19, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Matsunobu, A.; Imai, R.; Kamada, T.; Imaizumi, T.; Tsuji, H.; Tsujii, H.; Shioyama, Y.; Honda, H.; Tatezaki, S. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 2012, 118, 4555–4563. [Google Scholar] [CrossRef]
- Osu, N.; Kobayashi, D.; Shirai, K.; Musha, A.; Sato, H.; Hirota, Y.; Shibata, A.; Oike, T.; Ohno, T. Relative Biological Effectiveness of Carbon Ions for Head-and-Neck Squamous Cell Carcinomas According to Human Papillomavirus Status. J. Pers. Med. 2020, 10, 71. [Google Scholar] [CrossRef]
- Kobayashi, D.; Oike, T.; Shibata, A.; Niimi, A.; Kubota, Y.; Sakai, M.; Amornwhichet, N.; Yoshimoto, Y.; Hagiwara, Y.; Kimura, Y.; et al. Mitotic catastrophe is a putative mechanism underlying the weak correlation between sensitivity to carbon ions and cisplatin. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amornwichet, N.; Oike, T.; Shibata, A.; Nirodi, C.S.; Ogiwara, H.; Makino, H.; Kimura, Y.; Hirota, Y.; Isono, M.; Yoshida, Y.; et al. The EGFR mutation status affects the relative biological effectiveness of carbon-ion beams in non-small cell lung carcinoma cells. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Particle Therapy Co-Operative Group. Particle Therapy Facilities in Clinical Operation. Available online: https://www.ptcog.ch/index.php/facilities-in-operation (accessed on 30 July 2021).
- Oike, T.; Sato, H.; Noda, S.E.; Nakano, T. Translational research to improve the efficacy of carbon ion radiotherapy: Experience of Gunma University. Front. Oncol. 2016, 6, 139. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 38–53. [Google Scholar]
- Abdul-Aziz, A.; MacEwan, D.J.; Bowles, K.M.; Rushworth, S.A. Oxidative stress responses and NRF2 in human leukaemia. Oxid. Med. Cell. Longev. 2015, 2015, 454659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanito, M.; Agbaga, M.P.; Anderson, R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007, 42, 1838–1850. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). Int. J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Colombié, M.; Gouard, S.; Frindel, M.; Vidal, A.; Chérel, M.; Kraeber-Bodéré, F.; Rousseau, C.; Bourgeois, M. Focus on the controversial aspects of 64Cu-ATSM in tumoral hypoxia mapping by PET imaging. Front. Med. 2015, 2, 58. [Google Scholar] [CrossRef] [Green Version]
- Obata, A.; Yoshimi, E.; Waki, A.; Lewis, J.S.; Oyama, N.; Welch, M.J.; Saji, H.; Yonekura, Y.; Fujibayashi, Y. Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent Cu-diacetyl-bis (N 4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Ann. Nucl. Med. 2001, 15, 499–504. [Google Scholar] [CrossRef]
- Lewis, J.S.; McCarthy, D.W.; McCarthy, T.J.; Fujibayashi, Y.; Welch, M.J. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J. Nucl. Med. 1999, 40, 177–183. [Google Scholar]
- Dearling, J.L.; Packard, A.B. Some thoughts on the mechanism of cellular trapping of Cu (II)-ATSM. Nucl. Med. Biol. 2010, 37, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Zhao, M.; Shinohara, A.; Namikawa, O.; Ogura, T.; Masuda, N.; Takada, M.; Fukuoka, M.; Sone, S. Differential expressions of cyclin A and the retinoblastoma gene product in histological subtypes of lung cancer cell lines. J. Cancer Res. Clin. Oncol. 1997, 123, 533–538. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Oike, T.; Hirota, Y.; Dewi Maulany Darwis, N.; Shibata, A.; Ohno, T. Comparison of Clonogenic Survival Data Obtained by Pre-and Post-Irradiation Methods. J. Pers. Med. 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, K.; Murakami, M.; Hishikawa, Y.; Abe, M.; Akagi, T.; Yanou, T.; Kagiya, G.; Furusawa, Y.; Ando, K.; Nojima, K.; et al. Preclinical biological assessment of proton and carbon ion beams at Hyogo Ion Beam Medical Center. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 928–938. [Google Scholar] [CrossRef]
- Barazzuol, L.; Jeynes, J.C.; Merchant, M.J.; Wéra, A.C.; Barry, M.A.; Kirkby, K.J.; Suzuki, M. Radiosensitization of glioblastoma cells using a histone deacetylase inhibitor (SAHA) comparing carbon ions with X-rays. Int. J. Radiat. Biol. 2015, 91, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Shibata, A.; Moiani, D.; Arvai, A.S.; Perry, J.; Harding, S.M.; Genois, M.M.; Maity, R.; van Rossum-Fikkert, S.; Kertokalio, A.; Romoli, F.; et al. DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities. Mol. Cell. 2014, 53, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, T.; Desantis, A.; Bossi, G.; Di Agostino, S.; Sorino, C.; De Nicola, F.; Iezzi, S.; Franchitto, A.; Benassi, B.; Galanti, S.; et al. Che-1 promotes tumor cell survival by sustaining mutant p53 transcription and inhibiting DNA damage response activation. Cancer Cell. 2010, 18, 122–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, T.J.; Tian, H.; Joseph, I.B.; Menon, K.; Frost, D. Chemosensitization and radiosensitization of human lung and colon cancers by antimitotic agent, ABT-751, in athymic murine xenograft models of subcutaneous tumor growth. Cancer Chemother. Pharmacol. 2007, 59, 725–732. [Google Scholar] [CrossRef]
- Fujibayashi, Y.; Cutler, C.S.; Anderson, C.J.; McCarthy, D.W.; Jones, L.A.; Sharp, T.; Yonekura, Y.; Welch, M.J. Comparative studies of Cu-64-ATSM and C-11-acetate in an acute myocardial infarction model: Ex vivo imaging of hypoxia in rats. Nucl. Med. Biol. 1999, 26, 117–121. [Google Scholar] [CrossRef]
- Burgman, P.; O’Donoghue, J.A.; Lewis, J.S.; Welch, M.J.; Humm, J.L.; Ling, C.C. Cell line-dependent differences in uptake and retention of the hypoxia-selective nuclear imaging agent Cu-ATSM. Nucl. Med. Biol. 2005, 32, 623–630. [Google Scholar] [CrossRef]
- Floberg, J.M.; Wang, L.; Bandara, N.; Rashmi, R.; Mpoy, C.; Garbow, J.R.; Rogers, B.E.; Patti, G.J.; Schwarz, J.K. Alteration of Cellular Reduction Potential Will Change 64Cu-ATSM Signal with or without Hypoxia. J. Nucl. Med. 2020, 61, 427–432. [Google Scholar] [CrossRef]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol Phys. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- O’Donoghue, J.A.; Zanzonico, P.; Pugachev, A.; Wen, B.; Smith-Jones, P.; Cai, S.; Burnazi, E.; Finn, R.D.; Burgman, P.; Ruan, S.; et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu (II)-diacetyl-bis (N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1493–1502. [Google Scholar]
- Lewis, J.S.; Laforest, R.; Dehdashti, F.; Grigsby, P.W.; Welch, M.J.; Siegel, B.A. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J. Nucl. Med. 2008, 49, 1177–1182. [Google Scholar] [CrossRef] [Green Version]
- Sayin, V.I.; LeBoeuf, S.E.; Singh, S.X.; Davidson, S.M.; Biancur, D.; Guzelhan, B.S.; Alvarez, S.W.; Wu, W.L.; Karakousi, T.R.; Zavitsanou, A.M.; et al. Activation of the NRF2 antioxidant program generates an imbalance in central carbon metabolism in cancer. Elife 2017, 6, e28083. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Papagiannakopoulos, T.; Motohashi, H. Metabolic features of cancer cells in NRF2 addiction status. Biophys. Rev. 2020, 12, 435–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, M.; Rajerison, H.; Guerard, F.; Mougin-Degraef, M.; Barbet, J.; Michel, N.; Cherel, M.; Faivre-Chauvet, A. Contribution of (64Cu)-ATSM PET in molecular imaging of tumour hypoxia compared to classical (18F)-MISO—A selected review. Nucl. Med. Rev. 2011, 14, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérès, E.A.; Toutain, J.; Paty, L.P.; Divoux, D.; Ibazizène, M.; Guillouet, S.; Barré, L.; Vidal, A.; Cherel, M.; Bourgeois, M.; et al. 64 Cu-ATSM/64 Cu-Cl 2 and their relationship to hypoxia in glioblastoma: A preclinical study. EJNMMI Res. 2019, 9, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Karlsen, M.; Karlberg, A.M.; Redalen, K.R. Hypoxia imaging and theranostic potential of [64 Cu] [Cu (ATSM)] and ionic Cu (II) salts: A review of current evidence and discussion of the retention mechanisms. EJNMMI Res. 2020, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Carlin, S.; Zhang, H.; Reese, M.; Ramos, N.N.; Chen, Q.; Ricketts, S.A. A comparison of the imaging characteristics and microregional distribution of 4 hypoxia PET tracers. J. Nucl. Med. 2014, 55, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.; Tanaka, T.; Kobayashi, M.; Furukawa, T.; Mori, T.; Kudo, T.; Fujieda, S.; Fujibayashi, Y. Radio-copper-labeled Cu-ATSM: An indicator of quiescent but clonogenic cells under mild hypoxia in a Lewis lung carcinoma model. Nucl. Med. Biol. 2009, 36, 419–426. [Google Scholar] [CrossRef]
- Yoshii, Y.; Yoneda, M.; Ikawa, M.; Furukawa, T.; Kiyono, Y.; Mori, T.; Yoshii, H.; Oyama, N.; Okazawa, H.; Saga, T.; et al. Radiolabeled Cu-ATSM as a novel indicator of overreduced intracellular state due to mitochondrial dysfunction: Studies with mitochondrial DNA-less ρ0 cells and cybrids carrying MELAS mitochondrial DNA mutation. Nucl. Med. Biol. 2012, 39, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, R.; Ito, A.; Tomita, M.; Tsukada, T.; Yatagai, F.; Noguchi, M.; Matsumoto, Y.; Kase, Y.; Ando, K.; Okayasu, R.; et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat. Res. 2009, 171, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Schlaff, C.D.; Krauze, A.; Belard, A.; O’Connell, J.J.; Camphausen, K.A. Bringing the heavy: Carbon ion therapy in the radiobiological and clinical context. Radiother. Oncol. 2014, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tinganelli, W.; Durante, M. Carbon ion radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachankar, A.; Oike, T.; Hanaoka, H.; Kanai, A.; Sato, H.; Yoshida, Y.; Obinata, H.; Sakai, M.; Osu, N.; Hirota, Y.; et al. 64Cu-ATSM Predicts Efficacy of Carbon Ion Radiotherapy Associated with Cellular Antioxidant Capacity. Cancers 2021, 13, 6159. https://doi.org/10.3390/cancers13246159
Nachankar A, Oike T, Hanaoka H, Kanai A, Sato H, Yoshida Y, Obinata H, Sakai M, Osu N, Hirota Y, et al. 64Cu-ATSM Predicts Efficacy of Carbon Ion Radiotherapy Associated with Cellular Antioxidant Capacity. Cancers. 2021; 13(24):6159. https://doi.org/10.3390/cancers13246159
Chicago/Turabian StyleNachankar, Ankita, Takahiro Oike, Hirofumi Hanaoka, Ayaka Kanai, Hiro Sato, Yukari Yoshida, Hideru Obinata, Makoto Sakai, Naoto Osu, Yuka Hirota, and et al. 2021. "64Cu-ATSM Predicts Efficacy of Carbon Ion Radiotherapy Associated with Cellular Antioxidant Capacity" Cancers 13, no. 24: 6159. https://doi.org/10.3390/cancers13246159
APA StyleNachankar, A., Oike, T., Hanaoka, H., Kanai, A., Sato, H., Yoshida, Y., Obinata, H., Sakai, M., Osu, N., Hirota, Y., Takahashi, A., Shibata, A., & Ohno, T. (2021). 64Cu-ATSM Predicts Efficacy of Carbon Ion Radiotherapy Associated with Cellular Antioxidant Capacity. Cancers, 13(24), 6159. https://doi.org/10.3390/cancers13246159







