Chemokines in the Landscape of Cancer Immunotherapy: How They and Their Receptors Can Be Used to Turn Cold Tumors into Hot Ones?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Key Chemokine-Chemokine Receptor Interactions That Support Tumor Growth
2.1. The CXCR4-CXCL12 Pathway
2.2. The CCR2 Pathway
2.3. The CCR5 Pathway
2.4. The CXCR1/2 -CXCL8 Pathway
3. Key Chemokine-Chemokine Receptor Interactions That Limit Tumor Growth
3.1. CXCR3 and Its Ligands
3.2. Biased Signaling via CXCR3 Drives Activity of CD4+ and CD8+ Subsets in the Context of Cancer and Autoimmunity
3.3. CXCL10 and CXCL9 in Cancer Therapy: Could Systemic Administration of These Chemokines Induce Effector T Cells That Then Migrate to the Tumor Site to Limit Cancer Development?
3.4. Selections of Target Human Cancers for CXCL10/CXCL9 Immunotherapy
3.5. Why Do Cancer Cells Also Produce Chemokines That May Limit Tumor Growth?
4. Regulatory T Cells in Cancer Diseases, and Chemokine Receptor-Based Selective Depletion of These Cells for Cancer Immunotherapy
4.1. CCR4+ Tregs
4.2. CCR8+ Tregs
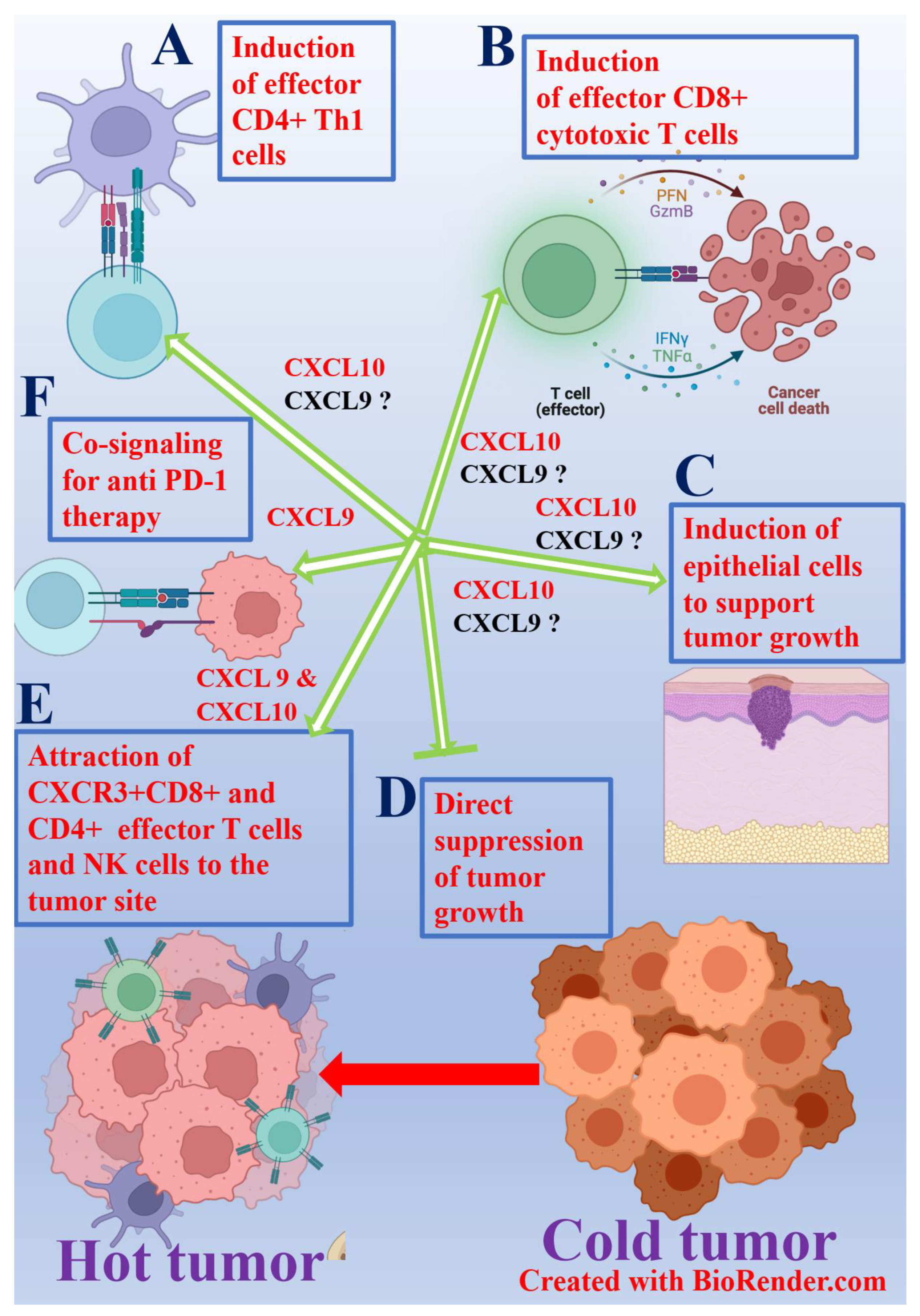
5. Could Chemokines Be Used to Turn Cold Tumors into Hot?
6. Conclusions
Funding
Conflicts of Interest
References
- Chow, M.T.; Luster, A.D. Chemokines in Cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luster, A.D. Chemokines—Chemotactic Cytokines That Mediate Inflammation. N. Engl. J. Med. 1998, 338, 436–445. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, P.M. Chemokines and the Molecular Basis of Cancer Metastasis. N. Engl. J. Med. 2001, 345, 833–835. [Google Scholar] [CrossRef] [Green Version]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer. 2004, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Poeta, V.M.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Bule, P.; Aguiar, S.I.; Aires-Da-Silva, F.; Dias, J.N.R. Chemokine-Directed Tumor Microenvironment Modulation in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9804. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef]
- Giuliano, S.; Guyot, M.; Grépin, R.; Pagès, G. The ELR+CXCL chemokines and their receptors CXCR1/CXCR2: A signaling axis and new target for the treatment of renal cell carcinoma. OncoImmunology 2014, 3, e28399. [Google Scholar] [CrossRef]
- Biasci, D.; Smoragiewicz, M.; Connell, C.M.; Wang, Z.; Gao, Y.; Thaventhiran, J.E.D.; Basu, B.; Magiera, L.; Johnson, T.I.; Bax, L.; et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl. Acad. Sci. USA 2020, 117, 28960–28970. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.J. The CXCL12–CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat. Rev. Cancer 2004, 4, 901–909. [Google Scholar] [CrossRef]
- Staller, P.; Sulitkova, J.; Lisztwan, J.; Moch, H.; Oakeley, E.J.; Krek, W. Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature 2003, 425, 307–311. [Google Scholar] [CrossRef]
- Schall, T.J.; Proudfoot, A.E.I. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat. Rev. Immunol. 2011, 11, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, D.P.; Medina-Ruiz, L.; Bartolini, R.; Schuette, F.; Hughes, C.E.; Pallas, K.; Vidler, F.; Macleod, M.K.L.; Kelly, C.J.; Lee, K.M.; et al. Chemokine Receptor Redundancy and Specificity Are Context Dependent. Immunity 2019, 50, 378–389.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A. The chemokine system: Redundancy for robust outputs. Immunol. Today 1999, 20, 254–257. [Google Scholar] [CrossRef]
- Noguchi, E.; Shien, T.; Iwata, H. Current status of PD-1/PD-L1 blockade immunotherapy in breast cancer. Jpn. J. Clin. Oncol. 2021, 51, 321–332. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin. Immunol. 2021, 226, 108707. [Google Scholar] [CrossRef]
- Versluis, J.M.; Long, G.; Blank, C.U. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat. Med. 2020, 26, 475–484. [Google Scholar] [CrossRef]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef]
- Robert, C.; Lanoy, E.; Besse, B. One or Two Immune Checkpoint Inhibitors? Cancer Cell 2019, 36, 579–581. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [Green Version]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.; Rodman, C.; Su, M.-J.; Melms, J.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.-R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997.e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nat. Cell Biol. 2021, 592, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 2020, 20, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Quezada, S.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010, 207, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017, 214, 895–904. [Google Scholar] [CrossRef]
- Nishino, M.; Sholl, L.M.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Anti–PD-1–Related Pneumonitis during Cancer Immunotherapy. N. Engl. J. Med. 2015, 373, 288–290. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleffel, S.; Posch, C.; Barthel, S.R.; Mueller, H.; Schlapbach, C.; Guenova, E.; Elco, C.P.; Lee, N.; Juneja, V.R.; Zhan, Q.; et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015, 162, 1242–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, M.K.; Postow, M.A.; Wolchok, J.D. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front. Oncol. 2015, 4, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Janjigian, Y.Y.; Wolchok, J.D.; Ariyan, C.E. Eradicating micrometastases with immune checkpoint blockade: Strike while the iron is hot. Cancer Cell 2021, 39, 738–742. [Google Scholar] [CrossRef]
- Koh, S.-B.; Ellisen, L.W. Immune activation and evolution through chemotherapy plus checkpoint blockade in triple-negative breast cancer. Cancer Cell 2021, 39, 1562–1564. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Toribio-Vázquez, C.; Rivas, J.G.; Yebes, A.; Carrión, D.M.; Quesada-Olarte, J.; Trelles, C.R.; Álvarez-Maestro, M.; Van Der Poel, H.; Martínez-Piñeiro, L. Immunotherapy toxicity. Diagnosis and treatment. Diagn. Treat. Arch. Esp Urol. 2020, 73, 906–917. [Google Scholar]
- Pirozzi, F.; Poto, R.; Aran, L.; Cuomo, A.; Galdiero, M.R.; Spadaro, G.; Abete, P.; Bonaduce, D.; Marone, G.; Tocchetti, C.G.; et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr. Oncol. Rep. 2021, 23, 13. [Google Scholar] [CrossRef]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events with Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef]
- Dougan, M.; Pietropaolo, M. Time to dissect the autoimmune etiology of cancer antibody immunotherapy. J. Clin. Investig. 2020, 130, 51–61. [Google Scholar] [CrossRef]
- Hwang, W.L.; Pike, L.R.G.; Royce, T.J.; Mahal, B.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, M.; Gort-Freitas, N.A.; Messemaker, M.; Bill, R.; Gungabeesoon, J.; Engblom, C.; Zilionis, R.; Garris, C.; Gerhard, G.M.; Kohl, A.; et al. Resident Kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci. Immunol. 2021, 6, eabi7083. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B. Converting Cold into Hot Tumors by Combining Immunotherapies. Cell 2017, 170, 1055–1056. [Google Scholar] [CrossRef] [Green Version]
- Sevenich, L. Turning “Cold” Into “Hot” Tumors—Opportunities and Challenges for Radio-Immunotherapy Against Primary and Metastatic Brain Cancers. Front. Oncol. 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.L. Blocking PI3K makes cold tumors hot. Science 2016, 354, 1246. [Google Scholar] [CrossRef] [Green Version]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nat. Cell Biol. 2015, 527, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, R.B.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Barash, U.; Zohar, Y.; Wildbaum, G.; Beider, K.; Nagler, A.; Karin, N.; Ilan, N.; Vlodavsky, I. Heparanase enhances myeloma progression via CXCL10 downregulation. Leukemia 2014, 28, 2178–2187. [Google Scholar] [CrossRef]
- Barsheshet, Y.; Wildbaum, G.; Levy, E.; Vitenshtein, A.; Akinseye, C.; Griggs, J.; Lira, S.A.; Karin, N. CCR8+FOXp3+ Treg cells as master drivers of immune regulation. Proc. Natl. Acad. Sci. USA 2017, 114, 6086–6091. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.M.; Summers, B.C.; Wang, Y.; Melikian, A.; Berahovich, R.; Miao, Z.; Penfold, M.E.T.; Sunshine, M.J.; Littman, D.R.; Kuo, C.J.; et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 2006, 203, 2201–2213. [Google Scholar] [CrossRef]
- Karin, N. The multiple faces of CXCL12 (SDF-1α) in the regulation of immunity during health and disease. J. Leukoc. Biol. 2010, 88, 463–473. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.R.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gangadhar, T.; Nandi, S.; Salgia, R. The role of chemokine receptor CXCR4 in lung cancer. Cancer Biol. Ther. 2010, 9, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Vela, M.; Aris, M.; Llorente, M.; Garcia-Sanz, J.A.; Kremer, L. Chemokine Receptor-Specific Antibodies in Cancer Immunotherapy: Achievements and Challenges. Front. Immunol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, C.; Zhao, H.; Chen, H. CXCR4 in breast cancer: Oncogenic role and therapeutic targeting. Drug Des. Dev. Ther. 2015, 9, 4953–4964. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F. The significance of cancer cell expression of the chemokine receptor CXCR. Semin. Cancer Biol. 2004, 14, 171–179. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hsiao, Y.-C.; Chen, Y.-J.; Wei, Y.-Y.; Lai, T.-H.; Tang, C.-H. Stromal cell-derived factor-1 enhances motility and integrin up-regulation through CXCR4, ERK and NF-κB-dependent pathway in human lung cancer cells. Biochem. Pharmacol. 2007, 74, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.C.; Makena, P.S.; Gorantla, V.; Sinclair, S.E.; Waters, C.M. CXCR4 regulates migration of lung alveolar epithelial cells through activation of Rac1 and matrix metalloproteinase-2. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, L846–L856. [Google Scholar] [CrossRef] [Green Version]
- Meiron, M.; Zohar, Y.; Anunu, R.; Wildbaum, G.; Karin, N. CXCL12 (SDF-1α) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J. Exp. Med. 2008, 205, 2643–2655. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ramjiawan, R.R.; Reiberger, T.; Ng, M.R.; Hato, T.; Huang, Y.; Ochiai, H.; Kitahara, S.; Unan, E.C.; Reddy, T.; et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015, 61, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.I. Chemokine receptors: Multifaceted therapeutic targets. Nat. Rev. Immunol. 2002, 2, 106–115. [Google Scholar] [CrossRef]
- Izikson, L.; Klein, R.S.; Charo, I.F.; Weiner, H.L.; Luster, A.D. Resistance to Experimental Autoimmune Encephalomyelitis in Mice Lacking the Cc Chemokine Receptor (Ccr2). J. Exp. Med. 2000, 192, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Wang, J.; Kivisakk, P.; Rollins, B.J.; Ransohoff, R.M. Absence of Monocyte Chemoattractant Protein 1 in Mice Leads to Decreased Local Macrophage Recruitment and Antigen-Specific T Helper Cell Type 1 Immune Response in Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 2001, 193, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, R.; Lu, H.; Butovsky, O.; Ohno, N.; Rietsch, A.M.; Cialic, R.; Wu, P.M.; Doykan, C.E.; Lin, J.; Cotleur, A.C.; et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014, 211, 1533–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, B.D.; Walker, A.; Harris, M.G.; Rayasam, A.; Sandor, M.; Fabry, Z. CCR2-Dependent Dendritic Cell Accumulation in the Central Nervous System during Early Effector Experimental Autoimmune Encephalomyelitis Is Essential for Effector T Cell Restimulation In Situ and Disease Progression. J. Immunol. 2015, 194, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Bannerman, P.; Ma, J.; Guo, F.; Miers, L.; Soulika, A.M.; Pleasure, D. Conditional Ablation of Astroglial CCL2 Suppresses CNS Accumulation of M1 Macrophages and Preserves Axons in Mice with MOG Peptide EAE. J. Neurosci. 2014, 34, 8175–8185. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Vadgama, J.V.; Wang, P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun. Signal. 2020, 18, 82. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Xia, R.; Wei, Y.; Wei, X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021, 54, e13115. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, J. Association of polymorphisms in the MCP-1 and CCR2 genes with the risk of cancer: A meta-analysis. Cytokine 2013, 64, 213–220. [Google Scholar] [CrossRef]
- Fein, M.R.; He, X.-Y.; Almeida, A.S.; Bružas, E.; Pommier, A.; Yan, R.; Eberhardt, A.; Fearon, D.T.; Van Aelst, L.; Wilkinson, J.E.; et al. Cancer cell CCR2 orchestrates suppression of the adaptive immune response. J. Exp. Med. 2020, 217, e20181551. [Google Scholar] [CrossRef]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Teng, K.-Y.; Han, J.; Zhang, X.; Hsu, S.-H.; He, S.; Wani, N.; Barajas, J.M.; Snyder, L.A.; Frankel, W.L.; Caligiuri, M.A.; et al. Blocking the CCL2–CCR2 Axis Using CCL2-Neutralizing Antibody Is an Effective Therapy for Hepatocellular Cancer in a Mouse Model. Mol. Cancer Ther. 2017, 16, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.Y.; Yuzhalin, A.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Bonapace, L.; Coissieux, M.-M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nat. Cell Biol. 2014, 515, 130–133. [Google Scholar] [CrossRef]
- Feng, L.; Qi, Q.; Wang, P.; Chen, H.; Chen, Z.; Meng, Z.; Liu, L. Serum level of CCL2 predicts outcome of patients with pancreatic cancer. Acta Gastroenterol. Belg. 2020, 83, 295–299. [Google Scholar]
- Fader, A.N.; Rasool, N.; Vaziri, S.A.J.; Kozuki, T.; Faber, P.W.; Elson, P.; Biscotti, C.V.; Michener, C.M.; Rose, P.G.; Rojas-Espaillat, L.; et al. CCL2 expression in primary ovarian carcinoma is correlated with chemotherapy response and survival outcomes. Anticancer Res. 2010, 30, 4791–4798. [Google Scholar] [PubMed]
- Arakaki, R.; Yamasaki, T.; Kanno, T.; Shibasaki, N.; Sakamoto, H.; Utsunomiya, N.; Sumiyoshi, T.; Shibuya, S.; Tsuruyama, T.; Nakamura, E.; et al. CCL 2 as a potential therapeutic target for clear cell renal cell carcinoma. Cancer Med. 2016, 5, 2920–2933. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, I.; Noack, A.; Makarevic, J.; Oppermann, E.; Waaga-Gasser, A.M.; Gasser, M.; Borgmann, H.; Huesch, T.; Gust, K.M.; Reiter, M.; et al. CCL2 Chemokine as a Potential Biomarker for Prostate Cancer: A Pilot Study. Cancer Res. Treat. 2015, 47, 306–312. [Google Scholar] [CrossRef]
- Eckstein, M.; Epple, E.; Jung, R.; Weigelt, K.; Lieb, V.; Sikic, D.; Stöhr, R.; Geppert, C.; Weyerer, V.; Bertz, S.; et al. CCL2 Expression in Tumor Cells and Tumor-Infiltrating Immune Cells Shows Divergent Prognostic Potential for Bladder Cancer Patients Depending on Lymph Node Stage. Cancers 2020, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, Y.; Cui, X.; Zhang, J.; Yang, Y.; Li, H.; Wu, H.; Li, J.; Wang, L.; Li, M.; et al. CCL2 expression correlates with Snail expression and affects the prognosis of patients with gastric cancer. Pathol. Res. Pract. 2017, 213, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.-D.; Zhan, Y.-T.; Zhu, Y.-H.; Li, Y.; Xie, D.; Guan, X.-Y. High levels of CCL2 or CCL4 in the tumor microenvironment predict unfavorable survival in lung adenocarcinoma. Thorac. Cancer 2018, 9, 775–784. [Google Scholar] [CrossRef]
- López, M.D.L.F.; Landskron, G.; Parada, D.; Dubois-Camacho, K.; Simian, D.; Martinez, M.; Romero, D.; Roa, J.C.; Chahuán, I.; Gutiérrez, R.; et al. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumor Biol. 2018, 40, 1010428318810059. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.O.; Ribeiro, F.L.L.; Batista, A.C.; Leles, C.R.; Alencar, R.D.C.G.; Silva, T.A. Association of CCL2 with Lymph Node Metastasis and Macrophage Infiltration in Oral Cavity and Lip Squamous Cell Carcinoma. Tumor Biol. 2008, 29, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhai, C.; Chang, Y.; Zhou, L.; Shi, T.; Tan, C.; Xu, L.; Xu, J. High expression of chemokine CCL2 is associated with recurrence after surgery in clear-cell renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 238.e19–238.e26. [Google Scholar] [CrossRef]
- Heiskala, M.; Leidenius, M.; Joensuu, K.; Heikkilä, P. High expression of CCL2 in tumor cells and abundant infiltration with CD14 positive macrophages predict early relapse in breast cancer. Virchows Archiv. 2019, 474, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef] [Green Version]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenberger, C.A.; Rabe, D.; Bainer, R.; Sankarasharma, D.; Chada, K.; Krausz, T.; Gilad, Y.; Becker, L.; Rosner, M.R. Metastasis Suppressors Regulate the Tumor Microenvironment by Blocking Recruitment of Prometastatic Tumor-Associated Macrophages. Cancer Res. 2015, 75, 4063–4073. [Google Scholar] [CrossRef] [Green Version]
- Suenaga, M.; Stintzing, S.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Okazaki, S.; Berger, M.D.; Miyamoto, Y.; Schirripa, M.; et al. Role of CCL5 and CCR5 gene polymorphisms in epidermal growth factor receptor signalling blockade in metastatic colorectal cancer: Analysis of the FIRE-3 trial. Eur. J. Cancer 2019, 107, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, M.K.; Novak, M.; Pestell, R.G.; Lah, T.T. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol. Oncol. 2019, 53, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 Signaling Promotes Invasion and Migration of CD133 + Ovarian Cancer Stem-Like Cells via NF-κB-Mediated MMP-9 Upregulation. Stem Cells 2012, 30, 2309–2319. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Xue, Y.; Lv, W.; Zhang, Y.; He, S. Critical roles of chemokine receptor CCR5 in regulating glioblastoma proliferation and invasion. Acta Biochim. Biophys. Sin. 2015, 47, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowska, E.; Przylipiak, A.; Zajkowska, M.; Piskór, B.M.; Borowik-Zaręba, A.; Ławicki, S. C-C motif chemokine ligand 5 and C-C chemokine receptor type 5: Possible diagnostic application in breast cancer patients. Acta Biochim. Pol. 2020, 67, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Guleria, K.; Sharma, S.; Manjari, I.; Uppal, M.S.; Singh, N.R.; Sambyal, V. p.R72P, PIN3 Ins16bp polymorphisms of TP53 and CCR5?32 in north Indian breast cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 3305–3311. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Hu, D.; Li, J.; Zhao, G.; Tang, W.; Cheng, H. Database Mining of Genes of Prognostic Value for the Prostate Adenocarcinoma Microenvironment Using the Cancer Gene Atlas. BioMed Res. Int. 2020, 2020, 5019793. [Google Scholar] [CrossRef]
- Fan, C.; Lu, W.; Li, K.; Zhao, C.; Wang, F.; Ding, G.; Wang, J. Identification of immune cell infiltration pattern and related critical genes in metastatic castration-resistant prostate cancer by bioinformatics analysis. Cancer Biomark. 2021, 32, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, S.; Schrama, D.; Keller, G.; Schadendorf, D.; Bröcker, E.-B.; Houben, R.; Zapatka, M.; Fink, W.; Kaufman, H.L.; Becker, J.C. Impact of the CCR5 gene polymorphism on the survival of metastatic melanoma patients receiving immunotherapy. Cancer Immunol. Immunother. 2008, 57, 685–691. [Google Scholar] [CrossRef]
- Suenaga, M.; Schirripa, M.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Cremolini, C.; Antoniotti, C.; Borelli, B.; Mashima, T.; et al. Gene Polymorphisms in the CCL5/CCR5 Pathway as a Genetic Biomarker for Outcome and Hand–Foot Skin Reaction in Metastatic Colorectal Cancer Patients Treated With Regorafenib. Clin. Color. Cancer 2018, 17, e395–e414. [Google Scholar] [CrossRef]
- Banin-Hirata, B.K.; Losi-Guembarovski, R.; Oda, J.M.M.; De Oliveira, C.E.C.; Campos, C.Z.; Mazzuco, T.L.; Borelli, S.D.; Ceribelli, J.R.; Watanabe, M.A.E. CCR2-V64I genetic polymorphism: A possible involvement in HER2+ breast cancer. Clin. Exp. Med. 2015, 16, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Butrym, A.; Kryczek, I.; Dlubek, D.; Jaskula, E.; Lange, A.; Jurczyszyn, A.; Mazur, G. High expression of CC chemokine receptor 5 (CCR5) promotes disease progression in patients with B-cell non-Hodgkin lymphomas. Curr. Probl. Cancer 2018, 42, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Chengcheng, L.; Wenwen, Q.; Ningyue, G.; Fangyuan, Z.; Runtong, X.; Zhenxiao, T.; Fenglei, X.; Yiming, Q.; Miaoqing, Z.; Xiaoming, L.; et al. Identification of the Immune-Related Genes in Tumor Microenvironment That Associated with the Recurrence of Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 2340. [Google Scholar] [CrossRef]
- Gao, J.; Tang, T.; Zhang, B.; Li, G. A Prognostic Signature Based on Immunogenomic Profiling Offers Guidance for Esophageal Squamous Cell Cancer Treatment. Front. Oncol. 2021, 11, 603634. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Meng, M.; Wang, W.; He, S.; Zhao, Y.; Gao, H.; Tang, W.; Liu, S.; Lin, Z.; et al. Identification of prognosis-related genes in the cervical cancer immune microenvironment. Gene 2021, 766, 145119. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, F.; Li, W.; Zhou, C.; Guan, Z.; Fan, H. Association of chemotactic factor receptor 5 gene with breast cancer. Genet. Mol. Res. 2013, 12, 5289–5300. [Google Scholar] [CrossRef] [PubMed]
- Zambra, F.M.B.; Biolchi, V.; Brum, I.S.; Chies, J. CCR2 and CCR5 genes polymorphisms in benign prostatic hyperplasia and prostate cancer. Hum. Immunol. 2013, 74, 1003–1008. [Google Scholar] [CrossRef]
- Watanabe, M.A.E.; Aoki, M.N.; Herrera, A.C.D.S.D.A.; Amarante, M.K.; Carneiro, J.L.D.V.; Fungaro, M.H. CCR5 and p53 codon 72 gene polymorphisms: Implications in breast cancer development. Int. J. Mol. Med. 2009, 23, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Novak, M.; Krajnc, M.K.; Hrastar, B.; Breznik, B.; Majc, B.; Mlinar, M.; Rotter, A.; Porčnik, A.; Mlakar, J.; Stare, K.; et al. CCR5-Mediated Signaling is Involved in Invasion of Glioblastoma Cells in Its Microenvironment. Int. J. Mol. Sci. 2020, 21, 4199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Qian, Z.; Han, Y. CCR5 is Associated with Immune Cell Infiltration and Prognosis of Lung Cancer. J. Thorac. Oncol. 2019, 14, e102–e103. [Google Scholar] [CrossRef]
- Kodama, T.; Koma, Y.-I.; Arai, N.; Kido, A.; Urakawa, N.; Nishio, M.; Shigeoka, M.; Yokozaki, H. CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab. Investig. 2020, 100, 1140–1157. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.B.; Janini, J.; Paranaiba, L.; Lozano-Burgos, C.; Olivero, P.; Gonzalez-Arriagada, W. Prognostic value of immunoexpression of CCR4, CCR5, CCR7 and CXCR4 in squamous cell carcinoma of tongue and floor of the mouth. Med. Oral Patol. Oral Cirugia Bucal 2019, 24, e354–e363. [Google Scholar] [CrossRef]
- Ryu, H.; Baek, S.W.; Moon, J.Y.; Jo, I.; Kim, N.; Lee, H.J. C-C motif chemokine receptors in gastric cancer (Review). Mol. Clin. Oncol. 2017, 8, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Guo, L.; Gao, M.; Li, J.; Xiang, S. Research Trends and Regulation of CCL5 in Prostate Cancer. OncoTargets Ther. 2021, 14, 1417–1427. [Google Scholar] [CrossRef]
- Shen, Z.; Li, T.; Chen, D.; Jia, S.; Yang, X.; Liang, L.; Chai, J.; Cheng, X.; Yang, X.; Sun, M. The CCL5/CCR5 axis contributes to the perineural invasion of human salivary adenoid cystic carcinoma. Oncol. Rep. 2013, 31, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Sachan, R.; Jain, M.; Mittal, B. CCR5-Δ32 Polymorphism and Susceptibility to Cervical Cancer: Association With Early Stage of Cervical Cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2008, 17, 87–91. [Google Scholar] [CrossRef]
- Stucky, A.; Sedghizadeh, P.; Mahabady, S.; Chen, X.; Zhang, C.; Zhang, G.; Zhang, X.; Zhong, J.F. Single-cell genomic analysis of head and neck squamous cell carcinoma. Oncotarget 2017, 8, 73208–73218. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.-J.; Chien, M.-H.; Lin, C.-W.; Chung, T.-T.; Zavras, A.-I.; Tsai, C.-M.; Chen, M.-K.; Yang, S.-F. Effect of CC chemokine ligand 5 and CC chemokine receptor 5 genes polymorphisms on the risk and clinicopathological development of oral cancer. Oral Oncol. 2010, 46, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Yan, J.; Liu, H.; Li, L.; Wu, B.; Guo, C.; Zhang, M. Identification of Prognostic Related Genes of Tumor Microenvironment Derived From Esophageal Cancer Patients. Pathol. Oncol. Res. 2021, 27, 589662. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Hu, G.; Wu, J.; Lv, L. A new clinical prognostic nomogram for liver cancer based on immune score. PLoS ONE 2020, 15, e0236622. [Google Scholar] [CrossRef]
- Srivastava, A.; Pandey, S.N.; Choudhuri, G.; Mittal, B. CCR5 Δ32 Polymorphism: Associated with Gallbladder Cancer Susceptibility. Scand. J. Immunol. 2008, 67, 516–522. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Carruba, G.; Calabrò, M.; Campisi, I.; Di Carlo, D.; Lio, D.; Colonna-Romano, G.; Candore, G.; Caruso, C. CCR5 Proinflammatory Allele in Prostate Cancer Risk: A pilot study in patients and centenarians from Sicily. Ann. N. Y. Acad. Sci. 2009, 1155, 289–292. [Google Scholar] [CrossRef]
- Hawila, E.; Razon, H.; Wildbaum, G.; Blattner, C.; Sapir, Y.; Shaked, Y.; Umansky, V.; Karin, N. CCR5 Directs the Mobilization of CD11b+Gr1+Ly6Clow Polymorphonuclear Myeloid Cells from the Bone Marrow to the Blood to Support Tumor Development. Cell Rep. 2017, 21, 2212–2222. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Karin, N. The Development and Homing of Myeloid-Derived Suppressor Cells: From a Two-Stage Model to a Multistep Narrative. Front. Immunol. 2020, 11, 557586. [Google Scholar] [CrossRef]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Blattner, C.; Fleming, V.; Weber, R.; Himmelhan, B.; Altevogt, P.; Gebhardt, C.; Schulze, T.J.; Razon, H.; Hawila, E.; Wildbaum, G.; et al. CCR5+ Myeloid-Derived Suppressor Cells Are Enriched and Activated in Melanoma Lesions. Cancer Res. 2018, 78, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, B.; Qin, J.; Zhou, H.; Majumdar, A.P.N.; Peng, F. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol. Immunotoxicol. 2018, 40, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Nawab, O.; Patel, T.; Kossenkov, A.V.; Halama, N.; Jaeger, D.; Pestell, R.G. Recent Advances Targeting CCR5 for Cancer and Its Role in Immuno-Oncology. Cancer Res. 2019, 79, 4801–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Swamydas, M.; Gao, J.-L.; Break, T.J.; Johnson, M.D.; Jaeger, M.; Rodriguez, C.A.; Lim, J.K.; Green, N.M.; Collar, A.L.; Fischer, B.G.; et al. CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci. Transl. Med. 2016, 8, 322ra10. [Google Scholar] [CrossRef] [Green Version]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef] [PubMed]
- Blaser, B.; Moore, J.L.; Hagedorn, E.J.; Li, B.; Riquelme, R.; Lichtig, A.; Yang, S.; Zhou, Y.; Tamplin, O.J.; Binder, V.; et al. CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J. Exp. Med. 2017, 214, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [Green Version]
- Rajarathnam, K.; Desai, U.R. Structural Insights Into How Proteoglycans Determine Chemokine-CXCR1/CXCR2 Interactions: Progress and Challenges. Front. Immunol. 2020, 11, 660. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Wu, X.; Wang, K.; Yu, J.; Luo, B.; Luo, G.; Wang, W.; Wang, H.; Li, J.; et al. CXCR1 promotes malignant behavior of gastric cancer cells in vitro and in vivo in AKT and EKR1/2 phosphorylation. Int. J. Oncol. 2016, 48, 2184–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandolini, L.; Cristiano, L.; Fidoamore, A.; De Pizzol, M.; Di Giacomo, E.; Florio, T.M.; Confalone, G.; Galante, A.; Cinque, B.; Benedetti, E.; et al. Targeting CXCR1 on breast cancer stem cells: Signaling pathways and clinical application modelling. Oncotarget 2015, 6, 43375–43394. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zhang, S.; Meng, Q.; Zhou, F.; Pan, B.; Liu, F.; Yu, Y. CXCR1 correlates to poor outcomes of EGFR-TKI against advanced non-small cell lung cancer by activating chemokine and JAK/STAT pathway. Pulm. Pharmacol. Ther. 2021, 67, 102001. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, X.; Luo, G.; Yu, M.; Zhang, X.; Xu, N. Increased CXCL8 Expression Is Negatively Correlated with the Overall Survival of Patients with ER-Negative Breast Cancer. Anticancer Res. 2017, 37, 4845–4852. [Google Scholar] [CrossRef]
- Yi, M.; Peng, C.; Xia, B.; Gan, L. CXCL8 facilitates the survival and Paclitaxel-resistance of triple-negative breast cancers. Clin. Breast Cancer 2021. [Google Scholar] [CrossRef]
- Zayed, H. The identification of highly upregulated genes in claudin-low breast cancer through an integrative bioinformatics approach. Comput. Biol. Med. 2020, 127, 103806. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, M.; Kwantwi, L.B.; Bi, X.; Zhang, C.; Cheng, Z.; Ding, X.; Su, T.; Wang, H.; Wu, Q. Breast cancer cells promote self-migration by secreting interleukin 8 to induce NET formation. Gene 2020, 754, 144902. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Suman, K.H.; Nair, N.; Majeed, J.; Tripathi, V. An updated review on the role of the CXCL8-CXCR1/2 axis in the progression and metastasis of breast cancer. Mol. Biol. Rep. 2021, 48, 6551–6561. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausovà, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Dong, S.; Ge, C.; Xiao, Y.; Li, R.; Ma, X.; Xue, Y.; Zhang, Q.; Lv, J.; et al. Association of CXCR1 and 2 expressions with gastric cancer metastasis in ex vivo and tumor cell invasion in vitro. Cytokine 2014, 69, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, W.; Wang, K.; Yu, J.; Luo, B.; Luo, G.; Wang, W.; Wang, H.; Li, J.; Wen, J. Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. Int. J. Oncol. 2016, 48, 1341–1352. [Google Scholar] [CrossRef]
- Varney, M.L.; Johansson, S.L.; Singh, R.K. Distinct Expression of CXCL8 and Its Receptors CXCR1 and CXCR2 and Their Association with Vessel Density and Aggressiveness in Malignant Melanoma. Am. J. Clin. Pathol. 2006, 125, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nannuru, K.C.; Sadanandam, A.; Varney, M.L.; Singh, R.K. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br. J. Cancer 2009, 100, 1638–1646. [Google Scholar] [CrossRef] [Green Version]
- Sapoznik, S.; Ortenberg, R.; Galore-Haskel, G.; Kozlovski, S.; Levy, D.; Avivi, C.; Barshack, I.; Cohen, C.J.; Besser, M.J.; Schachter, J.; et al. CXCR1 as a novel target for directing reactive T cells toward melanoma: Implications for adoptive cell transfer immunotherapy. Cancer Immunol. Immunother. 2012, 61, 1833–1847. [Google Scholar] [CrossRef]
- Singh, S.; Sadanandam, A.; Nannuru, K.C.; Varney, M.L.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small-Molecule Antagonists for CXCR2 and CXCR1 Inhibit Human Melanoma Growth by Decreasing Tumor Cell Proliferation, Survival, and Angiogenesis. Clin. Cancer Res. 2009, 15, 2380–2386. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Saxena, S.; Varney, M.; Singh, R. CXCR1/2 Chemokine Network Regulates Melanoma Resistance to Chemotherapies Mediated by NF-κB. Curr. Mol. Med. 2018, 17, 1. [Google Scholar] [CrossRef]
- Gabellini, C.; Trisciuoglio, D.; Desideri, M.; Candiloro, A.; Ragazzoni, Y.; Orlandi, A.; Zupi, G.; Del Bufalo, D. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur. J. Cancer 2009, 45, 2618–2627. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, S.; Varney, M.L.; Singh, R.K. Targeting CXCR1/CXCR2 receptor antagonism in malignant melanoma. Expert Opin. Ther. Targets 2010, 14, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Sadanandam, A.; Varney, M.L.; Nannuru, K.C.; Singh, R.K. Small interfering RNA-mediated CXCR1 or CXCR2 knock-down inhibits melanoma tumor growth and invasion. Int. J. Cancer 2010, 126, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.-M.; Li, J. A small-molecule antagonist of CXCR1 and CXCR2 inhibits cell proliferation, migration and invasion in melanoma via PI3K/AKT pathway. Med. Clín. 2019, 152, 425–430. [Google Scholar] [CrossRef]
- Kemp, D.M.; Pidich, A.; Larijani, M.; Jonas, R.; Lash, E.; Sato, T.; Terai, M.; De Pizzol, M.; Allegretti, M.; Igoucheva, O.; et al. Ladarixin, a dual CXCR1/2 inhibitor, attenuates experimental melanomas harboring different molecular defects by affecting malignant cells and tumor microenvironment. Oncotarget 2017, 8, 14428–14442. [Google Scholar] [CrossRef] [Green Version]
- Oghumu, S.; Varikuti, S.; Terrazas, C.; Kotov, D.; Nasser, M.W.; Powell, C.A.; Ganju, R.K.; Satoskar, A.R. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunity 2014, 143, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, R.; Singh, S.; Karls, R.K.; Quinn, F.D.; Taub, D.D.; Lillard, J.W. CXCL10+ T cells and NK cells assist in the recruitment and activation of CXCR3+ and CXCL11+ leukocytes during Mycobacteria-enhanced colitis. BMC Immunol. 2008, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, D.S.; Bernard, N.; Nie, C.Q.; Schofield, L. NK Cells Stimulate Recruitment of CXCR3+T Cells to the Brain duringPlasmodium berghei-Mediated Cerebral Malaria. J. Immunol. 2007, 178, 5779–5788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Fu, H.; Yang, Y.; Yu, H.; Ai, X.; Lei, Y.; Bao, W.; Tang, Y. Modulation of CXCR1 and CXCR3 expression on NK cells via Tim-3 in a murine model of primary biliary cholangitis. Mol. Immunol. 2021, 135, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, P.S.; Macedo, P.; Kilty, I.C.; Barnes, P.J.; Donnelly, L.E. Effect of JAK Inhibitors on Release of CXCL9, CXCL10 and CXCL11 from Human Airway Epithelial Cells. PLoS ONE 2015, 10, e0128757. [Google Scholar] [CrossRef] [Green Version]
- Korniejewska, A.; McKnight, A.J.; Johnson, Z.; Watson, M.L.; Ward, S.G. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunity 2011, 132, 503–515. [Google Scholar] [CrossRef]
- Luster, A.D.; Ravetch, J.V. Biochemical cherecterization of gamma interferon inducible cytokine (IP-10). J. Exp. Med. 1987, 166, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.E.; Strick, C.A.; Paradis, T.J.; Ogborne, K.T.; Loetscher, M.; Gladue, R.P.; Lin, W.; Boyd, J.G.; Moser, B.; Wood, D.E.; et al. Interferon–inducible T Cell Alpha Chemoattractant (I-TAC): A Novel Non-ELR CXC Chemokine with Potent Activity on Activated T Cells through Selective High Affinity Binding to CXCR. J. Exp. Med. 1998, 187, 2009–2021. [Google Scholar] [CrossRef]
- Groom, J.R.; Luster, A.D. CXCR3 in T cell function. Exp. Cell Res. 2011, 317, 620–631. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Ferguson, S.S.G.; Daaka, Y.; Miller, W.E.; Maudsley, S.; Della Rocca, G.J.; Lin, F.-T.; Kawakatsu, H.; Owada, K.; Luttrell, D.K.; et al. β-Arrestin-Dependent Formation of β 2 Adrenergic Receptor-Src Protein Kinase Complexes. Science 1999, 283, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Suomivuori, C.-M.; Latorraca, N.R.; Wingler, L.M.; Eismann, S.; King, M.C.; Kleinhenz, A.L.W.; Skiba, M.A.; Staus, D.P.; Kruse, A.C.; Lefkowitz, R.J.; et al. Molecular mechanism of biased signaling in a prototypical G protein–coupled receptor. Science 2020, 367, 881–887. [Google Scholar] [CrossRef]
- Brox, R.; Milanos, L.; Saleh, N.; Baumeister, P.; Buschauer, A.; Hofmann, D.; Heinrich, M.R.; Clark, T.; Tschammer, N. Molecular Mechanisms of Biased and Probe-Dependent Signaling at CXC-Motif Chemokine Receptor CXCR3 Induced by Negative Allosteric Modulators. Mol. Pharmacol. 2018, 93, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Corbisier, J.; Gales, C.; Huszagh, A.; Parmentier, M.; Springael, J.-Y. Biased Signaling at Chemokine Receptors. J. Biol. Chem. 2015, 290, 9542–9554. [Google Scholar] [CrossRef] [Green Version]
- Hauser, M.A.; Legler, D.F. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J. Leukoc. Biol. 2016, 99, 869–882. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.-Y.; Park, C.; Harrison, K.; Kehrl, J.H. Biased S1PR1 Signaling in B Cells Subverts Responses to Homeostatic Chemokines, Severely Disorganizing Lymphoid Organ Architecture. J. Immunol. 2019, 203, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.S.; Larsen, O.; Allmen, E.U.-V.; Lückmann, M.; Legler, D.F.; Frimurer, T.M.; Veldkamp, C.T.; Hjortø, G.M.; Rosenkilde, M.M. Biased Signaling of CCL21 and CCL19 Does Not Rely on N-Terminal Differences, but Markedly on the Chemokine Core Domains and Extracellular Loop 2 of CCR. Front. Immunol. 2019, 10, 2156. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Biased signaling of G protein-coupled receptors—From a chemokine receptor CCR7 perspective. Gen. Comp. Endocrinol. 2017, 258, 4–14. [Google Scholar] [CrossRef]
- Rajagopal, S.; Bassoni, D.L.; Campbell, J.J.; Gerard, N.P.; Gerard, C.; Wehrman, T.S. Biased Agonism as a Mechanism for Differential Signaling by Chemokine Receptors. J. Biol. Chem. 2013, 288, 35039–35048. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Fernández, J.L.; Criado-García, O. The Chemokine Receptor CCR7 Uses Distinct Signaling Modules With Biased Functionality to Regulate Dendritic Cells. Front. Immunol. 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Getschman, A.; Volkman, B.F.; Dwinell, M.B. Exploiting agonist biased signaling of chemokines to target cancer. Mol. Carcinog. 2017, 56, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Roy, I.; McAllister, D.M.; Gorse, E.; Dixon, K.; Piper, C.; Zimmerman, N.P.; Getschman, A.; Tsai, S.; Engle, D.D.; Evans, D.B.; et al. Pancreatic Cancer Cell Migration and Metastasis Is Regulated by Chemokine-Biased Agonism and Bioenergetic Signaling. Cancer Res. 2015, 75, 3529–3542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteen, A.; Elarsen, O.; Ethiele, S.; Rosenkilde, M.M. Biased and G Protein-Independent Signaling of Chemokine Receptors. Front. Immunol. 2014, 5, 277. [Google Scholar] [CrossRef] [Green Version]
- Steen, A.; Thiele, S.; Guo, D.; Hansen, L.S.; Frimurer, T.M.; Rosenkilde, M.M. Biased and Constitutive Signaling in the CC-chemokine Receptor CCR5 by Manipulating the Interface between Transmembrane Helices 6. J. Biol. Chem. 2013, 288, 12511–12521. [Google Scholar] [CrossRef] [Green Version]
- Karin, N.; Wildbaum, G.; Thelen, M. Biased signaling pathways via CXCR3 control the development and function of CD4+T cell subsets. J. Leukoc. Biol. 2015, 99, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Zohar, Y.; Wildbaum, G.; Novak, R.; Salzman, A.L.; Thelen, M.; Alon, R.; Barsheshet, Y.; Karp, C.L.; Karin, N. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J. Clin. Investig. 2014, 124, 2009–2022. [Google Scholar] [CrossRef] [Green Version]
- Karin, N.; Wildbaum, G. The Role of Chemokines in Shaping the Balance Between CD4+ T Cell Subsets and Its Therapeutic Implications in Autoimmune and Cancer Diseases. Front. Immunol. 2015, 6, 609. [Google Scholar] [CrossRef] [Green Version]
- Wildbaum, G.; Netzer, N.; Karin, N. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2002, 168, 5885–5892. [Google Scholar] [CrossRef] [Green Version]
- Salomon, I.; Netzer, N.; Wildbaum, G.; Schif-Zuck, S.; Maor, G.; Karin, N. Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J. Immunol. 2002, 169, 2685–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groom, J.; Richmond, J.; Murooka, T.; Sorensen, E.; Sung, J.H.; Bankert, K.; von Andrian, U.H.; Moon, J.J.; Mempel, T.R.; Luster, A.D. CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4+ T Helper 1 Cell Differentiation. Immunity 2012, 37, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunderson, A.J.; Yamazaki, T.; Mccarty, K.; Fox, N.; Phillips, M.; Alice, A.; Blair, T.; Whiteford, M.; O’Brien, D.; Ahmad, R.; et al. TGFβ suppresses CD8+ T cell expression of CXCR3 and tumor trafficking. Nat. Commun. 2020, 11, 1749. [Google Scholar] [CrossRef] [Green Version]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900.e10. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 2019, 50, 1498–1512.e5. [Google Scholar] [CrossRef]
- Alanio, C.; Da Silva, R.B.; Michonneau, D.; Bousso, P.; Ingersoll, M.A.; Albert, M.L. CXCR3/CXCL10 Axis Shapes Tissue Distribution of Memory Phenotype CD8+ T Cells in Nonimmunized Mice. J. Immunol. 2017, 200, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Mikucki, M.E.; Fisher, D.T.; Matsuzaki, J.; Skitzki, J.J.; Gaulin, N.B.; Muhitch, J.B.; Ku, A.W.; Frelinger, J.G.; Odunsi, K.; Gajewski, T.F.; et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 2015, 6, 7458. [Google Scholar] [CrossRef]
- Pan, J.; Burdick, M.D.; Belperio, J.A.; Xue, Y.Y.; Gerard, C.; Sharma, S.; Dubinett, S.M.; Strieter, R.M. CXCR3/CXCR3 Ligand Biological Axis Impairs RENCA Tumor Growth by a Mechanism of Immunoangiostasis. J. Immunol. 2006, 176, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural Innate and Adaptive Immunity to Cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walser, T.C.; Ma, X.; Kundu, N.; Dorsey, R.; Goloubeva, O.; Fulton, A.M. Immune-mediated Modulation of Breast Cancer Growth and Metastasis by the Chemokine Mig (CXCL9) in a Murine Model. J. Immunother. 2007, 30, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Arenberg, D.A.; White, E.S.; Burdick, M.D.; Strom, S.R.; Strieter, R.M. Improved survival in tumor-bearing SCID mice treated with interferon-gamma-inducible protein 10 (IP-10/CXCL10). Cancer Immunol. Immunother. 2001, 50, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Taslimi, Y.; Zahedifard, F.; Habibzadeh, S.; Taheri, T.; Abbaspour, H.; Sadeghipour, A.; Mohit, E.; Rafati, S. Antitumor Effect of IP-10 by Using Two Different Approaches: Live Delivery System and Gene Therapy. J. Breast Cancer 2016, 19, 34–44. [Google Scholar] [CrossRef]
- Gough, M.; Crittenden, M.; Thanarajasingam, U.; Sanchez-Perez, L.; Thompson, J.; Jevremovic, D.; Vile, R. Gene Therapy to Manipulate Effector T Cell Trafficking to Tumors for Immunotherapy. J. Immunol. 2005, 174, 5766–5773. [Google Scholar] [CrossRef] [Green Version]
- Mullins, I.M.; Slingluff, C.L.; Lee, J.K.; Garbee, C.F.; Shu, J.; Anderson, S.G.; Mayer, M.E.; Knaus, W.A.; Mullins, D. CXC Chemokine Receptor 3 Expression by Activated CD8+ T cells Is Associated with Survival in Melanoma Patients with Stage III Disease. Cancer Res. 2004, 64, 7697–7701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Karin, N. Chemokines and cancer: New immune checkpoints for cancer therapy. Curr. Opin. Immunol. 2018, 51, 140–145. [Google Scholar] [CrossRef]
- Peng, W.; Liu, C.; Xu, C.; Lou, Y.; Chen, J.; Yang, Y.; Yagita, H.; Overwijk, W.W.; Lizée, G.; Radvanyi, L.; et al. PD-1 Blockade Enhances T-cell Migration to Tumors by Elevating IFN-γ Inducible Chemokines. Cancer Res. 2012, 72, 5209–5218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, B.A.; Gingrich, J.; Kwon, E.D.; Madias, C.; Greenberg, N.M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997, 57, 3325–3330. [Google Scholar] [PubMed]
- Kato, M.; Takahashi, M.; Akhand, A.A.; Liu, W.; Dai, Y.; Shimizu, S.; Iwamoto, T.; Suzuki, H.; Nakashima, I. Transgenic mouse model for skin malignant melanoma. Oncogene 1998, 17, 1885–1888. [Google Scholar] [CrossRef]
- Bradford, J.R.; Wappett, M.; Beran, G.; Logie, A.; Delpuech, O.; Brown, H.; Boros, J.; Camp, N.J.; McEwen, R.; Mazzola, A.M.; et al. Whole transcriptome profiling of patient-derived xenograft models as a tool to identify both tumor and stromal specific biomarkers. Oncotarget 2016, 7, 20773–20787. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.M.; Xue, A.; Mittal, A.; Samra, J.S.; Smith, R.; Hugh, T.J. Patient-derived xenograft models of colorectal cancer in pre-clinical research: A systematic review. Oncotarget 2016, 7, 66212–66225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, R.; Patel, A.; Griffiths, L.M.; Dapper, J.; Stewart, E.A.; Houston, J.; Johnson, M.; Akers, W.J.; Furman, W.L.; Dyer, M.A. Next-generation humanized patient-derived xenograft mouse model for pre-clinical antibody studies in neuroblastoma. Cancer Immunol. Immunother. 2021, 70, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Rongvaux, A.; Taylor, A.; Jiang, T.; Tebaldi, T.; Balasubramanian, K.; Bagale, A.; Terzi, Y.K.; Gbyli, R.; Wang, X.; et al. A highly efficient and faithful MDS patient-derived xenotransplantation model for pre-clinical studies. Nat. Commun. 2019, 10, 366. [Google Scholar] [CrossRef] [Green Version]
- Strüder, D.; Momper, T.; Irmscher, N.; Krause, M.; Liese, J.; Schraven, S.; Zimpfer, A.; Zonnur, S.; Burmeister, A.-S.; Schneider, B.; et al. Establishment and characterization of patient-derived head and neck cancer models from surgical specimens and endoscopic biopsies. J. Exp. Clin. Cancer Res. 2021, 40, 246. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, Y.; Cai, S. CXCL10 expression and prognostic significance in stage II and III colorectal cancer. Mol. Biol. Rep. 2010, 37, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Liu, F.; Zhu, J.; Yang, L.; Cai, G.; Zhang, Z.; Huang, W.; Cai, S.; Xu, Y. CXCL10 mRNA expression predicts response to neoadjuvant chemoradiotherapy in rectal cancer patients. Tumor Biol. 2014, 35, 9683–9691. [Google Scholar] [CrossRef]
- Rainczuk, A.; Rao, J.R.; Gathercole, J.; Fairweather, N.J.; Chu, S.; Masadah, R.; Jobling, T.W.; Deb-Choudhury, S.; Dyer, J.; Stephens, A.N. Evidence for the antagonistic form of CXC-motif chemokine CXCL10 in serous epithelial ovarian tumours. Int. J. Cancer 2013, 134, 530–541. [Google Scholar] [CrossRef]
- Flores, R.J.; Kelly, A.J.; Li, Y.; Nakka, M.; Barkauskas, D.A.; Krailo, M.; Wang, L.L.; Perlaky, L.; Lau, C.C.; Hicks, M.J.; et al. A novel prognostic model for osteosarcoma using circulating CXCL10 and FLT3LG. Cancer 2017, 123, 144–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chen, J.; Guan, G.W.; Zhang, T.; Lu, F.M.; Chen, X.M. Expression and clinical significance of chemokine CXCL10 and its receptor CXCR3 in hepatocellular carcinoma. Beijing Da Xue Xue Bao Yi Xue Ban 2019, 51, 402–408. [Google Scholar] [CrossRef]
- Lieber, S.; Reinartz, S.; Raifer, H.; Finkernagel, F.; Dreyer, T.; Bronger, H.; Jansen, J.M.; Wagner, U.; Worzfeld, T.; Müller, R.; et al. Prognosis of ovarian cancer is associated with effector memory CD8+ T cell accumulation in ascites, CXCL9 levels and activation-triggered signal transduction in T cells. OncoImmunology 2018, 7, e1424672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.-K.; Deng, Z.-K.-; Chen, M.-T.; Qiu, S.-Q.; Xiao, Y.-S.; Qi, Y.-Z.; Xie, Q.; Wang, Z.-H.; Jia, S.-C.; Zeng, D.; et al. CXCL9 Is a Potential Biomarker of Immune Infiltration Associated With Favorable Prognosis in ER-Negative Breast Cancer. Front. Oncol. 2021, 11, 710286. [Google Scholar] [CrossRef]
- Li, Y.; Liang, M.; Lin, Y.; Lv, J.; Chen, M.; Zhou, P.; Fu, F.; Wang, C. Transcriptional Expressions of CXCL9/10/12/13 as Prognosis Factors in Breast Cancer. J. Oncol. 2020, 2020, 4270957. [Google Scholar] [CrossRef] [PubMed]
- Bolomsky, A.; Schreder, M.; Hübl, W.; Zojer, N.; Hilbe, W.; Ludwig, H. Monokine induced by interferon gamma (MIG/CXCL9) is an independent prognostic factor in newly diagnosed myeloma. Leuk. Lymphoma 2016, 57, 2516–2525. [Google Scholar] [CrossRef]
- Lunardi, S.; Jamieson, N.; Lim, S.Y.; Griffiths, K.L.; Carvalho-Gaspar, M.; Al-Assar, O.; Yameen, S.; Carter, R.C.; McKay, C.J.; Spoletini, G.; et al. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget 2014, 5, 11064–11080. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, A.M.; Raitman, I.; Feeley, L.; Pinnaduwage, D.; Nguyen, L.T.; O’Malley, F.P.; Ohashi, P.S.; Andrulis, I.L. Tumoral Lymphocytic Infiltration and Expression of the Chemokine CXCL10 in Breast Cancers from the Ontario Familial Breast Cancer Registry. Clin. Cancer Res. 2013, 19, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Casrouge, A.; Decalf, J.; Ahloulay, M.; Lababidi, C.; Mansour, H.; Vallet-Pichard, A.; Mallet, V.; Mottez, E.; Mapes, J.; Fontanet, A.; et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J. Clin. Investig. 2011, 121, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.E.; De Lemos, C.; Moos, T.; Christensen, J.; Thomsen, A.R. CXCL10 Is the Key Ligand for CXCR3 on CD8+Effector T Cells Involved in Immune Surveillance of the Lymphocytic Choriomeningitis Virus-Infected Central Nervous System. J. Immunol. 2006, 176, 4235–4243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastenmüller, W.; Brandes, M.; Wang, Z.; Herz, J.; Egen, J.G.; Germain, R.N. Peripheral Prepositioning and Local CXCL9 Chemokine-Mediated Guidance Orchestrate Rapid Memory CD8+ T Cell Responses in the Lymph Node. Immunity 2013, 38, 502–513. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-C.; Huang, Y.-H.; Su, C.-W.; Wang, Y.-J.; Huo, T.-I.; Lee, K.-C.; Lin, H.-C. CXCL9 Associated with Sustained Virological Response in Chronic Hepatitis B Patients Receiving Peginterferon Alfa-2a Therapy: A Pilot Study. PLoS ONE 2013, 8, e76798. [Google Scholar] [CrossRef] [PubMed]
- Ploquin, M.J.; Madec, Y.; Casrouge, A.; Huot, N.; Passaes, C.; Lécuroux, C.; Essat, A.; Boufassa, F.; Jacquelin, B.; Jochems, S.P.; et al. Elevated Basal Pre-infection CXCL10 in Plasma and in the Small Intestine after Infection Are Associated with More Rapid HIV/SIV Disease Onset. PLoS Pathog. 2016, 12, e1005774. [Google Scholar] [CrossRef] [Green Version]
- Wuest, T.R.; Carr, D.J. Dysregulation of CXCR3 Signaling due to CXCL10 Deficiency Impairs the Antiviral Response to Herpes Simplex Virus 1 Infection. J. Immunol. 2008, 181, 7985–7993. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Xu, L.; Che, X.; Wen, T.; Fan, Y.; Li, C.; Wang, S.; Cheng, Y.; Wang, X.; et al. CXCL9/10/11, a regulator of PD-L1 expression in gastric cancer. BMC Cancer 2018, 18, 462. [Google Scholar] [CrossRef]
- Doron, H.; Amer, M.; Ershaid, N.; Blazquez, R.; Shani, O.; Lahav, T.G.; Cohen, N.; Adler, O.; Hakim, Z.; Pozzi, S.; et al. Inflammatory Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 Signaling Axis. Cell Rep. 2019, 28, 1785–1798.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front. Immunol. 2018, 8, 1970. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. Biological Functions of Regulatory T Cells. Adv. Immunol. 2011, 112, 137–176. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef]
- Cieri, N.; Camisa, B.; Cocchiarella, F.; Forcato, M.; Oliveira, G.; Provasi, E.; Bondanza, A.; Bordignon, C.; Peccatori, J.; Ciceri, F.; et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013, 121, 573–584. [Google Scholar] [CrossRef]
- Li, J.; Huston, G.; Swain, S.L. IL-7 Promotes the Transition of CD4 Effectors to Persistent Memory Cells. J. Exp. Med. 2003, 198, 1807–1815. [Google Scholar] [CrossRef]
- Shinoda, K.; Hirahara, K.; Iinuma, T.; Ichikawa, T.; Suzuki, A.S.; Sugaya, K.; Tumes, D.J.; Yamamoto, H.; Hara, T.; Tani-Ichi, S.; et al. Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, E2842–E2851. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.U.; Khosla, R.; David, P.; Rastogi, A.; Vyas, A.; Singh, D.; Bhardwaj, A.; Sahney, A.; Maiwall, R.; Sarin, S.K.; et al. CD4+CD25+CD127low Regulatory T Cells Play Predominant Anti-Tumor Suppressive Role in Hepatitis B Virus-Associated Hepatocellular Carcinoma. Front. Immunol. 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, C.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP. Nat. Genet. 2001, 27, 20–21. [Google Scholar] [CrossRef]
- Cinier, J.; Hubert, M.; Besson, L.; Di Roio, A.; Rodriguez, C.; Lombardi, V.; Caux, C.; Ménétrier-Caux, C. Recruitment and Expansion of Tregs Cells in the Tumor Environment—How to Target Them? Cancers 2021, 13, 1850. [Google Scholar] [CrossRef] [PubMed]
- Berlato, C.; Khan, M.N.; Schioppa, T.; Thompson, R.; Maniati, E.; Montfort, A.; Jangani, M.; Canosa, M.; Kulbe, H.; Hagemann, U.B.; et al. A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. J. Clin. Investig. 2017, 127, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.-K.; Peterson, E.; Sun, J.; Goudie, C.; Drapkin, R.I.; Liu, J.F.; Matulonis, U.; Zhu, Q.; Marasco, W.A. Anti-CCR4 monoclonal antibody enhances antitumor immunity by modulating tumor-infiltrating Tregs in an ovarian cancer xenograft humanized mouse model. OncoImmunology 2015, 5, e1090075. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Ueda, R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006, 97, 1139–1146. [Google Scholar] [CrossRef]
- Kurose, K.; Ohue, Y.; Wada, H.; Iida, S.; Ishida, T.; Kojima, T.; Doi, T.; Suzuki, S.; Isobe, M.; Funakoshi, T.; et al. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin. Cancer Res. 2015, 21, 4327–4336. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Cho, Y.-S.; Lee, J.Y.; Kook, M.C.; Park, J.-W.; Nam, B.-H.; Bae, J.-M. The Chemokine Receptor CCR4 is Expressed and Associated With a Poor Prognosis in Patients With Gastric Cancer. Ann. Surg. 2009, 249, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Ou, Z.-L.; Yu, S.-J.; Gu, X.-L.; Yang, C.; Chen, A.-X.; Di, G.-H.; Shen, Z.-Z.; Shao, Z.-M. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res. Treat. 2011, 131, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, X.; Li, L.; Wu, X.; Yan, J.; Yang, H.; Song, F. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-β pathway in human non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017, 488, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Murakami, K.; Inoue, A.; Yonezawa, T.; Matsuki, N. CCR4 Blockade Depletes Regulatory T Cells and Prolongs Survival in a Canine Model of Bladder Cancer. Cancer Immunol. Res. 2019, 7, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Maolake, A.; Izumi, K.; Shigehara, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Takezawa, Y.; Machioka, K.; Narimoto, K.; Namiki, M.; et al. Tumor-associated macrophages promote prostate cancer migration through activation of the CCL22-CCR4 axis. Oncotarget 2016, 8, 9739–9751. [Google Scholar] [CrossRef] [Green Version]
- Olkhanud, P.B.; Baatar, D.; Bodogai, M.; Hakim, F.; Gress, R.; Anderson, R.; Deng, J.; Xu, M.; Briest, S.; Biragyn, A. Breast Cancer Lung Metastasis Requires Expression of Chemokine Receptor CCR4 and Regulatory T Cells. Cancer Res. 2009, 69, 5996–6004. [Google Scholar] [CrossRef] [Green Version]
- Inngjerdingen, M.; Damaj, B.; Maghazachi, A. Human NK Cells Express CC Chemokine Receptors 4 and 8 and Respond to Thymus and Activation-Regulated Chemokine, Macrophage-Derived Chemokine, and I. J. Immunol. 2000, 164, 4048–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.A.; Chang, D.S.; Colvin, R.A.; Byrne, M.H.; McCully, M.L.; Moser, B.; Lira, S.A.; Charo, I.F.; Luster, A.D. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ TH2 cells. Nat. Immunol. 2011, 12, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.-J.; Hou, X.-X.; Wang, X.-Q.; Li, D.-J. The CCL17-CCR4 axis between endometrial stromal cells and macrophages contributes to the high levels of IL-6 in ectopic milieu. Am. J. Reprod. Immunol. 2017, 78, e12644. [Google Scholar] [CrossRef]
- Shono, Y.; Suga, H.; Kamijo, H.; Fujii, H.; Oka, T.; Miyagaki, T.; Shishido-Takahashi, N.; Sugaya, M.; Sato, S. Expression of CCR3 and CCR4 Suggests a Poor Prognosis in Mycosis Fungoides and Sézary Syndrome. Acta Derm. Venereol. 2019, 99, 809–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, M.; Zhu, Y.; Zhang, X.; Yang, Y.; Wang, C. CCR4 Expression Is Associated With Poor Prognosis in Patients With Early Stage (pN0) Oral Tongue Cancer. J. Oral Maxillofac. Surg. 2018, 77, 426–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Kanao, K.; Suzuki, S.; Muramatsu, H.; Morinaga, S.; Kajikawa, K.; Kobayashi, I.; Nishikawa, G.; Kato, Y.; Zennami, K.; et al. Increased infiltration of CCR4-positive regulatory T cells in prostate cancer tissue is associated with a poor prognosis. Prostate 2019, 79, 1658–1665. [Google Scholar] [CrossRef]
- Yonekura, K.; Kanzaki, T.; Gunshin, K.; Kawakami, N.; Takatsuka, Y.; Nakano, N.; Tokunaga, M.; Kubota, A.; Takeuchi, S.; Kanekura, T.; et al. Effect of anti-CCR4 monoclonal antibody (mogamulizumab) on adult T-cell leukemia-lymphoma: Cutaneous adverse reactions may predict the prognosis. J. Dermatol. 2014, 41, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, C.M.; Chiu, B.-C.; Stolberg, V.R.; Hu, J.; Zeibecoglou, K.; Lukacs, N.W.; Lira, S.A.; Kunkel, S.L.; Chensue, S.W. CCR8 Is Expressed by Antigen-Elicited, IL-10-Producing CD4+CD25+ T Cells, Which Regulate Th2-Mediated Granuloma Formation in Mice. J. Immunol. 2005, 174, 1962–1970. [Google Scholar] [CrossRef] [Green Version]
- Roos, R.S.; Loetscher, M.; Legler, D.F.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Identification of CCR8, the Receptor for the Human CC Chemokine I. J. Biol. Chem. 1997, 272, 17251–17254. [Google Scholar] [CrossRef] [Green Version]
- Inngjerdingen, M.; Damaj, B.; Maghazachi, A.A. Expression and regulation of chemokine receptors in human natural killer cells. Blood 2001, 97, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Chensue, S.W.; Lukacs, N.W.; Yang, T.-Y.; Shang, X.; Frait, K.A.; Kunkel, S.L.; Kung, T.; Wiekowski, M.T.; Hedrick, J.A.; Cook, D.; et al. Aberrant in Vivo T Helper Type 2 Cell Response and Impaired Eosinophil Recruitment in Cc Chemokine Receptor 8 Knockout Mice. J. Exp. Med. 2001, 193, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Soler, D.; Chapman, T.R.; Poisson, L.R.; Wang, L.; Cote-Sierra, J.; Ryan, M.; McDonald, A.; Badola, S.; Fedyk, E.; Coyle, A.J.; et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J. Immunol. 2006, 177, 6940–6951. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.A.; Ling, M.; Leung, J.; Shreffler, W.G.; Luster, A.D. Identification of human CCR8 as a CCL18 receptor. J. Exp. Med. 2013, 210, 1889–1898. [Google Scholar] [CrossRef]
- Biber, K.; Zuurman, M.W.; Homan, H.; Boddeke, H.W.G.M. Expression of L-CCR in HEK 293 cells reveals functional responses to CCL2, CCL5, CCL7, and CCL. J. Leukoc. Biol. 2003, 74, 243–251. [Google Scholar] [CrossRef]
- Howard, O.M.; Dong, H.F.; Shirakawa, A.K.; Oppenheim, J.J. LEC induces chemotaxis and adhesion by interacting with CCR1. CCR Blood 2000, 96, 840–845. [Google Scholar] [CrossRef]
- Strasly, M.; Doronzo, G.; Capello, P.; Valdembri, D.; Arese, M.; Mitola, S.; Moore, P.; Alessandri, G.; Giovarelli, M.; Bussolino, F. CCL16 activates an angiogenic program in vascular endothelial cells. Blood 2004, 103, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.D.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Zhou, Q.; Zeng, H.; Zhang, H.; Liu, Z.; Shao, J.; Wang, Z.; Xiong, Y.; Wang, J.; Bai, Q.; et al. CCR8 blockade primes anti-tumor immunity through intratumoral regulatory T cells destabilization in muscle-invasive bladder cancer. Cancer Immunol. Immunother. 2020, 69, 1855–1867. [Google Scholar] [CrossRef]
- Villarreal, D.O.; L’Huillier, A.; Armington, S.; Mottershead, C.; Filippova, E.V.; Coder, B.D.; Petit, R.G.; Princiotta, M.F. Targeting CCR8 Induces Protective Antitumor Immunity and Enhances Vaccine-Induced Responses in Colon Cancer. Cancer Res. 2018, 78, 5340–5348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eruslanov, E.; Stoffs, T.L.; Kim, W.-J.; Daurkin, I.; Gilbert, S.M.; Su, L.-M.; Vieweg, J.; Daaka, Y.; Kusmartsev, S. Expansion of CCR8+ Inflammatory Myeloid Cells in Cancer Patients with Urothelial and Renal Carcinomas. Clin. Cancer Res. 2013, 19, 1670–1680. [Google Scholar] [CrossRef] [Green Version]
- Rytlewski, J.; Milhem, M.M.; Monga, V. Turning ‘Cold’ tumors ‘Hot’: Immunotherapies in sarcoma. Ann. Transl. Med. 2021, 9, 1039. [Google Scholar] [CrossRef]
- Yarmarkovich, M.; Maris, J.M. When Cold Is Hot: Immune Checkpoint Inhibition Therapy for Rhabdoid Tumors. Cancer Cell 2019, 36, 575–576. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Warming “Cold” Melanoma with TLR9 Agonists. Cancer Discov. 2018, 8, 670. [CrossRef] [Green Version]
- Kishton, R.J.; Lynn, R.C.; Restifo, N.P. Strength in Numbers: Identifying Neoantigen Targets for Cancer Immunotherapy. Cell 2020, 183, 591–593. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362.e24. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.K.; van Buuren, M.M.; Dang, K.K.; Hubbard-Lucey, V.M.; Sheehan, K.C.; Campbell, K.M.; Lamb, A.; Ward, J.P.; Sidney, J.; Blazquez, A.B.; et al. Key Parameters of Tumor Epitope Immunogenicity Revealed Through a Consortium Approach Improve Neoantigen Prediction. Cell 2020, 183, 818–834.e13. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.; Simoni, Y.; Penny, H.L.; Becht, E.; Loh, C.Y.; Gubin, M.M.; Ward, J.; Wong, S.C.; Schreiber, R.D.; Newell, E.W. Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8+ T cells. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Matsuki, M.; Hirohashi, Y.; Nakatsugawa, M.; Murai, A.; Kubo, T.; Hashimoto, S.; Tokita, S.; Murata, K.; Kanaseki, T.; Tsukahara, T.; et al. Tumor-infiltrating CD8+ T cells recognize a heterogeneously expressed functional neoantigen in clear cell renal cell carcinoma. Cancer Immunol. Immunother. 2021, 1–14. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Mints, M.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Łuksza, M.; Riaz, N.; Makarov, V.; Balachandran, V.P.; Hellmann, M.D.; Solovyov, A.; Rizvi, N.A.; Merghoub, T.; Levine, A.J.; Chan, T.A.; et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nat. Cell Biol. 2017, 551, 517–520. [Google Scholar] [CrossRef]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanic, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Verdegaal, E.M.E.; de Miranda, N.F.C.C.; Visser, M.; Harryvan, T.; van Buuren, M.M.; Andersen, R.S.; Hadrup, S.R.; van der Minne, C.E.; Schotte, R.; Spits, H.; et al. Neoantigen landscape dynamics during human melanoma–T cell interactions. Nat. Cell Biol. 2016, 536, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Tumour immunology: A triple blow for cancer. Nat. Rev. Immunol. 2015, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Immunotherapy: A triple blow for cancer. Nat. Rev. Cancer 2015, 15, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Kallies, A.; Zehn, D.; Utzschneider, D. Precursor exhausted T cells: Key to successful immunotherapy? Nat. Rev. Immunol. 2019, 20, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Disease | Prgnostic Association | Reference |
|---|---|---|
| Colorectal cancer (CRC) | low transcription of CXCL10 and poor prognosis in stages II and III CRC examined by snap-frozen CRC tissues by RT-PCR. | [218] |
| Rectal Cancer | Patients that are CXCL10high (RT PCR) display a better response to chemoradiotherapy, suggesting a synergistic beneficial effect of both | [219] |
| Epithelial ovarian carcinoma (HGSOC) | In patients with this disease high levels of a CXCL10 antagonist could be associated with a poor prognosis | [220] |
| Osteosarcoma (OS) | Better survival in patients with high levels of CXCL10 in circulating blood | [221] |
| Hepatocellular carcinoma (HCC) | High levels of CXCL10 in tumor tissues were associated with better prognostic and overall survival | [222] |
| Ovarian carcinoma | High levels of CXCL9 are associated with effector CD8+ T cell recruitment and good prognosis | [223] |
| ER-Negative Breast Cancer | Good prognosis associated with immune cells infiltration in suggesting CXCL9 as a potential biomarker for the prognosis of this disease | [224] |
| Breast cancer | High expression of CXCL9 and CXCL10 is associated with a good prognosis | [225] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karin, N. Chemokines in the Landscape of Cancer Immunotherapy: How They and Their Receptors Can Be Used to Turn Cold Tumors into Hot Ones? Cancers 2021, 13, 6317. https://doi.org/10.3390/cancers13246317
Karin N. Chemokines in the Landscape of Cancer Immunotherapy: How They and Their Receptors Can Be Used to Turn Cold Tumors into Hot Ones? Cancers. 2021; 13(24):6317. https://doi.org/10.3390/cancers13246317
Chicago/Turabian StyleKarin, Nathan. 2021. "Chemokines in the Landscape of Cancer Immunotherapy: How They and Their Receptors Can Be Used to Turn Cold Tumors into Hot Ones?" Cancers 13, no. 24: 6317. https://doi.org/10.3390/cancers13246317





