Multi-Marker Immunofluorescent Staining and PD-L1 Detection on Circulating Tumour Cells from Ovarian Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Optimisation of Antibodies for Immunostaining
2.3. Spiking Experiment and Enrichment Platform
2.4. Immunostaining of Spiked Cells and CTCs on Glass Slides Using the Fluorescent Quenching and Re-Staining Method
2.5. Isolation and Detection of CTCs from the Blood of Patients and Healthy Controls
2.6. Statistical Analysis
3. Results
3.1. Phenotypic Characterisation of OC Cell Lines
3.2. Immunocytochemistry of Spiked Cell Lines
3.3. Recovery Rate of Spiked Cells
3.4. CTC Detection from Patient Blood
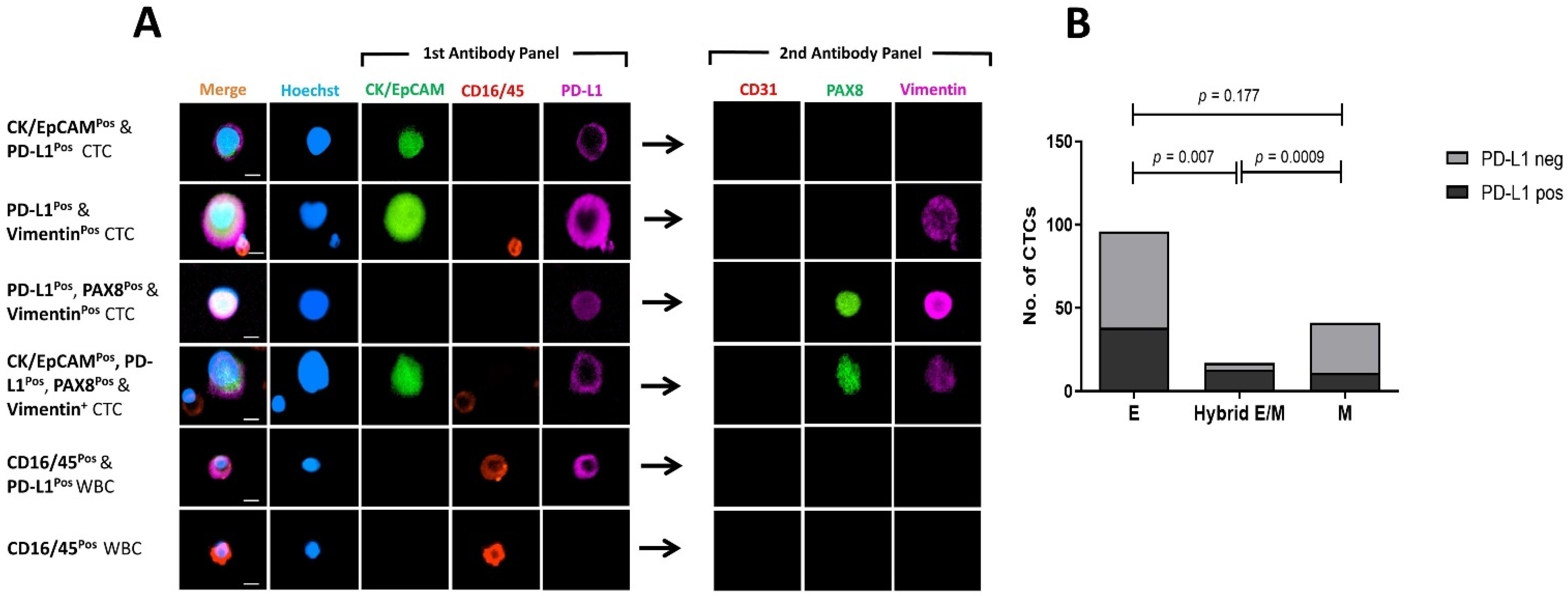
3.5. Marker Co-Expression and Exclusion of CD31pos Endothelial Cells
3.6. Comparing PD-L1 Expression on Mesenchymal and Epithelial CTCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantel, K.; Brakenhoff, R.H. Dissecting the metastatic cascade. Nat. Rev. Cancer 2004, 4, 448–456. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Balasubramanian, P.; Chen, A.P.; Kummar, S.; Evrard, Y.A.; Kinders, R.J. Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin Oncol. 2016, 43, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Chebouti, I.; Kasimir-Bauer, S.; Buderath, P.; Wimberger, P.; Hauch, S.; Kimmig, R.; Kuhlmann, J.D. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 2017, 8, 48820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjunath, Y.; Upparahalli, S.V.; Avella, D.M.; Deroche, C.B.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Smith, C.J.; Li, G.; Kaifi, J.T. PD-L1 expression with epithelial mesenchymal transition of circulating tumor cells is associated with poor survival in curatively resected non-small cell lung cancer. Cancers 2019, 11, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.C.; Robinson, P.S.; Wagner, C.; O’Shannessy, D.J. The Parsortix™ cell separation system—A versatile liquid biopsy platform. Cytom. Part A 2018, 93, 1234–1239. [Google Scholar] [CrossRef]
- Hvichia, G.; Parveen, Z.; Wagner, C.; Janning, M.; Quidde, J.; Stein, A.; Müller, V.; Loges, S.; Neves, R.; Stoecklein, N. A novel microfluidic platform for size and deformability based separation and the subsequent molecular characterization of viable circulating tumor cells. Int. J. Cancer 2016, 138, 2894–2904. [Google Scholar] [CrossRef]
- Scher, H.I.; Graf, R.P.; Schreiber, N.A.; McLaughlin, B.; Jendrisak, A.; Wang, Y.; Lee, J.; Greene, S.; Krupa, R.; Lu, D. Phenotypic heterogeneity of circulating tumor cells informs clinical decisions between AR signaling inhibitors and taxanes in metastatic prostate cancer. Cancer Res. 2017, 77, 5687–5698. [Google Scholar] [CrossRef] [Green Version]
- Gorges, T.M.; Tinhofer, I.; Drosch, M.; Röse, L.; Zollner, T.M.; Krahn, T.; Von Ahsen, O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asante, D.-B.; Calapre, L.; Ziman, M.; Meniawy, T.M.; Gray, E.S. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer Lett. 2020, 468, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Po, J.W.; Roohullah, A.; Lynch, D.; DeFazio, A.; Harrison, M.; Harnett, P.R.; Kennedy, C.; de Souza, P.; Becker, T.M. Improved ovarian cancer EMT-CTC isolation by immunomagnetic targeting of epithelial EpCAM and mesenchymal N-cadherin. J. Circ. Biomark. 2018, 7, 1849454418782617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.L.; Alpaugh, R.K.; Tsai, S.; Tang, C.-M.; Stefansson, S. Multi-Phenotypic subtyping of circulating tumor cells using sequential fluorescent quenching and restaining. Sci. Rep. 2016, 6, 33488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-h.; Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [Green Version]
- Tsao, S.-W.; Mok, S.C.; Fey, E.G.; Fletcher, J.A.; Wan, T.S.; Chew, E.-C.; Muto, M.G.; Knapp, R.C.; Berkowitz, R.S. Characterization of human ovarian surface epithelial cells immortalized by human papilloma viral oncogenes (HPV-E6E7 ORFs). Exp. Cell Res. 1995, 218, 499–507. [Google Scholar] [CrossRef]
- Adler, E.; Mhawech-Fauceglia, P.; Gayther, S.A.; Lawrenson, K. PAX8 expression in ovarian surface epithelial cells. Hum. Pathol. 2015, 46, 948–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, Y.; Li, J.; Yuan, Z.; Yuan, B.; Zhang, T.; Cragun, J.M.; Kong, B.; Zheng, W. PAX8: A sensitive and specific marker to identify cancer cells of ovarian origin for patients prior to neoadjuvant chemotherapy. J. Hematol. Oncol. 2013, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Gowrishankar, K.; Gunatilake, D.; Gallagher, S.J.; Tiffen, J.; Rizos, H.; Hersey, P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS ONE 2015, 10, e0123410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherian, S.; Levin, G.; Lo, W.Y.; Mauck, M.; Kuhn, D.; Lee, C.; Wood, B.L. Evaluation of an 8-color flow cytometric reference method for white blood cell differential enumeration. Cytom. B Clin. Cytom. 2010, 78, 319–328. [Google Scholar] [CrossRef]
- Adams, D.L.; Adams, D.K.; He, J.; Kalhor, N.; Zhang, M.; Xu, T.; Gao, H.; Reuben, J.M.; Qiao, Y.; Komaki, R. Sequential tracking of PD-L1 expression and RAD50 induction in circulating tumor and stromal cells of lung cancer patients undergoing radiotherapy. Clin. Cancer Res. 2017, 23, 5948–5958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakuła, M.; Mikuła-Pietrasik, J.; Witucka, A.; Kostka-Jeziorny, K.; Uruski, P.; Moszyński, R.; Naumowicz, E.; Sajdak, S.; Tykarski, A.; Książek, K. The epithelial-mesenchymal transition initiated by malignant ascites underlies the transmesothelial invasion of ovarian cancer cells. Int. J. Mol. Sci. 2019, 20, 137. [Google Scholar] [CrossRef] [Green Version]
- Oyer, J.L.; Gitto, S.B.; Altomare, D.A.; Copik, A.J. PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology 2018, 7, e1509819. [Google Scholar] [CrossRef] [PubMed]
- Obermayr, E.; Maritschnegg, E.; Agreiter, C.; Pecha, N.; Speiser, P.; Helmy-Bader, S.; Danzinger, S.; Krainer, M.; Singer, C.; Zeillinger, R. Efficient leukocyte depletion by a novel microfluidic platform enables the molecular detection and characterization of circulating tumor cells. Oncotarget 2018, 9, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, C.; Joosse, S.A.; Schneegans, S.; Wilken, O.J.; Janning, M.; Loreth, D.; Müller, V.; Prieske, K.; Banys-Paluchowski, M.; Horst, L.J. Pre-analytical and analytical variables of label-independent enrichment and automated detection of circulating tumor Cells in cancer patients. Cancers 2020, 12, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, S.O.; Pantel, K. A new era in liquid biopsy: From genotype to phenotype. Clin. Chem. 2020, 66, 89–96. [Google Scholar] [CrossRef]
- Kim, M.; Suh, D.H.; Choi, J.Y.; Bu, J.; Kang, Y.-T.; Kim, K.; No, J.H.; Kim, Y.B.; Cho, Y.-H. Post-debulking circulating tumor cell as a poor prognostic marker in advanced stage ovarian cancer: A prospective observational study. Medicine 2019, 98, e15354. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kim, M.; Choi, J.Y.; Bu, J.; Kang, Y.-T.; Kwon, B.S.; Lee, B.; Kim, K.; No, J.H.; Kim, Y.-B. Circulating tumor cells in the differential diagnosis of adnexal masses. Oncotarget 2017, 8, 77195. [Google Scholar] [CrossRef] [Green Version]
- Pearl, M.L.; Dong, H.; Tulley, S.; Zhao, Q.; Golightly, M.; Zucker, S.; Chen, W.-T. Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs). Gynecol. Oncol. 2015, 137, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Kindelberger, D.; Doyle, C.; Lowe, A.; Barry, W.T.; Matulonis, U.A. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol. Oncol. 2013, 131, 352–356. [Google Scholar] [CrossRef]
- Guo, Y.-X.; Neoh, K.H.; Chang, X.-H.; Sun, Y.; Cheng, H.-Y.; Ye, X.; Ma, R.-Q.; Han, R.P.; Cui, H. Diagnostic value of HE4+ circulating tumor cells in patients with suspicious ovarian cancer. Oncotarget 2018, 9, 7522. [Google Scholar] [CrossRef]
- Xu, L.; Mao, X.; Guo, T.; Chan, P.Y.; Shaw, G.; Hines, J.; Stankiewicz, E.; Wang, Y.; Oliver, R.T.D.; Ahmad, A.S. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 2017, 23, 5112–5122. [Google Scholar] [CrossRef] [Green Version]
- Morrison, G.J.; Cunha, A.T.; Jojo, N.; Xu, Y.; Xu, Y.; Kwok, E.; Robinson, P.; Dorff, T.; Quinn, D.; Carpten, J. Cancer transcriptomic profiling from rapidly enriched circulating tumor cells. Int. J. Cancer 2020, 146, 2845–2854. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, C.; Le Moulec, S.; Billiot, F.; Loriot, Y.; Ngo-Camus, M.; Vielh, P.; Fizazi, K.; Massard, C.; Farace, F. Vimentin and Ki67 expression in circulating tumour cells derived from castrate-resistant prostate cancer. BMC Cancer 2016, 16, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jou, H.-J.; Chou, L.-Y.; Chang, W.-C.; Ho, H.-C.; Zhang, W.-T.; Ling, P.-Y.; Tsai, K.-H.; Chen, S.-H.; Chen, T.-H.; Lo, P.-H. An automatic platform based on nanostructured microfluidic chip for isolating and identification of circulating tumor cells. Micromachines 2021, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, J.; Jin, Y.; Cheng, S.; Huang, S.; Zhang, N.; Wang, Y. Development and validation for prognostic nomogram of epithelial ovarian cancer recurrence based on circulating tumor cells and epithelial–mesenchymal transition. Sci. Rep. 2021, 11, 6540. [Google Scholar] [CrossRef]
- Gening, S.O.; Abakumova, T.V.; Gafurbaeva, D.U.; Rizvanov, A.A.; Antoneeva, I.I.; Miftakhova, R.R.; Peskov, A.B.; Gening, T.P. The Detection of Stem-Like Circulating Tumor Cells Could Increase the Clinical Applicability of Liquid Biopsy in Ovarian Cancer. Life 2021, 11, 815. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 2019, 176, 98–112.e114. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Muinelo-Romay, L.; Cebey-López, V.; Pereira-Veiga, T.; Martínez-Pena, I.; Abreu, M.; Abalo, A.; Lago-Lestón, R.M.; Abuín, C.; Palacios, P. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-clusters) as Predictors of Patient Outcomes. Cancers 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, E.J.; Cho, Y.; Kim, S.; Chung, H.H.; Park, N.H.; Song, Y.-S. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Bonome, T.; Lee, J.-Y.; Park, D.-C.; Radonovich, M.; Pise-Masison, C.; Brady, J.; Gardner, G.J.; Hao, K.; Wong, W.H.; Barrett, J.C. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005, 65, 10602–10612. [Google Scholar] [CrossRef] [Green Version]
- Ostrand-Rosenberg, S.; Horn, L.A.; Haile, S.T. The programmed death-1 immune-suppressive pathway: Barrier to antitumor immunity. J. Immunol. 2014, 193, 3835–3841. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Doo, D.W.; Norian, L.A.; Arend, R.C. Checkpoint inhibitors in ovarian cancer: A review of preclinical data. Gynecol. Oncol. Rep. 2019, 29, 48–54. [Google Scholar] [CrossRef]
- Arend, R.C.; Jackson-Fisher, A.; Jacobs, I.A.; Chou, J.; Monk, B.J. Ovarian cancer: New strategies and emerging targets for the treatment of patients with advanced disease. Cancer Biol. Ther. 2021, 22, 89–105. [Google Scholar] [CrossRef]
- Khattak, M.A.; Reid, A.; Freeman, J.; Pereira, M.; McEvoy, A.; Lo, J.; Frank, M.H.; Meniawy, T.; Didan, A.; Spencer, I. PD-L1 Expression on Circulating Tumor Cells May Be Predictive of Response to Pembrolizumab in Advanced Melanoma: Results from a Pilot Study. Oncologist 2020, 25, e520. [Google Scholar] [CrossRef] [Green Version]
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Mourlanette, J.; Gouin, S.; Dormoy, I.; Favre, G. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018, 120, 108–112. [Google Scholar] [CrossRef]
- Anantharaman, A.; Friedlander, T.; Lu, D.; Krupa, R.; Premasekharan, G.; Hough, J.; Edwards, M.; Paz, R.; Lindquist, K.; Graf, R. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 2016, 16, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 2018, 7, e1438111. [Google Scholar] [CrossRef]
- Tsao, M.S.; Kerr, K.M.; Kockx, M.; Beasley, M.B.; Borczuk, A.C.; Botling, J.; Bubendorf, L.; Chirieac, L.; Chen, G.; Chou, T.Y.; et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018, 13, 1302–1311. [Google Scholar] [CrossRef] [Green Version]
- Alsuliman, A.; Colak, D.; Al-Harazi, O.; Fitwi, H.; Tulbah, A.; Al-Tweigeri, T.; Al-Alwan, M.; Ghebeh, H. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: Significance in claudin-low breast cancer cells. Mol. Cancer 2015, 14, 149. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; Diao, L.; Cuentas, E.R.P.; Denning, W.L.; Chen, L.; Fan, Y.H.; Byers, L.A.; Wang, J.; Papadimitrakopoulou, V.A.; Behrens, C. Epithelial–mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin. Cancer Res. 2016, 22, 3630–3642. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.-H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Georganaki, M.; van Hooren, L.; Dimberg, A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front. Immunol. 2018, 9, 3081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Liu, Y.; Zhang, T.; Wang, Z.; Gu, M.; Li, Y.; Wang, D.D.; Li, W.; Lin, P.P. PD-L1+ aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett. 2020, 469, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.; Landin, J.; Szczerba, B.M.; Castro-Giner, F.; Gkountela, S.; Donato, C.; Krol, I.; Scherrer, R.; Balmelli, C.; Malinovska, A. Denosumab treatment is associated with the absence of circulating tumor cells in patients with breast cancer. Breast Cancer Res. 2018, 20, 141. [Google Scholar] [CrossRef]
- Aya-Bonilla, C.A.; Morici, M.; Hong, X.; McEvoy, A.C.; Sullivan, R.J.; Freeman, J.; Calapre, L.; Khattak, M.A.; Meniawy, T.; Millward, M. Detection and prognostic role of heterogeneous populations of melanoma circulating tumour cells. Br. J. Cancer 2020, 122, 1059–1067. [Google Scholar] [CrossRef]
- Chaves-Moreira, D.; Morin, P.J.; Drapkin, R. Unraveling the mysteries of PAX8 in reproductive tract cancers. Cancer Res. 2021, 81, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Bellahcene, A.; Bachelier, R.; Detry, C.; Lidereau, R.; Clezardin, P.; Castronovo, V. Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells. Breast Cancer Res. Treat. 2007, 101, 135–148. [Google Scholar] [CrossRef]
- Tan, C.-C.; Li, G.-X.; Tan, L.-D.; Du, X.; Li, X.-Q.; He, R.; Wang, Q.-S.; Feng, Y.-M. Breast cancer cells obtain an osteomimetic feature via epithelial-mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction. Oncotarget 2016, 7, 79688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelep, C.; Eberhardt, J. Automated rare single cell picking with the ALS cellcelector™. Cytom. Part A 2018, 93, 1267–1270. [Google Scholar] [CrossRef] [Green Version]
- Beasley, A.; Isaacs, T.; Khattak, M.A.; Freeman, J.B.; Allcock, R.; Chen, F.K.; Pereira, M.R.; Yau, K.; Bentel, J.; Vermeulen, T. Clinical application of circulating tumor cells and circulating tumor DNA in uveal melanoma. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Zheng, X.; Jin, B.; Zhang, F.; Zhu, L.; Cui, L. Effects of CD44 and E-cadherin overexpression on the proliferation, adhesion and invasion of ovarian cancer cells. Exp. Ther. Med. 2017, 14, 5557–5563. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| PID | Disease Status | Age (Years) | FIGO Stage | CA-125 (kU/L) | BRCA Status | Tumour PAX8 | Treatment | Lymphovascular Involvement | CRS/Patient Outcome | Ascites (Volume) | CTC Count |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OC 714 | Recurrent | 50 | IIIC | 114 | WT | Positive | Surgery/AC | NR | NA | Absent | 5 |
| OC 1248 | ND | 71 | IVA | 354 | WT | Positive | NACT | No | CRS 3 | Large | 0 |
| OC 1251 | ND | 68 | IIIC | 1858 | WT | Positive | NACT | Yes | CRS 2 | Large | 10 |
| OC 1313 | ND | 55 | IIIC | 51 | WT | Positive | NACT | Yes | CRS 1 | Small | 0 |
| OC 1350 | ND | 72 | IIIC | 554 | WT | Positive (Diffuse) | NACT | Yes | CRS 3 | Small | 0 |
| OC 1354 | ND | 74 | IIIC | 169 | WT | Positive | NACT | Yes | CRS 1 | Absent | 3 |
| OC 1362 | ND | 68 | IVA | 1001 | WT | Positive (Diffuse) | NACT | No | PD1st | Absent | 8 |
| OC 1409 | ND | 66 | IIIC | 429 | Mutant | Positive | NACT | Yes | CSR 2 | Small | 49 |
| OC 1418 | ND | 73 | IVB | 1200 | WT | Positive | NACT | Yes | CRS 2 | Medium | 0 |
| OC 1423 | ND | 53 | IVB | 313 | WT | Positive (Patchy) | NACT | No | CRS 2 | Small | 19 |
| OC 1382 | ND | 68 | IC | 570 | WT | NR | Surgery | Yes | Good | Small | 41 |
| OC 1388 | Recurrent | 79 | IIIA | 10 | WT | Positive | Surgery/AC | NR | PD† | NR | 13 |
| OC 1400 | ND | 65 | IA # | 27 | WT | Negative | Surgery | No | Good | Absent | 0 |
| OC 1436 | ND | 82 | IIIC | 125 | WT | Positive | Surgery | Yes | NA | Absent | 2 |
| OC 1458 | ND | 83 | IVA | 1056 | WT | Positive | NACT | NR | PD†a | Small | 0 |
| OC 1364 | ND | 58 | IIIC | 576 | WT | NR | NACT | NR | PD1st | NR | 7 |
| PID | Disease Status | CK/EpCAM | Vimentin | PAX8 | Total Count (CTCs) | PD-L1 |
|---|---|---|---|---|---|---|
| OC 714 | Recurrent | 2 | 4 | 3 | 5 | 0 |
| OC 1248 | ND | 0 | 0 | 0 | 0 | 0 |
| OC 1251 | ND | 5 | 7 | 1 | 10 | 3 |
| OC 1313 | ND | 0 | 0 | 0 | 0 | 0 |
| OC 1350 | ND | 0 | 0 | 0 | 0 | 0 |
| OC 1354 | ND | 3 | 3 | 0 | 3 | 0 |
| OC 1362 | ND | 5 | 3 | 0 | 8 | 6 |
| OC 1409 | ND | 47 | 14 | 2 | 49 | 45 |
| OC 1418 | ND | 0 | 0 | 0 | 0 | 0 |
| OC 1423 | ND | 2 | 17 | 1 | 19 | 0 |
| OC 1382 | ND | 39 | 3 | 1 | 41 | 0 |
| OC 1388 | Recurrent | 3 | 6 | 1 | 13 | 7 |
| OC 1400 | ND | 0 | 0 | 0 | 0 | 0 |
| OC 1436 | ND | 2 | 0 | 0 | 2 | 0 |
| OC 1458 | ND | 0 | 0 | 0 | 0 | 0 |
| OC 1364 | ND | 5 | 1 | 2 | 7 | 1 |
| TOTAL | 113 | 58 | 11 | 157 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asante, D.-B.; Morici, M.; Mohan, G.R.K.A.; Acheampong, E.; Spencer, I.; Lin, W.; van Miert, P.; Gibson, S.; Beasley, A.B.; Ziman, M.; et al. Multi-Marker Immunofluorescent Staining and PD-L1 Detection on Circulating Tumour Cells from Ovarian Cancer Patients. Cancers 2021, 13, 6225. https://doi.org/10.3390/cancers13246225
Asante D-B, Morici M, Mohan GRKA, Acheampong E, Spencer I, Lin W, van Miert P, Gibson S, Beasley AB, Ziman M, et al. Multi-Marker Immunofluorescent Staining and PD-L1 Detection on Circulating Tumour Cells from Ovarian Cancer Patients. Cancers. 2021; 13(24):6225. https://doi.org/10.3390/cancers13246225
Chicago/Turabian StyleAsante, Du-Bois, Michael Morici, Ganendra R. K. A. Mohan, Emmanuel Acheampong, Isaac Spencer, Weitao Lin, Paula van Miert, Samantha Gibson, Aaron B. Beasley, Melanie Ziman, and et al. 2021. "Multi-Marker Immunofluorescent Staining and PD-L1 Detection on Circulating Tumour Cells from Ovarian Cancer Patients" Cancers 13, no. 24: 6225. https://doi.org/10.3390/cancers13246225







