Epithelial-to-Mesenchymal Transition Mediates Resistance to Maintenance Therapy with Vinflunine in Advanced Urothelial Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Clinicopathological Baseline Characteristics
2.2. FFPE-Tissue RNA Extraction
2.3. Microarray Gene Expression Profiling
2.4. Functional Enrichment Analysis
2.5. Quantitative RT-PCR (qRT-PCR)
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Analysis of Combined Drug Effects
2.9. Colony-Formation Assay
2.10. Western Blotting
2.11. Apoptosis Assay
2.12. Statistical Analysis
3. Results
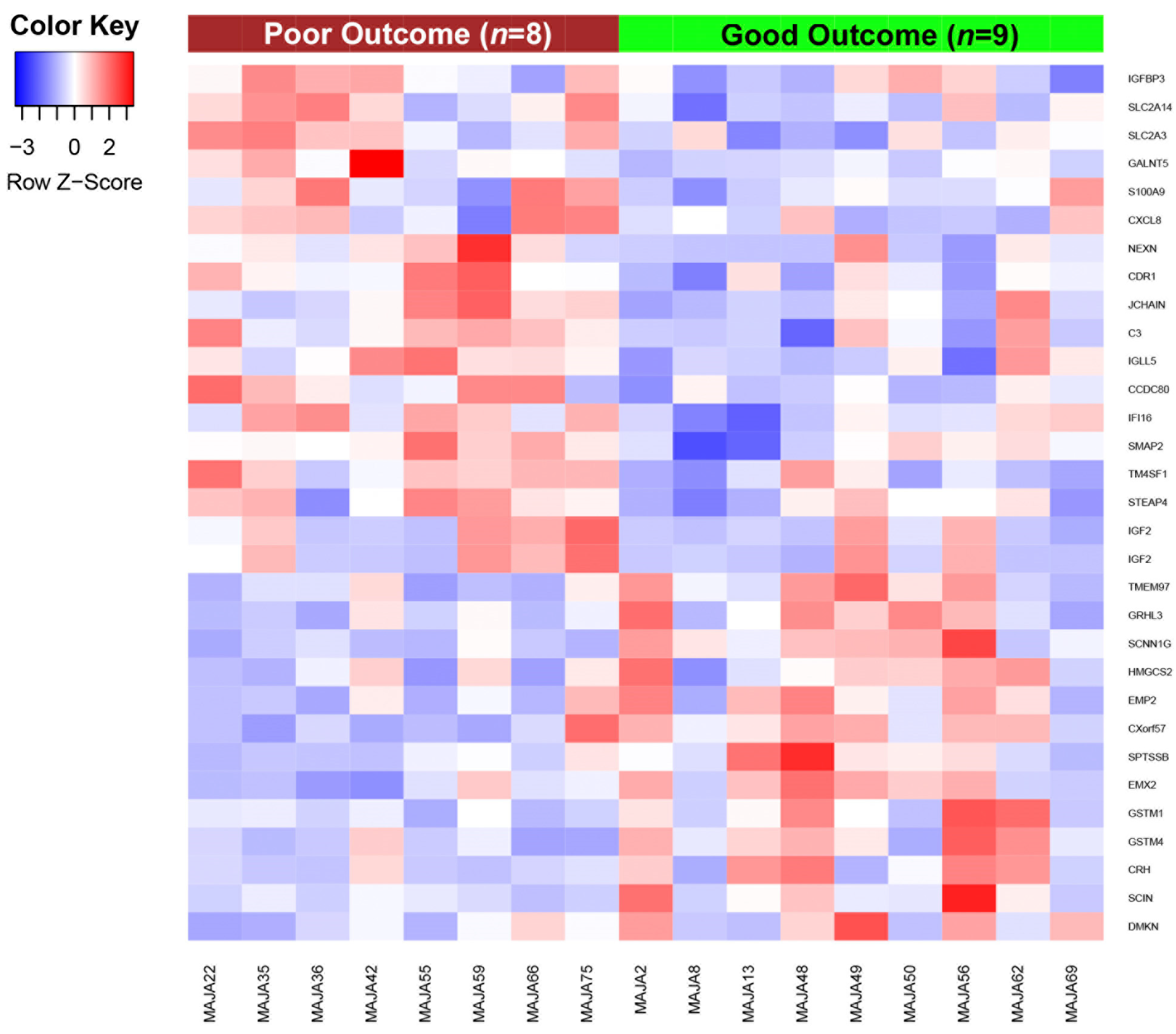
3.1. Differential Gene Expression Patterns between Patients with Good and Poor Outcome to Vinflunine Treatment
3.2. Epithelial-to-Mesenchymal Transition (EMT) Pathway Mediate Vinflunine Resistance in aUCC Patients
3.3. Downregulation of EMT Markers Enhances Vinflunine Sensitivity in UCC Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- Bellmunt, J.; von der Maase, H.; Mead, G.M.; Skoneczna, I.; De Santis, M.; Daugaard, G.; Boehle, A.; Chevreau, C.; Paz-Ares, L.; Laufman, L.R.; et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012, 30, 1107–1113. [Google Scholar] [CrossRef]
- Garcia, J.; Santome, L.; Anido, U.; Fernandez-Calvo, O.; Afonso-Afonso, J.; Lazaro, M.; Medina, A.; Vazquez Estevez, S. Metastatic Bladder Cancer: Second-Line Treatment and Recommendations of the Genitourinary Tumor Division of the Galician Oncologic Society (SOG-GU). Curr. Oncol. Rep. 2016, 18, 72. [Google Scholar] [CrossRef]
- Culine, S.; Theodore, C.; De Santis, M.; Bui, B.; Demkow, T.; Lorenz, J.; Rolland, F.; Delgado, F.M.; Longerey, B.; James, N. A hase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimen. Br. J. Cancer. 2006, 94, 1395–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughn, D.J.; Srinivas, S.; Stadler, W.M.; Pili, R.; Petrylak, D.; Sternberg, C.N.; Smith, D.C.; Ringuette, S.; de Wit, E.; Pautret, V.; et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: Results of a large phase 2 study. Cancer 2009, 115, 4110–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt, J.; Theodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Duran, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Garcia-Donas, J.; Font, A.; Perez-Valderrama, B.; Virizuela, J.A.; Climent, M.A.; Hernando-Polo, S.; Arranz, J.A.; Del Mar Llorente, M.; Lainez, N.; Villa-Guzman, J.C.; et al. Maintenance therapy with vinflunine plus best supportive care versus best supportive care alone in patients with advanced urothelial carcinoma with a response after first-line chemotherapy (MAJA; SOGUG 2011/02): A multicentre, randomised, controlled, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 672–681a. [Google Scholar]
- van der Heijden, M.S.; Loriot, Y.; Duran, I.; Ravaud, A.; Retz, M.; Vogelzang, N.J.; Nelson, B.; Wang, J.; Shen, X.; Powles, T. Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur. Urol. 2021, 80, 7–11. [Google Scholar] [CrossRef]
- Font, A.; Luque, R.; Villa, J.C.; Domenech, M.; Vazquez, S.; Gallardo, E.; Virizuela, J.A.; Beato, C.; Morales-Barrera, R.; Gelabert, A.; et al. The Challenge of Managing Bladder Cancer and Upper Tract Urothelial Carcinoma: A Review with Treatment Recommendations from the Spanish Oncology Genitourinary Group (SOGUG). Target Oncol. 2019, 14, 15–32. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Li, H.; Che, J.; Cao, B. Meta-analysis comparing maintenance strategies with continuous therapy and complete chemotherapy-free interval strategies in the treatment of metastatic colorectal cancer. Oncotarget 2016, 7, 33418–33428. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.; Vella, E.T.; Coakley, N.; Cheng, S.; Gregg, R.; Ung, Y.C.; Ellis, P.M. The Use of Systemic Treatment in the Maintenance of Patients with Non-Small Cell Lung Cancer: A Systematic Review. J. Thorac. Oncol. 2016, 11, 989–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt Molins, J.; Garcia-Donas Jimenez, J.; Valderrama, B.P.; Virizuela Echaburu, J.A.; Hernando-Polo, S.; Climent Duran, M.A.; Villa-Guzman, J.C.; Arranz Arija, J.A.; Ostiategui, M.L.; Milagro, N.L.; et al. Final Overall Survival Analysis of the SOGUG Phase 2 MAJA Study: Maintenance Vinflunine Versus Best Supportive Care After First-Line Chemotherapy in Advanced Urothelial Carcinoma. Clin. Genitourin. Cancer 2020, 18, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Kruczynski, A.; Barret, J.M.; Etievant, C.; Colpaert, F.; Fahy, J.; Hill, B.T. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem. Pharmacol. 1998, 55, 635–648. [Google Scholar] [CrossRef]
- Kruczynski, A.; Etievant, C.; Perrin, D.; Chansard, N.; Duflos, A.; Hill, B.T. Characterization of cell death induced by vinflunine, the most recent Vinca alkaloid in clinical development. Br. J. Cancer 2002, 86, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio, L.M.; Pulido, E.G.; Gallego, G.A. Vinflunine: A new vision that may translate into antiangiogenic and antimetastatic activity. Anticancer Drugs 2012, 23, 1–11. [Google Scholar] [CrossRef]
- Braguer, D.; Barret, J.M.; McDaid, H.; Kruczynski, A. Antitumor activity of vinflunine: Effector pathways and potential for synergies. Semin. Oncol. 2008, 35, S13–S21. [Google Scholar] [CrossRef]
- Kruczynski, A.; Hill, B.T. Vinflunine, the latest Vinca alkaloid in clinical development. A review of its preclinical anticancer properties. Crit. Rev. Oncol. Hematol. 2001, 40, 159–173. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome. Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [Green Version]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, H.; Irizarry, R.; Carvalho, B.; Speed, T.P. Estimation and assessment of raw copy numbers at the single locus level. Bioinformatics 2008, 24, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; Finotello, F.; List, M. Immunedeconv: An R Package for Unified Access to Computational Methods for Estimating Immune Cell Fractions from Bulk RNA-Sequencing Data. Methods Mol. Biol. 2020, 2120, 223–232. [Google Scholar] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Cardus, A.; Martinez-Balibrea, E.; Bandres, E.; Malumbres, R.; Gines, A.; Manzano, J.L.; Taron, M.; Garcia-Foncillas, J.; Abad, A. Pharmacogenomic approach for the identification of novel determinants of acquired resistance to oxaliplatin in colorectal cancer. Mol. Cancer Ther. 2009, 8, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Earl, J.; Rico, D.; Carrillo-de-Santa-Pau, E.; Rodriguez-Santiago, B.; Mendez-Pertuz, M.; Auer, H.; Gomez, G.; Grossman, H.B.; Pisano, D.G.; Schulz, W.A. The UBC-40 Urothelial Bladder Cancer cell line index: A genomic resource for functional studies. BMC Genomics 2015, 16, 403. [Google Scholar]
- Ruiz de Porras, V.; Bystrup, S.; Martinez-Cardus, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-kappaB signalling pathway. Sci. Rep. 2016, 6, 24675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz de Porras, V.; Wang, X.C.; Palomero, L.; Marin-Aguilera, M.; Sole-Blanch, C.; Indacochea, A.; Jimenez, N.; Bystrup, S.; Bakht, M.; Conteduca, V.; et al. Taxane-induced Attenuation of the CXCR2/BCL-2 Axis Sensitizes Prostate Cancer to Platinum-based Treatment. Eur. Urol. 2021, 79, 722–733. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- De Wever, O.; Pauwels, P.; De Craene, B.; Sabbah, M.; Emami, S.; Redeuilh, G.; Gespach, C.; Bracke, M.; Berx, G. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008, 130, 481–494. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, L.A.; Castosa, R.; Haz-Conde, M.; Rodriguez, M.; Blanco, M.; Valladares, M.; Figueroa, A. Role of the microtubule-targeting drug vinflunine on cell-cell adhesions in bladder epithelial tumour cells. BMC Cancer 2014, 14, 507. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, A.; Majeed, M.; Sahebkar, A. Curcumin: A potent agent to reverse epithelial-to-mesenchymal transition. Cell Oncol. 2019, 42, 405–421. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Jia, Z.; Gao, Y.; Zhao, C.; Yao, Y. Curcumin inhibits bladder cancer progression via regulation of beta-catenin expression. Tumour Biol. 2017, 39, 1010428317702548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutz, J.; Janicova, A.; Woidacki, K.; Chun, F.K.; Blaheta, R.A.; Relja, B. Curcumin-A Viable Agent for Better Bladder Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 3761. [Google Scholar] [CrossRef]
- Park, B.H.; Lim, J.E.; Jeon, H.G.; Seo, S.I.; Lee, H.M.; Choi, H.Y.; Jeon, S.S.; Jeong, B.C. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016, 7, 63870–63886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz de Porras, V.; Layos, L.; Martinez-Balibrea, E. Curcumin: A therapeutic strategy for colorectal cancer? Semin. Cancer Biol. 2021, 73, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Yang, X.; Zheng, M.; Zhao, Q.; Zhang, K.; Li, Z.; Zhang, H.; Zhang, S. Role of Transmembrane 4 L Six Family 1 in the Development and Progression of Cancer. Front Mol. Biosci. 2020, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wang, G.; Qian, K.; Chen, L.; Ju, L.; Qian, G.; Wu, C.L.; Dan, H.C.; Jiang, W.; Wu, M.; et al. TM4SF1 regulates apoptosis, cell cycle and ROS metabolism via the PPARgamma-SIRT1 feedback loop in human bladder cancer cells. Cancer Lett. 2018, 414, 278–293. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, J.; Di, Z.; Yuan, W.; Zhou, Z.; Liu, Z.; Han, S.; Liu, Y.; Ying, G.; Shu, X.; et al. TM4SF1 promotes EMT and cancer stemness via the Wnt/beta-catenin/SOX2 pathway in colorectal cancer. J. Exp. Clin. Cancer Res. 2020, 39, 232. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Xu, H.; Chen, D.; Liu, W.; Wang, J. Over-expression of TM4SF1 improves cell metastasis and growth by activating ERK1/2 signaling pathway in human prostate cancer. J. BUON. 2019, 24, 2531–2538. [Google Scholar]
- Ye, L.; Pu, C.; Tang, J.; Wang, Y.; Wang, C.; Qiu, Z.; Xiang, T.; Zhang, Y.; Peng, W. Transmembrane-4 L-six family member-1 (TM4SF1) promotes non-small cell lung cancer proliferation, invasion and chemo-resistance through regulating the DDR1/Akt/ERK-mTOR axis. Respir. Res. 2019, 20, 106. [Google Scholar] [CrossRef]
- Xue, L.; Yu, X.; Jiang, X.; Deng, X.; Mao, L.; Guo, L.; Fan, J.; Fan, Q.; Wang, L.; Lu, S.H. TM4SF1 promotes the self-renewal of esophageal cancer stem-like cells and is regulated by miR-141. Oncotarget 2017, 8, 19274–19284. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Ohashi, S.; Wong, G.S.; Ahmadi, A.; Kalman, R.A.; Budo, D.; Klein-Szanto, A.J.; Herlyn, M.; Diehl, J.A.; Nakagawa, H. Insulin-like growth factor-binding protein-3 promotes transforming growth factor-β1-mediated epithelial-to-mesenchymal transition and motility in transformed human esophageal cells. Carcinogenesis 2010, 31, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Koochekpour, S.; Strup, S.E.; Kyprianou, N. Reversion of epithelial-mesenchymal transition by a novel agent DZ-50 via IGF binding protein-3 in prostate cancer cells. Oncotarget 2017, 8, 78507–78519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [Green Version]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Marin-Aguilera, M.; Codony-Servat, J.; Reig, O.; Lozano, J.J.; Fernandez, P.L.; Pereira, M.V.; Jimenez, N.; Donovan, M.; Puig, P.; Mengual, L.; et al. Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol. Cancer Ther. 2014, 13, 1270–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConkey, D.J.; Choi, W.; Marquis, L.; Martin, F.; Williams, M.B.; Shah, J.; Svatek, R.; Das, A.; Adam, L.; Kamat, A.; et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009, 28, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal 2014, 7, re8. [Google Scholar] [CrossRef] [Green Version]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Zhang, Y.; Li, A.; Yu, P.; Song, L.; Liang, J.; Cao, N.; Gao, J.; Xu, R.; Ma, Y.; et al. Curcumin reverses hepatic epithelial mesenchymal transition induced by trichloroethylene by inhibiting IL-6R/STAT3. Toxicol. Mech. Methods 2021, 31, 589–599. [Google Scholar] [CrossRef]
- Zhao, Z.; Cheng, X.; Wang, Y.; Han, R.; Li, L.; Xiang, T.; He, L.; Long, H.; Zhu, B.; He, Y. Metformin inhibits the IL-6-induced epithelial-mesenchymal transition and lung adenocarcinoma growth and metastasis. PLoS ONE 2014, 9, e95884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullen, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]




| Variables | Patients with Good Outcome (n = 9) | Patients with Poor Outcome (n = 8) | p-Value |
|---|---|---|---|
| Median age, yrs (range) | 61 (47–74) | 68 (52–80) | ns |
| Gender Male Female | 7 2 | 6 2 | ns |
| ECOG PS 0 1 | 5 4 | 3 5 | ns |
| Hemoglobin < 10 gr/dL | 2 | 0 | ns |
| Liver metastases | 0 | 2 | ns |
| Number of poor prognostic factors 0 1 2 | 4 4 1 | 3 3 2 | ns |
| Response to cis/gem CR PR SD | 4 4 1 | 1 6 1 | ns |
| Status (at last visit) Alive Dead | 7 2 | 1 7 |
| Gene | Gene Description | Chromosome | FC * | p-Value * |
|---|---|---|---|---|
| Genes downregulated in patients with good outcome to vinflunine treatment | ||||
| C3+C18F2B3:D19 | Complement component 3 | chr19 | −2.0511 | 0.0066 |
| CDR1 | Cerebellar degeneration related protein 1 | chrX | −2.0409 | 0.0019 |
| IGFBP3 | Insulin like growth factor binding protein 3 | chr7 | −1.9304 | 0.0109 |
| IGF2 | Insulin-like growth factor 2 | chr11 | −1.9108 | 0.0343 |
| CCDC80 | Coiled-coil domain containing 80 | chr3 | −1.8935 | 0.0043 |
| JCHAIN | Joining chain of multimeric IgA and IgM | chr4 | −1.8610 | 0.0230 |
| CXCL8 | Chemokine (C-X-C motif) ligand 8 | chr4 | −1.8393 | 0.0399 |
| S100A9 | S100 calcium binding protein A9 | chr1 | −1.7811 | 0.0460 |
| TM4SF1 | Transmembrane 4 L six family member 1 | chr3 | −1.7805 | 0.0070 |
| IGLL5 | Immunoglobulin lambda-like polypeptide 5 | chr22 | −1.6382 | 0.0185 |
| Genes upregulated in patients with good outcome to vinflunine treatment | ||||
| GRHL3 | Grainyhead-like transcription factor 3 | chr1 | 1.5581 | 0.0011 |
| SCIN | Scinderin | chr7 | 1.5654 | 0.0120 |
| CXorf57 | Chromosome X open reading frame 57 | chrX | 1.5952 | 0.0066 |
| GSTM1 | Glutathione S-transferase mu 1 | chr1 | 1.5981 | 0.0105 |
| SCNN1G | Sodium channel non-voltage gated 1 gamma subunit | chr16 | 1.6793 | 0.0007 |
| EMX2 | Empty spiracles homeobox 2 | chr10 | 1.6874 | 0.0014 |
| DMKN | Dermokine | chr19 | 1.6882 | 0.0261 |
| TMEM97 | Transmembrane protein 97 | chr17 | 1.7270 | 0.0052 |
| CRH | Corticotropin releasing hormone | chr8 | 1.8411 | 0.0394 |
| SPTSSB | Serine palmitoyltransferase small subunit B | chr3 | 1.8950 | 0.0144 |
| Gene Set | No. Genes in Set | Gene Overlap | p-Value | FDR q-Value |
|---|---|---|---|---|
| Negatively enriched gene sets in patients with good outcome to vinflunine treatment | ||||
| EMT | 196 | 73 | 0.000 | 0.000 |
| IL6/JAK/STAT3 signaling | 87 | 39 | 0.000 | 0.001 |
| Coagulation | 136 | 46 | 0.000 | 0.003 |
| Allograft rejection | 198 | 68 | 0.002 | 0.008 |
| Interferon gamma response | 196 | 75 | 0.000 | 0.007 |
| Inflammatory response | 197 | 71 | 0.000 | 0.023 |
| Hypoxia | 196 | 67 | 0.000 | 0.031 |
| Angiogenesis | 36 | 11 | 0.040 | 0.035 |
| Complement | 194 | 71 | 0.000 | 0.035 |
| Kras signaling up | 195 | 65 | 0.004 | 0.036 |
| Myogenesis | 199 | 57 | 0.006 | 0.047 |
| Positively enriched gene sets in patients with good outcome to vinflunine treatment | ||||
| G2M checkpoint | 197 | 61 | 0.017 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Font, A.; Ruiz de Porras, V.; Valderrama, B.P.; Ramirez, J.L.; Nonell, L.; Virizuela, J.A.; Anido, U.; González-del-Alba, A.; Lainez, N.; Llorente, M.d.M.; et al. Epithelial-to-Mesenchymal Transition Mediates Resistance to Maintenance Therapy with Vinflunine in Advanced Urothelial Cell Carcinoma. Cancers 2021, 13, 6235. https://doi.org/10.3390/cancers13246235
Font A, Ruiz de Porras V, Valderrama BP, Ramirez JL, Nonell L, Virizuela JA, Anido U, González-del-Alba A, Lainez N, Llorente MdM, et al. Epithelial-to-Mesenchymal Transition Mediates Resistance to Maintenance Therapy with Vinflunine in Advanced Urothelial Cell Carcinoma. Cancers. 2021; 13(24):6235. https://doi.org/10.3390/cancers13246235
Chicago/Turabian StyleFont, Albert, Vicenç Ruiz de Porras, Begoña P. Valderrama, Jose Luis Ramirez, Lara Nonell, José Antonio Virizuela, Urbano Anido, Aránzazu González-del-Alba, Nuria Lainez, Maria del Mar Llorente, and et al. 2021. "Epithelial-to-Mesenchymal Transition Mediates Resistance to Maintenance Therapy with Vinflunine in Advanced Urothelial Cell Carcinoma" Cancers 13, no. 24: 6235. https://doi.org/10.3390/cancers13246235
APA StyleFont, A., Ruiz de Porras, V., Valderrama, B. P., Ramirez, J. L., Nonell, L., Virizuela, J. A., Anido, U., González-del-Alba, A., Lainez, N., Llorente, M. d. M., Jiménez, N., Mellado, B., García-Donas, J., & Bellmunt, J. (2021). Epithelial-to-Mesenchymal Transition Mediates Resistance to Maintenance Therapy with Vinflunine in Advanced Urothelial Cell Carcinoma. Cancers, 13(24), 6235. https://doi.org/10.3390/cancers13246235








