Modulation of the Gal-9/TIM-3 Immune Checkpoint with α-Lactose. Does Anomery of Lactose Matter?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Lactose: α- and β-Anomers
3. The TIM-3 Immune Checkpoint and Its Targeting with Antibodies
4. Small Molecules Targeting TIM-3/Gal-9 Checkpoint
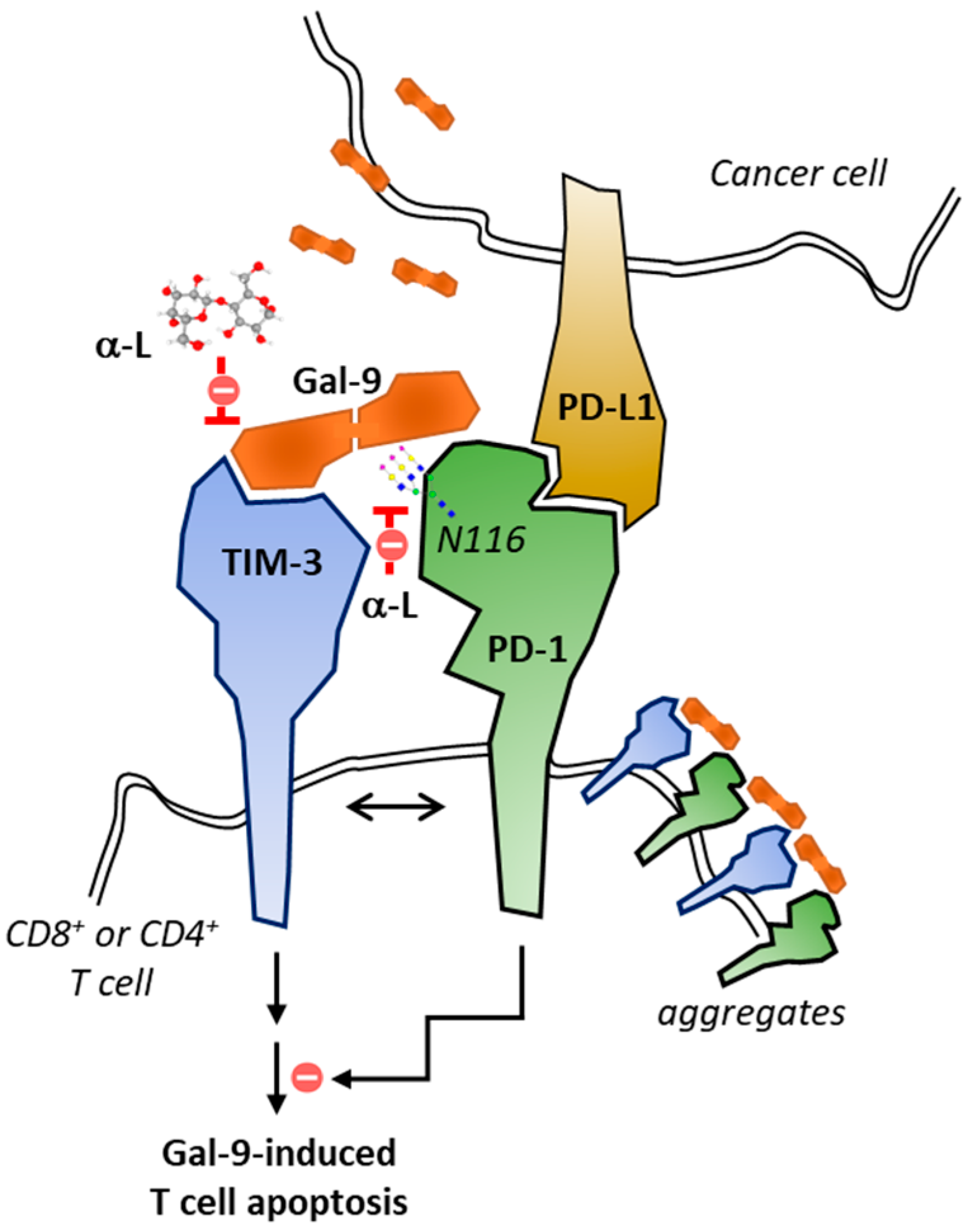
5. Tim-3/Gal-9 Signaling Blockade with α/β-Lactose
6. Lactose as a Dual Modulator of Gal-9/TIM-3 and PD1/PD-L1 Checkpoints
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Timmer, F.E.F.; Geboers, B.; Nieuwenhuizen, S.; Dijkstra, M.; Schouten, E.A.C.; Puijk, R.S.; de Vries, J.J.J.; van den Tol, M.P.; Bruynzeel, A.M.E.; Streppel, M.M.; et al. Pancreatic Cancer and Immunotherapy: A Clinical Overview. Cancers 2021, 13, 4138. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, J.; Gast, K.; Quiroga, D.; Lustberg, M.; Williams, N. Triple-negative breast cancer: Promising prognostic biomarkers currently in development. Expert Rev. Anticancer Ther. 2021, 21, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Desbaillets, N.; Hottinger, A.F. Immunotherapy in Glioblastoma: A Clinical Perspective. Cancers 2021, 13, 3721. [Google Scholar] [CrossRef]
- Corti, C.; Nicolò, E.; Curigliano, G. Novel immune targets for the treatment of triple-negative breast cancer. Expert Opin. Ther. Targets. 2021, 25, 815–834. [Google Scholar] [CrossRef]
- Tang, D.; Lotze, M.T. Tumor immunity times out: TIM-3 and HMGB1. Nat. Immunol. 2012, 13, 808–810. [Google Scholar] [CrossRef]
- Lee, J.B.; Ha, S.J.; Kim, H.R. Clinical Insights Into Novel Immune Checkpoint Inhibitors. Front. Pharmacol. 2021, 12, 681320. [Google Scholar] [CrossRef] [PubMed]
- Iżykowska, K.; Rassek, K.; Korsak, D.; Przybylski, G.K. Novel targeted therapies of T cell lymphomas. J. Hematol. Oncol. 2020, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.; Hung, E.; Singh, I.; Ben-Neriah, Y. Immunity in acute myeloid leukemia: Where the immune response and targeted therapy meet. Eur. J. Immunol. Online ahead of print. 2021. [Google Scholar] [CrossRef]
- Krejcik, J.; Barnkob, M.B.; Nyvold, C.G.; Larsen, T.S.; Barington, T.; Abildgaard, N. Harnessing the Immune System to Fight Multiple Myeloma. Cancers 2021, 13, 4546. [Google Scholar] [CrossRef]
- Mohsenzadegan, M.; Bavandpour, P.; Nowroozi, M.R.; Amini, E.; Kourosh-Arami, M.; Momeni, S.A.; Bokaie, S.; Sharifi, L. The Potential of T Cell Immunoglobulin and Mucin-Domain containing-3 (Tim-3) in Designing Novel Immunotherapy for Bladder Cancer. Endocr. Metab. Immune Disord. Drug Targets. Online ahead of print. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Liu, J.; Chen, G.; Qiao, G. The Emerging Role of T-Cell Immunoglobulin Mucin-3 in Breast Cancer: A Promising Target For Immunotherapy. Front. Oncol. 2021, 11, 723238. [Google Scholar] [CrossRef] [PubMed]
- Zang, K.; Hui, L.; Wang, M.; Huang, Y.; Zhu, X.; Yao, B. TIM-3 as a Prognostic Marker and a Potential Immunotherapy Target in Human Malignant Tumors: A Meta-Analysis and Bioinformatics Validation. Front. Oncol. 2021, 11, 579351. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Komrokji, R.S.; Brunner, A.M. TIM-3 pathway dysregulation and targeting in cancer. Expert Rev. Anticancer Ther. 2021, 21, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Asayama, T.; Taura, H.; Ishibashi, M.; Kuribayashi-Hamada, Y.; Onodera-Kondo, A.; Okuyama, N.; Yamada, A.; Shimizu, M.; Moriya, K.; Takahashi, H.; et al. Functional expression of Tim-3 on blasts and clinical impact of its ligand galectin-9 in myelodysplastic syndromes. Oncotarget 2017, 8, 88904–88917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, M.; Tan, J.; Zeng, C.; Li, Y.; Ganjalikhani-Hakemi, M. TIM-3 in Leukemia; Immune Response and Beyond. Front. Oncol. 2021, 11, 753677. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Wang, M.; Zhang, L.; Yu, L. One Stone, Two Birds: The Roles of Tim-3 in Acute Myeloid Leukemia. Front Immunol. 2021, 12, 618710. [Google Scholar] [CrossRef]
- Daver, N. Immune checkpoint inhibitors in acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2021, 34, 101247. [Google Scholar] [CrossRef]
- Platteau, C.; Lefebvre, J.; Affouard, F.; Derollez, P. Ab initio structure determination of the hygroscopic anhydrous form of alpha-lactose by powder X-ray diffraction. Acta Crystallogr. B 2004, 60, 453–460. [Google Scholar] [CrossRef]
- Platteau, C.; Lefebvre, J.; Affouard, F.; Willart, J.F.; Derollez, P.; Mallet, F. Structure determination of the stable anhydrous phase of alpha-lactose from X-ray powder diffraction. Acta Crystallogr. B 2005, 61, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, B.; Fu, L.; Chen, C.; Liu, W.; Tan, S. Tailoring α/β Ratio of Pollen-Like Anhydrous Lactose as Ingredient Carriers for Controlled Dissolution Rate. Crystals 2021, 11, 1049. [Google Scholar] [CrossRef]
- Jawad, R.; Drake, A.F.; Elleman, C.; Martin, G.P.; Warren, F.J.; Perston, B.B.; Ellis, P.R.; Hassoun, M.A.; Royall, P.G. Stability of sugar solutions: A novel study of the epimerization kinetics of lactose in water. Mol. Pharm. 2014, 11, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, T.; Yang, X.; Yue, F.; Fu, F. An exploration of the solvent- and acid-catalyzed mutarotation mechanisms of lactose in aqueous solution. New J. Chem. 2020, 44, 16421–16430. [Google Scholar] [CrossRef]
- Batra, A.; Desai, D.; Serajuddin, A.T.M. Conversion of alpha-lactose monohydrate to anhydrous form with superior tabletability by twin-screw extrusion at elevated temperature. Int. J. Pharm. 2020, 588, 119790. [Google Scholar] [CrossRef]
- López-Pablos, A.L.; Leyva-Porras, C.C.; Silva-Cázares, M.B.; Longoria-Rodríguez, F.E.; Pérez-García, S.A.; Vértiz-Hernández, A.A.; Saavedra-Leos, M.Z. Preparation and Characterization of High Purity Anhydrous β-Lactose from α-Lactose Monohydrate at Mild Temperature. Int. J. Polym. Sci. 2018, 2018, 5069063. [Google Scholar] [CrossRef] [Green Version]
- Lara-Mota, E.E.; Nicolás-Vázquez, M.I.; López-Martínez, L.A.; Espinosa-Solis, V.; Cruz-Alcantar, P.; Toxqui-Teran, A.; Saavedra-Leos, M.Z. Phenomenological study of the synthesis of pure anhydrous beta-lactose in alcoholic solution. Food Chem. 2021, 340, 128054. [Google Scholar] [CrossRef] [PubMed]
- EMA/CHMP/186428/2016. European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Information for the Package Leaflet Regarding Lactose used as an Excipient in Medicinal Products for Human Use. Public Consultation: 19 November 2018–31 May 2019. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-information-package-leaflet-regarding-lactose-used-excipient-medicinal-products-human-use_en.pdf (accessed on 12 October 2021).
- Della Bella, A.; Müller, M.; Soldati, L.; Elviri, L.; Bettini, R. Quantitative determination of micronization-induced changes in the solid state of lactose. Int. J. Pharm. 2016, 505, 383–393. [Google Scholar] [CrossRef]
- Mehta, P. Imagine the Superiority of Dry Powder Inhalers from Carrier Engineering. J. Drug Deliv. 2018, 2018, 5635010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, W.R.; Chang, R.Y.K.; Kwok, P.C.L.; Chen, D.; Chan, H.K. Spray drying lactose from organic solvent suspensions for aerosol delivery to the lungs. Int. J. Pharm. 2020, 591, 119984. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.J.; Wolff, K.; Nokhodchi, A.; Martin, G.P.; Royall, P.G. Variability in the α and β anomer content of commercially available lactose. Int. J. Pharm. 2019, 555, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Alzoubi, T.; Martin, G.P.; Barlow, D.J.; Royall, P.G. Stability of alpha-lactose monohydrate: The discovery of dehydration triggered solid-state epimerization. Int. J. Pharm. 2021, 604, 120715. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.T.; Tang, X.L.; Jiang, F.Y.; Yan, X.; Deng, J. Porous Lactose as a Novel Ingredient Carrier for the Improvement of Quercetin Solubility In Vitro. Bioinorg. Chem. Appl. 2021, 2021, 2586990. [Google Scholar] [CrossRef] [PubMed]
- Dominik, M.; Vraníková, B.; Svačinová, P.; Elbl, J.; Pavloková, S.; Prudilová, B.B.; Šklubalová, Z.; Franc, A. Comparison of Flow and Compression Properties of Four Lactose-Based Co-Processed Excipients: Cellactose® 80, CombiLac®, MicroceLac® 100, and StarLac®. Pharmaceutics 2021, 13, 1486. [Google Scholar] [CrossRef] [PubMed]
- Zellnitz, S.; Lamešić, D.; Stranzinger, S.; Pinto, J.T.; Planinšek, O.; Paudel, A. Spherical agglomerates of lactose as potential carriers for inhalation. Eur. J. Pharm. Biopharm. 2021, 159, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, M.; Biasucci, G.; Maddalena, Y.; Di Scala, C.; De Caro, C.; Calignano, A.; Canani, R.B. Lactose Intolerance in Pediatric Patients and Common Misunderstandings About Cow’s Milk Allergy. Pediatr. Ann. 2021, 50, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose intolerance: An update on its pathogenesis, diagnosis, and treatment. Nutr. Res. 2021, 89, 23–34. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Deng, Y.; Jiang, C.; Deng, N. Fermentation, purification and immunogenicity of a recombinant tumor multi-epitope vaccine, VBP3. Protein Expr. Purif. 2020, 174, 105658. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, L.; Lin, H.; Mao, Z.; Bao, K.; Hao, P.; Pei, Z.; Li, J.; Hu, Z. Lactose induced redox-dependent senescence and activated Nrf2 pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 2034–2045. [Google Scholar]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Feizisani, F.; Shomali, N.; Abdelbasset, W.K.; Hemmatzadeh, M.; Gholizadeh, J.; Jadidi-Niaragh, F.; Bokov, D.O.; Janebifam, M.; Mohammadi, H. PD-1/PD-L1 Blockade: Prospectives for immunotherapy in Cancer and Autoimmunity. IUBMB Life. 2021, 73, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef] [Green Version]
- Dempke, W.C.M.; Fenchel, K.; Uciechowski, P.; Dale, S.P. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur. J. Cancer 2017, 74, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Ye, Z.; Cheung, A.M.S.; Goh, Y.P.S.; Oh, H.L.J.; Rajarethinam, R.; Yeo, S.P.; Soh, M.K.; Chan, E.H.L.; Tan, L.K.; et al. Effective Killing of Acute Myeloid Leukemia by TIM-3 Targeted Chimeric Antigen Receptor T Cells. Mol. Cancer Ther. 2021, 20, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Kuchroo, V.K. Tim-3, Lag-3, and TIGIT. Emerg. Concepts Target. Immune Checkp. Cancer Autoimmun. 2017, 410, 127–156. [Google Scholar]
- Saleh, R.; Toor, S.M.; Elkord, E. Targeting TIM-3 in solid tumors: Innovations in the preclinical and translational realm and therapeutic potential. Expert Opin. Ther. Targets. 2020, 24, 1251–1262. [Google Scholar] [CrossRef]
- Kandel, S.; Adhikary, P.; Li, G.; Cheng, K. The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy. Cancer Lett. 2021, 510, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cheng, S.; Fan, L.; Zhang, B.; Xu, S. TIM-3: An update on immunotherapy. Int. Immunopharmacol. 2021, 99, 107933. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Q.; Liu, H.; He, X.; Huang, F.; Wang, Y. Role of Tim-3 in regulating tumorigenesis, inflammation, and antitumor immunity therapy. Cancer Biomark. 2021, 32, 237–248. [Google Scholar] [CrossRef]
- Oweida, A.; Hararah, M.K.; Phan, A.; Binder, D.; Bhatia, S.; Lennon, S.; Bukkapatnam, S.; Van Court, B.; Uyanga, N.; Darragh, L.; et al. Resistance to Radiotherapy and PD-L1 Blockade Is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin. Cancer Res. 2018, 24, 5368–5380. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Li, Z. Targeting Tim-3 in Cancer With Resistance to PD-1/PD-L1 Blockade. Front. Oncol. 2021, 11, 731175. [Google Scholar] [CrossRef]
- Yasinska, I.M.; Sakhnevych, S.S.; Pavlova, L.; Teo Hansen Selnø, A.; Teuscher Abeleira, A.M.; Benlaouer, O.; Gonçalves Silva, I.; Mosimann, M.; Varani, L.; Bardelli, M.; et al. The Tim-3-Galectin-9 Pathway and Its Regulatory Mechanisms in Human Breast Cancer. Front. Immunol. 2019, 10, 1594. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Liu, X.; Wang, X.; Yan, F.; Wang, P.; Jiang, Y.; Du, J.; Yang, Z. TIGIT(+) TIM-3(+) NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis B virus-related hepatocellular carcinoma. Oncoimmunology 2021, 10, 1942673. [Google Scholar] [CrossRef]
- Solinas, C.; De Silva, P.; Bron, D.; Willard-Gallo, K.; Sangiolo, D. Significance of TIM3 expression in cancer: From biology to the clinic. Semin. Oncol. 2019, 46, 372–379. [Google Scholar] [CrossRef]
- Gonçalves Silva, I.; Yasinska, I.M.; Sakhnevych, S.S.; Fiedler, W.; Wellbrock, J.; Bardelli, M.; Varani, L.; Hussain, R.; Siligardi, G.; Ceccone, G.; et al. The Tim-3-galectin-9 Secretory Pathway is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells. EBioMedicine 2017, 22, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Han, D.; Gao, S.; Zhang, W.; Yu, H.; Liu, P.; Fu, R.; Li, L.; Shao, Z. CD8+ T cells exhaustion induced by myeloid-derived suppressor cells in myelodysplastic syndromes patients might be through TIM3/Gal-9 pathway. J. Cell. Mol. Med. 2020, 24, 1046–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dama, P.; Tang, M.; Fulton, N.; Kline, J.; Liu, H. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J. Immunother. Cancer 2019, 7, 175. [Google Scholar] [CrossRef]
- Kikushige, Y.; Shima, T.; Takayanagi, S.; Urata, S.; Miyamoto, T.; Iwasaki, H.; Takenaka, K.; Teshima, T.; Tanaka, T.; Inagaki, Y.; et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010, 7, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Kikushige, Y.; Miyamoto, T.; Yuda, J.; Jabbarzadeh-Tabrizi, S.; Shima, T.; Takayanagi, S.; Niiro, H.; Yurino, A.; Miyawaki, K.; Takenaka, K.; et al. A TIM-3/Gal-9 Autocrine Stimulatory Loop Drives Self-Renewal of Human Myeloid Leukemia Stem Cells and Leukemic Progression. Cell Stem Cell 2015, 17, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Kikushige, Y.; Miyamoto, T. Identification of TIM-3 as a Leukemic Stem Cell Surface Molecule in Primary Acute Myeloid Leukemia. Oncology 2015, 89, 28–32. [Google Scholar] [CrossRef]
- Marchand, T.; Pinho, S. Leukemic Stem Cells: From Leukemic Niche Biology to Treatment Opportunities. Front. Immunol. 2021, 12, 775128. [Google Scholar] [CrossRef]
- Acharya, N.; Sabatos-Peyton, C.; Anderson, A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer 2020, 8, e000911. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Zeidan, A.M. Management of patients with higher-risk myelodysplastic syndromes after failure of hypomethylating agents: What is on the horizon? Best Pract. Res. Clin. Haematol. 2021, 34, 101245. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, F.; Zaynagetdinov, R.; Huang, H.; Sood, V.D.; Wang, H.; Zhao, X.; Jenkins, M.H.; Ji, Q.; Wang, Y.; et al. Identification and characterization of M6903, an antagonistic anti-TIM-3 monoclonal antibody. Oncoimmunology 2020, 9, 1744921. [Google Scholar] [CrossRef] [Green Version]
- Harding, J.J.; Moreno, V.; Bang, Y.J.; Hong, M.H.; Patnaik, A.; Trigo, J.; Szpurka, A.M.; Yamamoto, N.; Doi, T.; Fu, S.; et al. Blocking TIM-3 in Treatment-refractory Advanced Solid Tumors: A Phase Ia/b Study of LY3321367 with or without an Anti-PD-L1 Antibody. Clin. Cancer Res. 2021, 27, 2168–2178. [Google Scholar] [CrossRef]
- Jin, Y.J.; Yu, D.; Tian, X.L.; Li, H.X.; Zhou, X.C.; Kong, Y.; Zhang, W.; Zhang, L.; Lei, C.; Yang, Z.L.; et al. A novel and effective approach to generate germline-like monoclonal antibodies by integration of phage and mammalian cell display platforms. Acta Pharmacol. Sin. 2021, 1–9. [Google Scholar] [CrossRef]
- Dixon, K.O.; Tabaka, M.; Schramm, M.A.; Xiao, S.; Tang, R.; Dionne, D.; Anderson, A.C.; Rozenblatt-Rosen, O.; Regev, A.; Kuchroo, V.K. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 2021, 595, 101–106. [Google Scholar] [CrossRef]
- Gefen, T.; Castro, I.; Muharemagic, D.; Puplampu-Dove, Y.; Patel, S.; Gilboa, E. A TIM-3 Oligonucleotide Aptamer Enhances T Cell Functions and Potentiates Tumor Immunity in Mice. Mol. Ther. 2017, 25, 2280–2288. [Google Scholar] [CrossRef] [Green Version]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Zhong, T.; Zhao, C.; Wang, S.; Tao, D.; Ma, S.; Shou, C. The biologically functional identification of a novel TIM3-binding peptide P26 in vitro and in vivo. Cancer Chemother. Pharmacol. 2020, 86, 783–792. [Google Scholar] [CrossRef]
- Hollebecque, A.; Chung, H.C.; de Miguel, M.J.; Italiano, A.; Machiels, J.P.; Lin, C.C.; Dhani, N.C.; Peeters, M.; Moreno, V.; Su, W.C.; et al. Safety and Antitumor Activity of α-PD-L1 Antibody as Monotherapy or in Combination with α-TIM-3 Antibody in Patients with Microsatellite Instability-High/Mismatch Repair-Deficient Tumors. Clin. Cancer Res. 2021, 27, 6393–6404. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Al-Kali, A.; Borate, U.; Cluzeau, T.; DeZern, A.E.; Giagounidis, A.; Kobata, K.; Lyons, R.; Platzbecker, U.; Sallman, D.A.; et al. Sabatolimab (MBG453) Combination Treatment Regimens for Patients (Pts) with Higher-Risk Myelodysplastic Syndromes (HR-MDS): The MDS Studies in the Stimulus Immuno-Myeloid Clinical Trial Program. Blood 2021, 138, 4669. [Google Scholar] [CrossRef]
- Bruner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Vey, N.; Scholl, S.; Garcia-Manero, G.; Wermke, M.; Janssen, J.; Traer, E.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients with Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndrome (HR-MDS): Updated Results from a Phase 1b Study. Blood 2020, 136, 1–2. [Google Scholar] [CrossRef]
- Waight, J.; Iyer, P.; Breous-Nystrom, E.; Riordan, C.; Findeis, M.; Underwood, D.; Connolly, J.; Sanicola-Nadel, M.; Nastri, H.; Scherle, P.; et al. Abstract 3825: INCAGN02390, a novel antagonist antibody that targets the co-inhibitory receptor TIM-3. In Proceedings of the AACR Annual Meeting 2018, Chicago, IL, USA, 14–18 April 2018; 2018; 78, p. 3825. [Google Scholar] [CrossRef]
- Desai, J.; Meniawy, T.; Beagle, B.; Li, Z.Z.; Mu, S.; Wu, J.; Denlinger, C.S.; Messersmith, W.A. Bgb-A425, an investigational anti-TIM-3 monoclonal antibody, in combination with tislelizumab, an anti-PD-1 monoclonal antibody, in patients with advanced solid tumors: A phase I/II trial in progress. J. Clin. Oncol. 2020, 38, TPS3146. [Google Scholar] [CrossRef]
- Sabatos-Peyton, C.A.; Nevin, J.; Brock, A.; Venable, J.D.; Tan, D.J.; Kassam, N.; Xu, F.; Taraszka, J.; Wesemann, L.; Pertel, T.; et al. Blockade of Tim-3 binding to phosphatidylserine and CEACAM1 is a shared feature of anti-Tim-3 antibodies that have functional efficacy. Oncoimmunology 2017, 7, e1385690. [Google Scholar] [CrossRef] [Green Version]
- Santini, V. Advances in myelodysplastic syndrome. Curr. Opin. Oncol. 2021, 33, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, H.; Astaneh, M.; Tehrani, M.; Hossein-Nataj, H.; Zaboli, E.; Shekarriz, R.; Asgarian-Omran, H. Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8(+) T cells in early clinical stages of chronic lymphocytic leukemia. Immunol. Res. 2020, 68, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, T.; Akiba, H.; Nakayama, M.; Harada, N.; Okumura, K.; Homma, S.; Miyake, S. Cutting Edge: Anti-TIM-3 Treatment Exacerbates Pulmonary Inflammation and Fibrosis in Mice. J. Immunol. 2017, 199, 3733–3737. [Google Scholar] [CrossRef] [Green Version]
- Tembhre, M.K.; Parihar, A.S.; Sharma, A.; Gupta, S.; Chattopadhyay, P.; Sharma, V.K. Participation of T cell immunoglobulin and mucin domain-3 (TIM-3) and its ligand (galectin-9) in the pathogenesis of active generalized vitiligo. Immunol. Res. 2015, 62, 23–34. [Google Scholar] [CrossRef]
- Rahimi, A.; Hossein-Nataj, H.; Hajheydari, Z.; Aryanian, Z.; Shayannia, A.; Ajami, A.; Asgarian-Omran, H. Expression analysis of PD-1 and Tim-3 immune checkpoint receptors in patients with vitiligo; positive association with disease activity. Exp. Dermatol. 2019, 28, 674–681. [Google Scholar] [CrossRef]
- Koohini, Z.; Hossein-Nataj, H.; Mobini, M.; Hosseinian-Amiri, A.; Rafiei, A.; Asgarian-Omran, H. Analysis of PD-1 and Tim-3 expression on CD4+ T cells of patients with rheumatoid arthritis; negative association with DAS28. Clin. Rheumatol. 2018, 37, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Fujita, Y.; Asano, T.; Matsuoka, N.; Temmoku, J.; Sato, S.; Yashiro-Furuya, M.; Watanabe, H.; Migita, K. T cell immunoglobulin and mucin domain-3 is associated with disease activity and progressive joint damage in rheumatoid arthritis patients. Medicine 2020, 99, e22892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yu, G.; Han, Q.; Cui, C.; Zhang, B. TIM-3: An emerging target in the liver diseases. Scand. J. Immunol. 2020, 91, e12825. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, D.; Zhu, Y.; Pang, N.; Ding, J. The role of Tim-3/Galectin-9 pathway in T-cell function and prognosis of patients with human papilloma virus-associated cervical carcinoma. FASEB J. 2021, 35, e21401. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Song, Z.; Zhang, A. Small-Molecule Immuno-Oncology Therapy: Advances, Challenges and New Directions. Curr. Top. Med. Chem. 2019, 19, 180–185. [Google Scholar] [CrossRef]
- Khan, A.; Bresnick, A.; Cahill, S.; Girvin, M.; Almo, S.; Quinn, R. Advantages of Molecular Weight Identification during Native MS Screening. Planta Med. 2018, 84, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Bhattacharya, K.; Mandal, C.; Pal, B.C. Identification and quantification of the active component quercetin 3-O-rutinoside from Barringtonia racemosa, targets mitochondrial apoptotic pathway in acute lymphoblastic leukemia. J. Asian Nat. Prod. Res. 2010, 12, 639–648. [Google Scholar] [CrossRef]
- Mathew, N.S.; Kurrey, N.K.; Bettadaiah, B.K.; Negi, P.S. Anti-proliferative activity of Ensete superbum Roxb. Cheesman extract and its active principles on human colorectal cancer cell lines. J. Food Sci. 2021, 86, 5026–5040. [Google Scholar] [CrossRef]
- Xie, N.; Wang, Y.; Wang, Q.; Li, F.R.; Guo, B. Lipoteichoic acid of Bifidobacterium in combination with 5-fluorouracil inhibit tumor growth and relieve the immunosuppression. Bull. Cancer. 2012, 99, 55–63. [Google Scholar] [CrossRef]
- Guo, B.; Xie, N.; Wang, Y. Cooperative effect of Bifidobacteria lipoteichoic acid combined with 5-fluorouracil on hepatoma-22 cells growth and apoptosis. Bull. Cancer. 2015, 102, 204–212. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, S.; Cao, J.; Ye, J.; Huang, H.; Liao, D.; Yang, Y.; Chen, W.; Pu, R. Combined targeting of vascular endothelial growth factor C (VEGFC) and P65 using miR-27b-3p agomir and lipoteichoic acid in the treatment of gastric cancer. J. Gastrointest. Oncol. 2021, 12, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, M.; Gao, S.; Hong, L.; Hou, T.; Zhang, Y.; Zhu, Y.; Qian, F. Shikonin ameliorates lipoteichoic acid-induced acute lung injury via promotion of neutrophil apoptosis. Mol. Med. Rep. 2021, 23, 133. [Google Scholar] [CrossRef] [PubMed]
- Rietz, T.A.; Teuscher, K.B.; Mills, J.J.; Gogliotti, R.D.; Lepovitz, L.T.; Scaggs, W.R.; Yoshida, K.; Luong, K.; Lee, T.; Fesik, S.W. Fragment-Based Discovery of Small Molecules Bound to T-Cell Immunoglobulin and Mucin Domain-Containing Molecule 3 (TIM-3). J. Med. Chem. 2021, 64, 14757–14772. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. Structure and functions of T-cell immunoglobulin-domain and mucin- domain protein 3 in cancer. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Shi, J.; Sun, Y.; Zheng, F. Glycyrrhizin, a Potential Drug for Autoimmune Encephalomyelitis by Inhibiting High-Mobility Group Box 1. DNA Cell. Biol. 2018, 37, 941–946. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef]
- Kaur, I.; Behl, T.; Bungau, S.; Kumar, A.; Mehta, V.; Setia, D.; Uddin, M.S.; Zengin, G.; Aleya, L.; Arora, S. Exploring the therapeutic promise of targeting HMGB1 in rheumatoid arthritis. Life Sci. 2020, 258, 118164. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Bai, Y.; Zhang, R.; Meng, R.; Chen, F.; Wang, H.; Billiar, T.R.; Xiao, X.; Lu, B.; et al. A small molecule binding HMGB1 inhibits caspase-11-mediated lethality in sepsis. Cell Death Dis. 2021, 12, 402. [Google Scholar] [CrossRef]
- Wang, W.H.; Lin, C.Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.H.; Liu, F.T.; Wang, S.F. The role of galectins in virus infection-A systemic literature review. J. Microbiol. Immunol. Infect. 2020, 53, 925–935. [Google Scholar] [CrossRef]
- Zheng, Y.; Feng, W.; Wang, Y.J.; Sun, Y.; Shi, G.; Yu, Q. Galectins as potential emerging key targets in different types of leukemia. Eur. J. Pharmacol. 2019, 844, 73–78. [Google Scholar] [CrossRef]
- Türeci, O.; Schmitt, H.; Fadle, N.; Pfreundschuh, M.; Sahin, U. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin’s disease. J. Biol. Chem. 1997, 272, 6416–6422. [Google Scholar] [CrossRef] [Green Version]
- Wada, J.; Kanwar, Y.S. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J. Biol. Chem. 1997, 272, 6078–6086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Nishi, N.; Murata, T.; Usui, T.; Nakamura, T.; Wakatsuki, S.; Kato, R. Crystal structure of the galectin-9 N-terminal carbohydrate recognition domain from Mus musculus reveals the basic mechanism of carbohydrate recognition. J. Biol. Chem. 2006, 281, 35884–35893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Weyer, P.S.; Muehlfeit, M.; Klose, C.; Bonventre, J.V.; Walz, G.; Kuehn, E.W. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006, 351, 571–576. [Google Scholar] [CrossRef]
- John, S.; Mishra, R. Galectin-9: From cell biology to complex disease dynamics. J. Biosci. 2016, 41, 507–534. [Google Scholar] [CrossRef]
- Kocibalova, Z.; Guzyova, M.; Borovska, I.; Messingerova, L.; Copakova, L.; Sulova, Z.; Breier, A. Development of Multidrug Resistance in Acute Myeloid Leukemia Is Associated with Alterations of the LPHN1/GAL-9/TIM-3 Signaling Pathway. Cancers 2021, 13, 3629. [Google Scholar] [CrossRef]
- Katoh, S.; Ishii, N.; Nobumoto, A.; Takeshita, K.; Dai, S.Y.; Shinonaga, R.; Niki, T.; Nishi, N.; Tominaga, A.; Yamauchi, A.; et al. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 27–35. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Chatzopoulos, K.; Butterfield, J.T.; Sutor, S.L.; Leontovich, A.A.; Nevala, W.K.; Flotte, T.J.; Markovic, S.N. CD206-positive myeloid cells bind galectin-9 and promote a tumor-supportive microenvironment. J. Pathol. 2018, 245, 468–477. [Google Scholar] [CrossRef]
- Sudhakar, J.N.; Lu, H.H.; Chiang, H.Y.; Suen, C.S.; Hwang, M.J.; Wu, S.Y.; Shen, C.N.; Chang, Y.M.; Li, F.A.; Liu, F.T.; et al. Lumenal Galectin-9-Lamp2 interaction regulates lysosome and autophagy to prevent pathogenesis in the intestine and pancreas. Nat. Commun. 2020, 11, 4286. [Google Scholar] [CrossRef]
- Bitra, A.; Doukov, T.; Wang, J.; Picarda, G.; Benedict, C.A.; Croft, M.; Zajonc, D.M. Crystal structure of murine 4-1BB and its interaction with 4-1BBL support a role for galectin-9 in 4-1BB signaling. J. Biol. Chem. 2018, 293, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Bi, S.; Hong, P.W.; Lee, B.; Baum, L.G. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc. Natl. Acad. Sci. USA. 2011, 108, 10650–10655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, K.; Webb, N.E.; Pang, M.; Hernandez-Davies, J.E.; Lee, K.P.; Gonzalez, P.; Douglass, M.V.; Lee, B.; Baum, L.G. Galectin-9 binds to O-glycans on protein disulfide isomerase. Glycobiology 2017, 27, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- Schlichtner, S.; Meyer, N.H.; Yasinska, I.M.; Aliu, N.; Berger, S.M.; Gibbs, B.F.; Fasler-Kan, E.; Sumbayev, V.V. Functional role of galectin-9 in directing human innate immune reactions to Gram-negative bacteria and T cell apoptosis. Int. Immunopharmacol. 2021, 100, 108155. [Google Scholar] [CrossRef] [PubMed]
- Moar, P.; Tandon, R. Galectin-9 as a biomarker of disease severity. Cell. Immunol. 2021, 361, 104287. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Nakagawa, R.; Itoh, A.; Murakami, H.; Kashio, Y.; Abe, H.; Katoh, S.; Kontani, K.; Kihara, M.; Zhang, S.L.; et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J. Immunol. 2005, 175, 2974–2981. [Google Scholar] [CrossRef] [Green Version]
- Itoh, A.; Nonaka, Y.; Ogawa, T.; Nakamura, T.; Nishi, N. Galectin-9 induces atypical ubiquitination leading to cell death in PC-3 prostate cancer cells. Glycobiology 2019, 29, 22–35. [Google Scholar] [CrossRef]
- Morishita, A.; Nomura, K.; Tani, J.; Fujita, K.; Iwama, H.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Oura, K.; Chiyo, T.; et al. Galectin-9 suppresses the tumor growth of colon cancer in vitro and in vivo. Oncol. Rep. 2021, 45, 105. [Google Scholar] [CrossRef]
- Niki, T.; Fujita, K.; Rosen, H.; Hirashima, M.; Masaki, T.; Hattori, T.; Hoshino, K. Plasma Galectin-9 Concentrations in Normal and Diseased Condition. Cell Physiol. Biochem. 2018, 50, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, A.; Tsukada, J.; Mizobe, T.; Higashi, T.; Mouri, F.; Tanikawa, R.; Yamauchi, A.; Hirashima, M.; Tanaka, Y. Intracellular galectin-9 activates inflammatory cytokines in monocytes. Genes Cells. 2009, 14, 511–521. [Google Scholar] [CrossRef]
- Blair, B.B.; Funkhouser, A.T.; Goodwin, J.L.; Strigenz, A.M.; Chaballout, B.H.; Martin, J.C.; Arthur, C.M.; Funk, C.R.; Edenfield, W.J.; Blenda, A.V. Increased Circulating Levels of Galectin Proteins in Patients with Breast, Colon, and Lung Cancer. Cancers 2021, 13, 4819. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Q.; Zhang, Y.M.; Wu, L.Y.; Wu, D.; Chang, C.K. Research Progress in the Role of Tim3/galectin-9 in Hematological Malignancy--Review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 1360–1364. [Google Scholar] [PubMed]
- Merani, S.; Chen, W.; Elahi, S. The bitter side of sweet: The role of Galectin-9 in immunopathogenesis of viral infections. Rev. Med. Virol. 2015, 25, 175–186. [Google Scholar] [CrossRef]
- Si, Y.; Yao, Y.; Jaramillo Ayala, G.; Li, X.; Han, Q.; Zhang, W.; Xu, X.; Tai, G.; Mayo, K.H.; Zhou, Y.; et al. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129755. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.V.; André, S.; Gabius, H.J. The third dimension of reading the sugar code by lectins: Design of glycoclusters with cyclic scaffolds as tools with the aim to define correlations between spatial presentation and activity. Molecules 2013, 18, 4026–4053. [Google Scholar] [CrossRef] [Green Version]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin-Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Quintana, J.I.; Ardá, A.; Gimeno, A.; Jiménez-Barbero, J. Targeting Galectins With Glycomimetics. Front. Chem. 2020, 8, 593. [Google Scholar] [CrossRef]
- Freichel, T.; Heine, V.; Laaf, D.; Mackintosh, E.E.; Sarafova, S.; Elling, L.; Snyder, N.L.; Hartmann, L. Sequence-Defined Heteromultivalent Precision Glycomacromolecules Bearing Sulfonated/Sulfated Nonglycosidic Moieties Preferentially Bind Galectin-3 and Delay Wound Healing of a Galectin-3 Positive Tumor Cell Line in an In Vitro Wound Scratch Assay. Macromol. Biosci. 2020, 20, 2000163. [Google Scholar] [CrossRef]
- Heine, V.; Hovorková, M.; Vlachová, M.; Filipová, M.; Bumba, L.; Janoušková, O.; Hubálek, M.; Cvačka, J.; Petrásková, L.; Pelantová, H.; et al. Immunoprotective neo-glycoproteins: Chemoenzymatic synthesis of multivalent glycomimetics for inhibition of cancer-related galectin-3. Eur. J. Med. Chem. 2021, 220, 113500. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hartz, R.A.; Beno, B.R.; Ghosh, K.; Shukla, J.K.; Kumar, A.; Patel, D.; Kalidindi, N.; Lemos, N.; Gautam, S.S.; et al. Synthesis, Structure-Activity Relationships, and In Vivo Evaluation of Novel Tetrahydropyran-Based Thiodisaccharide Mimics as Galectin-3 Inhibitors. J. Med. Chem. 2021, 64, 6634–6655. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Sanam, S.; Alvala, R.; Alvala, M. An updated patent review of galectin-1 and galectin-3 inhibitors and their potential therapeutic applications (2016-present). Expert Opin. Ther. Pat. 2021, 31, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Capone, E.; Iacobelli, S.; Sala, G. Role of galectin 3 binding protein in cancer progression: A potential novel therapeutic target. J. Transl. Med. 2021, 19, 405. [Google Scholar] [CrossRef] [PubMed]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. The expression of Galectin-3 in endometrial cancer: A systematic review of the literature. Mol. Biol. Rep. 2021, 48, 5699–5705. [Google Scholar] [CrossRef]
- Boutas, I.; Potiris, A.; Makrakis, E.; Messaropoulos, P.; Papaioannou, G.K.; Kalantaridou, S.Ν. The expression of Galectin-3 in breast cancer and its association with metastatic disease: A systematic review of the literature. Mol. Biol. Rep. 2021, 48, 807–815. [Google Scholar] [CrossRef]
- Fujita, K.; Iwama, H.; Oura, K.; Tadokoro, T.; Samukawa, E.; Sakamoto, T.; Nomura, T.; Tani, J.; Yoneyama, H.; Morishita, A.; et al. Cancer Therapy Due to Apoptosis: Galectin-9. Int. J. Mol. Sci. 2017, 18, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, R.; Miyagaki, T.; Kamijo, H.; Oka, T.; Shishido-Takahashi, N.; Suga, H.; Sugaya, M.; Sato, S. Possible therapeutic applicability of galectin-9 in cutaneous T-cell lymphoma. J. Dermatol. Sci. 2019, 96, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, J.; Yamamoto, M.; Nagoshi, H.; Kobayashi, T.; Sasaki, N.; Shimura, Y.; Horiike, S.; Kimura, S.; Yamauchi, A.; Hirashima, M.; et al. Targeting activating transcription factor 3 by Galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol. Cancer Res. 2010, 8, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Chiyo, T.; Fujita, K.; Iwama, H.; Fujihara, S.; Tadokoro, T.; Ohura, K.; Matsui, T.; Goda, Y.; Kobayashi, N.; Nishiyama, N.; et al. Galectin-9 Induces Mitochondria-Mediated Apoptosis of Esophageal Cancer In Vitro and In Vivo in a Xenograft Mouse Model. Int. J. Mol. Sci. 2019, 20, 2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesmelova, I.V.; Ermakova, E.; Daragan, V.A.; Pang, M.; Menéndez, M.; Lagartera, L.; Solís, D.; Baum, L.G.; Mayo, K.H. Lactose binding to galectin-1 modulates structural dynamics, increases conformational entropy, and occurs with apparent negative cooperativity. J. Mol. Biol. 2010, 397, 1209–1230. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Feng, S.; Gao, J.; Wang, Y.; Zhang, Z.; Meng, Y.; Zhou, Y.; Tai, G.; Su, J. Human galectin-2 interacts with carbohydrates and peptides non-classically: New insight from X-ray crystallography and hemagglutination. Acta Biochim. Biophys. Sin. 2016, 48, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraboji, K.; Håkansson, M.; Genheden, S.; Diehl, C.; Qvist, J.; Weininger, U.; Nilsson, U.J.; Leffler, H.; Ryde, U.; Akke, M.; et al. The carbohydrate-binding site in galectin-3 is preorganized to recognize a sugarlike framework of oxygens: Ultra-high-resolution structures and water dynamics. Biochemistry 2012, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Bum-Erdene, K.; Leffler, H.; Nilsson, U.J.; Blanchard, H. Structural characterization of human galectin-4 C-terminal domain: Elucidating the molecular basis for recognition of glycosphingolipids, sulfated saccharides and blood group antigens. FEBS J. 2015, 282, 3348–3367. [Google Scholar] [CrossRef] [PubMed]
- Bum-Erdene, K.; Leffler, H.; Nilsson, U.J.; Blanchard, H. Structural characterisation of human galectin-4 N-terminal carbohydrate recognition domain in complex with glycerol, lactose, 3′-sulfo-lactose, and 2′-fucosyllactose. Sci. Rep. 2016, 6, 20289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.M.; Singh, T.; Wu, J.H.; Lensch, M.; André, S.; Gabius, H.J. Interaction profile of galectin-5 with free saccharides and mammalian glycoproteins: Probing its fine specificity and the effect of naturally clustered ligand presentation. Glycobiology 2006, 16, 524–537. [Google Scholar] [CrossRef] [Green Version]
- Barrès, C.; Blanc, L.; Bette-Bobillo, P.; André, S.; Mamoun, R.; Gabius, H.J.; Vidal, M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 2010, 115, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Gitt, M.A.; Colnot, C.; Poirier, F.; Nani, K.J.; Barondes, S.H.; Leffler, H. Galectin-4 and galectin-6 are two closely related lectins expressed in mouse gastrointestinal tract. J. Biol. Chem. 1998, 273, 2954–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houzelstein, D.; Reyes-Gomez, E.; Maurer, M.; Netter, P.; Higuet, D. Expression patterns suggest that despite considerable functional redundancy, galectin-4 and -6 play distinct roles in normal and damaged mouse digestive tract. J. Histochem. Cytochem. 2013, 61, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, E.; Miller, M.C.; Nesmelova, I.V.; López-Merino, L.; Berbís, M.A.; Nesmelov, Y.; Tkachev, Y.V.; Lagartera, L.; Daragan, V.A.; André, S.; et al. Lactose binding to human galectin-7 (p53-induced gene 1) induces long-range effects through the protein resulting in increased dimer stability and evidence for positive cooperativity. Glycobiology 2013, 23, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Ideo, H.; Matsuzaka, T.; Nonaka, T.; Seko, A.; Yamashita, K. Galectin-8-N-domain recognition mechanism for sialylated and sulfated glycans. J. Biol. Chem. 2011, 286, 11346–11355. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Song, C.; Si, Y.; Cui, L.; Yang, T.; Li, Y.; Wang, H.; Tai, G.; Zhou, Y. Identification of key amino acid residues determining ligand binding specificity, homodimerization and cellular distribution of human galectin-10. Glycobiology 2019, 29, 85–93. [Google Scholar] [CrossRef]
- Sakthivel, D.; Littler, D.; Shahine, A.; Troy, S.; Johnson, M.; Rossjohn, J.; Piedrafita, D.; Beddoe, T. Cloning, expression, purification and crystallographic studies of galectin-11 from domestic sheep (Ovis aries). Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 993–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.Y.; Hsu, D.K.; Yu, L.; Ni, J.; Liu, F.T. Cell cycle regulation by galectin-12, a new member of the galectin superfamily. J. Biol. Chem. 2001, 276, 20252–20260. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Wang, Y.; Si, Y.; Gao, J.; Song, C.; Cui, L.; Wu, R.; Tai, G.; Zhou, Y. Galectin-13, a different prototype galectin, does not bind β-galacto-sides and forms dimers via intermolecular disulfide bridges between Cys-136 and Cys-138. Sci. Rep. 2018, 8, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Cui, L.; Si, Y.; Song, C.; Li, Y.; Yang, T.; Wang, H.; Mayo, K.H.; Tai, G.; Zhou, Y. Resetting the ligand binding site of placental protein 13/galectin-13 recovers its ability to bind lactose. Biosci. Rep. 2018, 38, BSR20181787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.; Li, Y.; Yang, T.; Li, X.; Ayala, G.J.; Mayo, K.H.; Tai, G.; Su, J.; Zhou, Y. Structure-function studies of galectin-14, an important effector molecule in embryology. FEBS J. 2021, 288, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.A.; Adelson, D.L.; Bazer, F.W.; Burghardt, R.C.; Meeusen, E.N.; Spencer, T.E. Discovery and characterization of an epithelial-specific galectin in the endometrium that forms crystals in the trophectoderm. Proc. Natl. Acad. Sci USA. 2004, 101, 7982–7987. [Google Scholar] [CrossRef] [Green Version]
- Sehrawat, S.; Reddy, P.B.; Rajasagi, N.; Suryawanshi, A.; Hirashima, M.; Rouse, B.T. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010, 6, e1000882. [Google Scholar] [CrossRef]
- De Kivit, S.; Kraneveld, A.D.; Knippels, L.M.; van Kooyk, Y.; Garssen, J.; Willemsen, L.E. Intestinal epithelium-derived galectin-9 is involved in the immunomodulating effects of nondigestible oligosaccharides. J. Innate Immun. 2013, 5, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Fang, Q. Effect of Galectin-9/Tim-3 pathway on the polarization of M1/M2 subtype in murine macrophages induced by lipopolysaccharide. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018, 30, 836–841. [Google Scholar] [PubMed]
- Zhang, W.; Zhang, Y.; He, Y.; Wang, X.; Fang, Q. Lipopolysaccharide mediates time-dependent macrophage M1/M2 polarization through the Tim-3/Galectin-9 signalling pathway. Exp. Cell Res. 2019, 376, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, H.; Yu, L.; Liu, M.; Zuo, Z.; Han, Q.; Zhang, J.; Tian, Z.; Zhang, C. Intestinal Lamina Propria CD4+ T Cells Promote Bactericidal Activity of Macrophages via Galectin-9 and Tim-3 Interaction during Salmonella enterica Serovar Typhimurium Infection. Infect. Immun. 2018, 86, e00769-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraman, P.; Sada-Ovalle, I.; Beladi, S.; Anderson, A.C.; Dardalhon, V.; Hotta, C.; Kuchroo, V.K.; Behar, S.M. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 2010, 207, 2343–2354. [Google Scholar] [CrossRef]
- Qin, A.; Zhong, T.; Zou, H.; Wan, X.; Yao, B.; Zheng, X.; Yin, D. Critical role of Tim-3 mediated autophagy in chronic stress induced immunosuppression. Cell. Biosci. 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, S.; Su, X.Z.; Song, J.; Lu, F. Blockage of Galectin-receptor Interactions by alpha-lactose Exacerbates Plasmodium berghei-induced Pulmonary Immunopathology. Sci Rep. 2016, 6, 32024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, N.; Zhang, T.; Chen, R.; Feng, Y.; Sang, X.; Yang, N.; Chen, Q. Tim-3 signaling blockade with alpha-lactose induces compensatory TIGIT expression in Plasmodium berghei ANKA-infected mice. Parasit. Vectors 2019, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sundararajan, A.; Suryawanshi, A.; Kumar, N.; Veiga-Parga, T.; Kuchroo, V.K.; Thomas, P.G.; Sangster, M.Y.; Rouse, B.T. T cell immunoglobulin and mucin protein-3 (Tim-3)/Galectin-9 interaction regulates influenza A virus-specific humoral and CD8 T-cell responses. Proc. Natl. Acad. Sci. USA. 2011, 108, 19001–19006. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.J.; Rogers, M.C.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Multiple Inhibitory Pathways Contribute to Lung CD8+ T Cell Impairment and Protect against Immunopathology during Acute Viral Respiratory Infection. J. Immunol. 2016, 197, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Deng, H.; Li, P.; Zhang, J.; Zhang, J.; Wang, D.; Li, S.; Luo, Y.; Wei, Z.; Bi, G.; et al. alpha-Lactose Improves the Survival of Septic Mice by Blockade of TIM-3 Signaling to Prevent NKT Cell Apoptosis and Attenuate Cytokine Storm. Shock 2017, 47, 337–345. [Google Scholar] [CrossRef]
- Wei, Z.; Li, P.; Yao, Y.; Deng, H.; Yi, S.; Zhang, C.; Wu, H.; Xie, X.; Xia, M.; He, R.; et al. Alpha-lactose reverses liver injury via blockade of Tim-3-mediated CD8 apoptosis in sepsis. Clin. Immunol. 2018, 192, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Yu, D.; Hu, Y.; Jin, W.; Qin, Y.; Kong, D.; Wang, H.; Li, G.; Alessandrini, A.; et al. Galectin-9 is required for endometrial regenerative cells to induce long-term cardiac allograft survival in mice. Stem Cell Res. Ther. 2020, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shiratori, B.; Chagan-Yasutan, H.; Matsumoto, M.; Niki, T.; Tanaka, M.; Takahashi, Y.; Usami, O.; Ashino, Y.; Hattori, T. Secretion of IFN-gamma Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis. Int. J. Mol. Sci. 2017, 18, 1382. [Google Scholar] [CrossRef] [Green Version]
- Tanino, Y.; Hashimoto, T.; Ojima, T.; Mizuno, M. F-fucoidan from Saccharina japonica is a novel inducer of galectin-9 and exhibits anti-allergic activity. J. Clin. Biochem. Nutr. 2016, 59, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paasela, M.; Kolho, K.L.; Vaarala, O.; Honkanen, J. Lactose inhibits regulatory T-cell-mediated suppression of effector T-cell interferon-gamma and IL-17 production. Br. J. Nutr. 2014, 112, 1819–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, K.; Kukita, A.; Li, Y.J.; Uehara, N.; Zhang, J.Q.; Takahashi, I.; Kukita, T. Regulation of osteoclastogenesis through Tim-3: Possible involvement of the Tim-3/galectin-9 system in the modulation of inflammatory bone destruction. Lab. Invest. 2014, 94, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zheng, M.H.; Qu, Y.; Li, J.F.; Lu, A.G.; Li, J.W.; Wang, M.L.; Liu, B.Y. Galectin-9 isoforms influence the adhesion between colon carcinoma LoVo cells and human umbilical vein endothelial cells in vitro by regulating the expression of E-selectin in LoVo cells. Zhonghua Zhong Liu Za Zhi 2009, 31, 95–98. [Google Scholar]
- Lhuillier, C.; Barjon, C.; Baloche, V.; Niki, T.; Gelin, A.; Mustapha, R.; Claër, L.; Hoos, S.; Chiba, Y.; Ueno, M.; et al. Characterization of neutralizing antibodies reacting with the 213-224 amino-acid segment of human galectin-9. PLoS ONE 2018, 13, e0202512. [Google Scholar] [CrossRef]
- Lhuillier, C.; Barjon, C.; Niki, T.; Gelin, A.; Praz, F.; Morales, O.; Souquere, S.; Hirashima, M.; Wei, M.; Dellis, O.; et al. Impact of Exogenous Galectin-9 on Human T Cells: Contribution of the T cell receptor complex to antigen-independent activation but not to apoptosis induction. J. Biol. Chem. 2015, 290, 16797–16811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleason, M.K.; Lenvik, T.R.; McCullar, V.; Felices, M.; O’Brien, M.S.; Cooley, S.A.; Verneris, M.R.; Cichocki, F.; Holman, C.J.; Panoskaltsis-Mortari, A.; et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–3072. [Google Scholar] [CrossRef]
- Fu, X.; Cebo, M.; Ikegami, T.; Lämmerhofer, M. Separation of carbohydrate isomers and anomers on poly-N-(1H-tetrazole-5-yl)-methacrylamide-bonded stationary phase by hydrophilic interaction chromatography as well as determination of anomer interconversion energy barriers. J. Chromatogr. A. 2020, 1620, 460981. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, E.; Zhang, Z.; Zhao, G.; Cao, H. Association between Tim-3 and Gal-9 expression and gastric cancer prognosis. Oncol. Rep. 2018, 40, 2115–2126. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhu, C.; Cao, H.; Zhang, Z.; Zhao, E. The Association Between Immune Characteristic and Clinical Pathology in Chinese Patients with Adenocarcinoma of Esophagogastric Junction. Cancer Manag. Res. 2020, 12, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.; Conaway, M.R.; Chinn, Z.; Duska, L.; Stoler, M.; Mills, A.M. Looking past PD-L1: Expression of immune checkpoint TIM-3 and its ligand galectin-9 in cervical and vulvar squamous neoplasia. Mod. Pathol. 2020, 33, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Taghiloo, S.; Allahmoradi, E.; Ebadi, R.; Tehrani, M.; Hosseini-Khah, Z.; Janbabaei, G.; Shekarriz, R.; Asgarian-Omran, H. Upregulation of Galectin-9 and PD-L1 Immune Checkpoints Molecules in Patients with Chronic Lymphocytic Leukemia. Asian Pac. J. Cancer Prev. 2017, 18, 2269–2274. [Google Scholar] [PubMed]
- Saleh, R.; Toor, S.M.; Khalaf, S.; Elkord, E. Breast Cancer Cells and PD-1/PD-L1 Blockade Upregulate the Expression of PD-1, CTLA-4, TIM-3 and LAG-3 Immune Checkpoints in CD4+ T Cells. Vaccines 2019, 7, 149. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Toor, S.M.; Al-Ali, D.; Sasidharan Nair, V.; Elkord, E. Blockade of PD-1, PD-L1, and TIM-3 Altered Distinct Immune- and Cancer-Related Signaling Pathways in the Transcriptome of Human Breast Cancer Explants. Genes 2020, 11, 703. [Google Scholar] [CrossRef]
- Kikushige, Y. TIM-3 in normal and malignant hematopoiesis: Structure, function, and signaling pathways. Cancer Sci. 2021, 112, 3419–3426. [Google Scholar] [CrossRef]
- Lima, C.D.L.; Coelho, H.; Gimeno, A.; Trovão, F.; Diniz, A.; Dias, J.S.; Jiménez-Barbero, J.; Corzana, F.; Carvalho, A.L.; Cabrita, E.J.; et al. Structural Insights into the Molecular Recognition Mechanism of the Cancer and Pathogenic Epitope, LacdiNAc by Immune-Related Lectins. Chemistry 2021, 27, 7951–7958. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, C.; Zhang, L.; Wang, S.; Ma, M.; Zhao, J.; Song, Z.; Wang, F.; Qu, X.; Li, F.; et al. Development of M10, myricetin-3-O-beta-d-lactose sodium salt, a derivative of myricetin as a potent agent of anti-chronic colonic inflammation. Eur. J. Med. Chem. 2019, 174, 9–15. [Google Scholar] [CrossRef]
- Wang, F.; Song, Z.Y.; Qu, X.J.; Li, F.; Zhang, L.; Li, W.B.; Cui, S.X. M10, a novel derivative of Myricetin, prevents ulcerative colitis and colorectal tumor through attenuating robust endoplasmic reticulum stress. Carcinogenesis 2018, 39, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Yang, J.; Qu, X.J.; Meng, J.; Miao, R.R.; Cui, S.X. M10, a Myricetin-3-O-b-D-Lactose Sodium Salt, Prevents Ulcerative Colitis Through Inhibiting Necroptosis in Mice. Front. Pharmacol. 2020, 11, 557312. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.R.; Zhan, S.; Hu, X.T.; Yuan, W.M.; Wu, L.J.; Cui, S.X.; Qu, X.J. Myricetin and M10, a myricetin-3-O-beta-d-lactose sodium salt, modify composition of gut microbiota in mice with ulcerative colitis. Toxicol. Lett. 2021, 346, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Li, N.; Li, M.; Ma, S.; Tan, S.; Guo, X.; Wang, Z.; Wu, Z.; Gao, L.; et al. Spatial distribution and functional analysis define the action pathway of Tim-3/Tim-3 ligands in tumor development. Mol. Ther. 2021. [Google Scholar] [CrossRef]



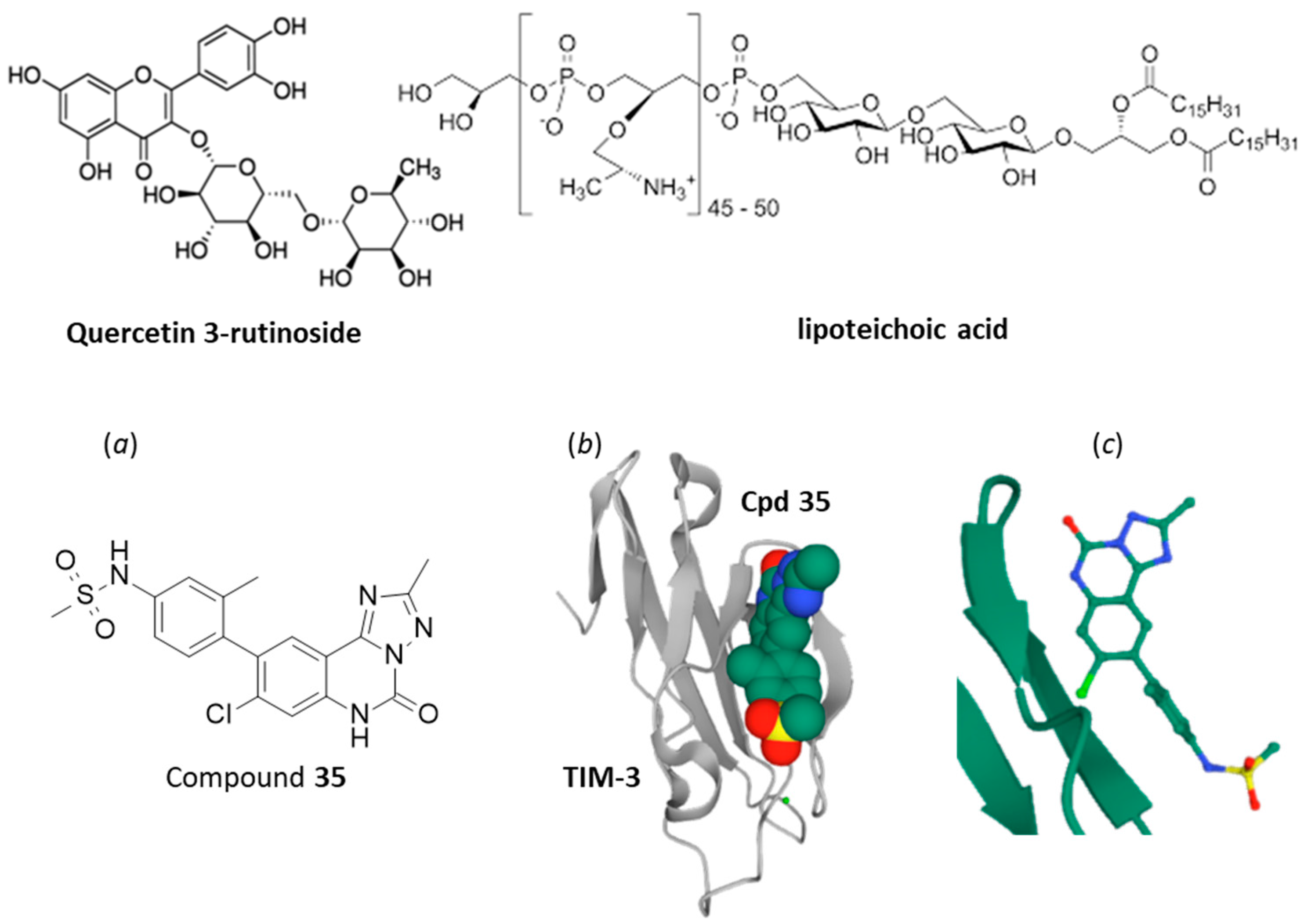


| Anti-TIM-3 Antibodies | Current Development | References |
|---|---|---|
| LY3321367 (Eli Lilly; Indianapolis, IN, USA) | Phase 1 trial in patients with relapsed/refractory solid tumor, alone or in combination with an anti-PD-L1 mAb. NCT03099109 * | [66,72] |
| Sabatolimab (MBG453, Novartis, Basel, Switzerland) | Fast track designation by US FDA for the treatment of patients with myelodysplastic syndromes (MDS) | [73] |
| Phase 1 trial in patients with advanced solid tumor, alone or in combination with anti-PD-1 spartalizumab. | [63] | |
| Phase 1 trial in patients with Acute Myeloid Leukemia (AML). NCT04812548, NCT04623216, NCT04878432, NCT04150029 * | [74] | |
| BMS-986258 (ONO 7807, BMS, Lawrenceville, NJ, USA) | Fully human anti-TIM-3 mAb, tested in combination with anti-PD-1 nivolumab in patients with advanced solid tumors (Phase 1). NCT03446040 * | |
| Cobolimab (TSR-022, GSK, Brentford, UK) | Anti-TIM-3 mAb in combination with anti-PD-1 in patients with liver cancer or with a melanoma (Phase 1). NCT04655976, NCT03680508, NCT04139902, NCT02817633 * | |
| Sym023 (Symphogen; Ballerup, Denmark) | Fully human anti-TIM-3 mAb, in patients with advanced solid tumor malignancies or lymphomas (Phase 1). NCT03489343 * | |
| INCAGN02390 (Incyte, Agenus; Wilmington, DE, USA) | Phase 1 study to determine the safety, tolerability, and preliminary efficacy in participants with advanced malignancies. Fully human Fc-engineered IgG1k. NCT03652077, NCT04370704. | [75] |
| RO7121661 (Roche; Basel, Switzerland) | Anti-PD-1/TIM-3 bispecific mAbs, tested in patients with advanced and/or metastatic solid tumors (Phase 1). NCT03708328 * | |
| BGB-A425 (BeiGene Ltd., Beijing, China) | Humanized anti-TIM-3 mAb, in combination with anti-PD-1 mAb tislelizumab, in patients with advanced solid tumors (phase I/II trial). NCT03744468 * | [76] |
| M6903 | Fully human anti-TIM-3 mAb, without effector function, which blocks binding of TIM-3 to the 3 ligands phosphatidylserine, CEACAM1, and Gal-9. Experimental laboratories studies. | [65] |
| F38.2E2 | Anti-human TIM-3 antibody capable of blocking binding of TIM-3 to phosphatidylserine and CEACAM1. Experimental tool. | [77] |
| Galectins | Lactose Binding | +/− | References |
|---|---|---|---|
| Gal-1 | Lactose binds the two carbohydrate recognition domains of the Gal-1 dimer. | + | [143] |
| Gal-2 | Lactose binds to only one of the carbohydrate recognition domain subunits of the Gal-2 dimer structure. | + | [144] |
| Gal-3 | Crystal structure of the carbohydrate recognition domain of Gal-3 in complex with lactose. | + | [145,146] |
| Gal-4 | Analysis of lactose and derivatives binding to C-terminal carbohydrate recognition domain of human Gal-4. | + | [147,148] |
| Gal-5 | A little-studied galectin (apparently specific for rat). Lactose binding described. | + | [149,150] |
| Gal-6 | Lactose binding not reported but very likely considering the strong homology with Gal-4. | ? | [151,152] |
| Gal-7 | Lactose binding induces stabilization of the Gal-7 dimer. | + | [153] |
| Gal-8 | Binding of lactose to human galectin-8-N-domain | + | [154] |
| Gal-9 | Structure of murine Gal-9 n-ter CRD bound to lactose. | + | [107] |
| Gal-10 | Gal-10 forms a novel dimeric structure and binds lactose. | + | [155] |
| Gal-11 | Gal-11 is only expressed in ruminants. Binding to lactose suggested. | + | [156] |
| Gal-12 | Binding of lactose to Gal-12 | + | [157] |
| Gal-13 | Wild-type Gal-13 and its variant R53H do not bind lactose. Engineering of variant R53H can lead to lactose binding. | − | [158,159] |
| Gal-14 | Lactose does not interact with this lectin, or very weakly. | − | [160] |
| Gal-15 | Binding of lactose to Gal-15 | + | [161] |
| Gal-16 | Gal-16 exists as a monomer and lacks the ability to bind lactose. | − | [128] |
| Cell System or Animal Model | Effect of Gal-9 | References |
|---|---|---|
| Endometrial regenerative cells (ERC) | ERC express Gal-9 and play a major role in immune modulation. Lactose blocks Gal-9 immunomodulatory effect in ERC, and thereby modulate the proliferative rate of stimulated CD4+ T and CD8+ T cells, cocultured with ERC. | [175] |
| Mice infected with the malaria pathogen Plasmodium berghei | Blockade of Tim-3/Gal-9 with α-lactose induces a compensatory expression of the immunosuppressive molecule TIGIT. | [170] |
| Bone marrow derived macrophages (BMDM). | Downregulation of Gal-9 and TIM-3 protein expression and soluble Gal-9 secretion in LPS-induced BMDM. | [165] |
| Prostate cancer cells (PC-3) | Addition of lactose induces solubilization of membrane-bound Gal-9. | [121] |
| Salmonella enterica intestinal infection mouse model | Blocking Tim-3/Gal-9 interaction with α-lactose attenuates the bactericidal activity of intracellular S. typhimurium by macrophages. | [166] |
| Pleural fluid cells (PFC) | Gal-9 stimulates interferon-γ synthesis in PFC and lactose inhibits this effect. | [176] |
| Intestinal epithelial cells (IEC) and mouse model. | Lactose binding to Gal-9 inhibits the anti-allergy properties of the sulfated polysaccharide F-fucoidan from Saccharina japonica. | [177] |
| Co-cultures of human peripheral blood mononuclear cell (PBMC)-derived Treg and effector T cells (Teff). | Lactose inhibits the down-regulation induced by Treg of the secretion of IFN-γ and IL-17 in PBMC-Teff co-cultures. Lactose inhibits human Treg-mediated suppression of Th1 and Th17 immune responses. | [178] |
| Intestinal epithelial cells (IEC) | Neutralization of Gal-9 with lactose prevents the induction of IFN-γ secretion and suppresses the production of IL-10 by PBMC. | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C.; Thuru, X.; Quesnel, B. Modulation of the Gal-9/TIM-3 Immune Checkpoint with α-Lactose. Does Anomery of Lactose Matter? Cancers 2021, 13, 6365. https://doi.org/10.3390/cancers13246365
Bailly C, Thuru X, Quesnel B. Modulation of the Gal-9/TIM-3 Immune Checkpoint with α-Lactose. Does Anomery of Lactose Matter? Cancers. 2021; 13(24):6365. https://doi.org/10.3390/cancers13246365
Chicago/Turabian StyleBailly, Christian, Xavier Thuru, and Bruno Quesnel. 2021. "Modulation of the Gal-9/TIM-3 Immune Checkpoint with α-Lactose. Does Anomery of Lactose Matter?" Cancers 13, no. 24: 6365. https://doi.org/10.3390/cancers13246365
APA StyleBailly, C., Thuru, X., & Quesnel, B. (2021). Modulation of the Gal-9/TIM-3 Immune Checkpoint with α-Lactose. Does Anomery of Lactose Matter? Cancers, 13(24), 6365. https://doi.org/10.3390/cancers13246365








