Circulating Exosomal Integrin β3 Is Associated with Intracranial Failure and Survival in Lung Cancer Patients Receiving Cranial Irradiation for Brain Metastases: A Prospective Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
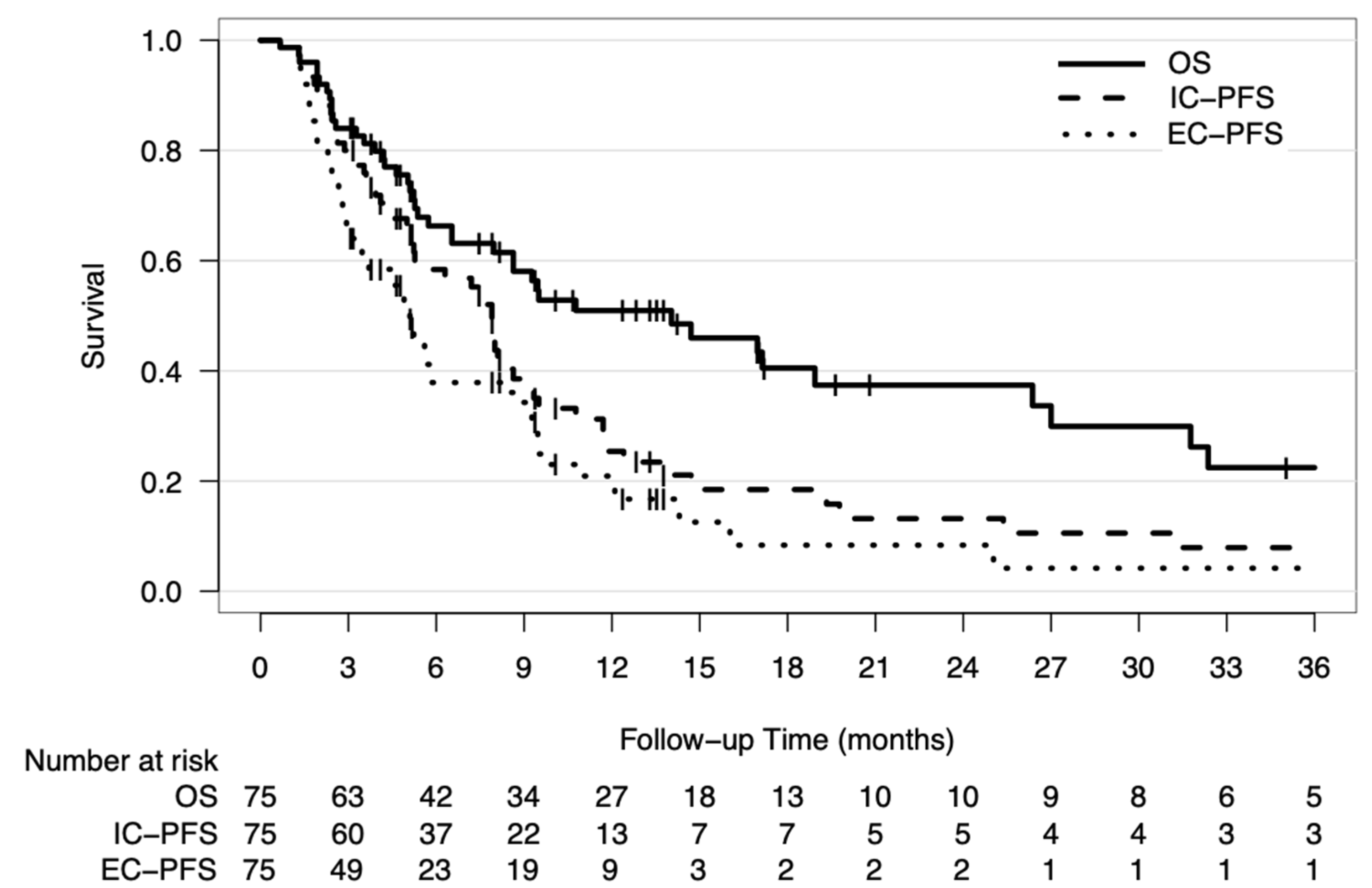
2.1. Patient Characteristics and Outcomes
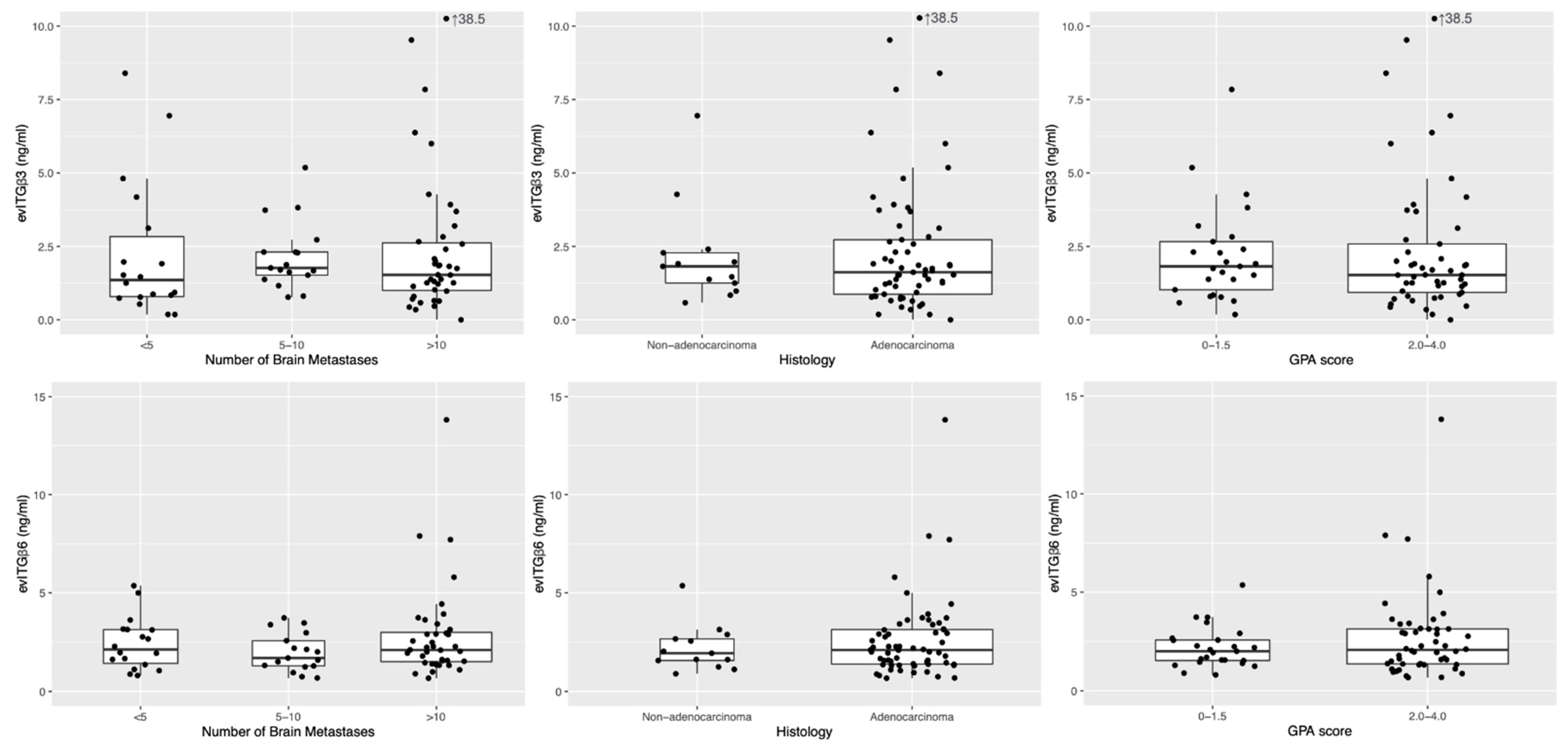
2.2. Expression of Circulating Extracellular Vesicles Integrins
2.3. Expression of Circulating EV Integrins Independently Predicted Outcomes for BM
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5, 5. [Google Scholar] [CrossRef]
- Eichler, A.F.; Chung, E.; Kodack, D.P.; Loeffler, J.S.; Fukumura, D.; Jain, R.K. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 2011, 8, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro-Oncology 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.B.; Hansen, H.H.; Hansen, M.; Dombernowsky, P. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J. Clin. Oncol. 1988, 6, 1474–1480. [Google Scholar] [CrossRef]
- Peters, S.; Bexelius, C.; Munk, V.; Leighl, N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat. Rev. 2016, 45, 139–162. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. 1997, 37, 745–751. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Sharma, M.; Venur, V.A.; Schmitt, P.; Kotecha, R.; Chao, S.T.; Suh, J.H.; Angelov, L.; Mohammadi, A.M.; Vogelbaum, M.A.; et al. Impact of EGFR mutation and ALK rearrangement on the outcomes of non-small cell lung cancer patients with brain metastasis. Neuro-Oncology 2020, 22, 267–277. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients with Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G.; Aznavoorian, S.; Liotta, L.A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 1993, 9, 541–573. [Google Scholar] [CrossRef] [PubMed]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Malanchi, I.; Santamaria-Martinez, A.; Susanto, E.; Peng, H.; Lehr, H.A.; Delaloye, J.F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011, 481, 85–89. [Google Scholar] [CrossRef]
- Oskarsson, T.; Acharyya, S.; Zhang, X.H.; Vanharanta, S.; Tavazoie, S.F.; Morris, P.G.; Downey, R.J.; Manova-Todorova, K.; Brogi, E.; Massague, J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011, 17, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.A.; Goodman, S.L.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro-Oncology 2013, 15, 1664–1672. [Google Scholar] [CrossRef]
- Vogetseder, A.; Thies, S.; Ingold, B.; Roth, P.; Weller, M.; Schraml, P.; Goodman, S.L.; Moch, H. alphav-Integrin isoform expression in primary human tumors and brain metastases. Int. J. Cancer 2013, 133, 2362–2371. [Google Scholar] [CrossRef]
- Lorger, M.; Krueger, J.S.; O’Neal, M.; Staflin, K.; Felding-Habermann, B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc. Natl. Acad. Sci. USA 2009, 106, 10666–10671. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Kovanda, A.K.; Melchardt, T.; Bartsch, R.; Hainfellner, J.A.; Sipos, B.; Schittenhelm, J.; Zielinski, C.C.; Widhalm, G.; Dieckmann, K.; et al. alphavbeta3, alphavbeta5 and alphavbeta6 integrins in brain metastases of lung cancer. Clin. Exp. Metastasis 2014, 31, 841–851. [Google Scholar] [CrossRef]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Singh, A.; Zerlanko, B.J.; Iozzo, R.V.; Languino, L.R. The alphavbeta6 integrin is transferred intercellularly via exosomes. J. Biol. Chem. 2015, 290, 4545–4551. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.T.; Keen, J.H.; Languino, L.R. Exosome-mediated Transfer of alphavbeta3 Integrin from Tumorigenic to Nontumorigenic Cells Promotes a Migratory Phenotype. Mol. Cancer Res. 2016, 14, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Krishn, S.R.; Singh, A.; Bowler, N.; Duffy, A.N.; Friedman, A.; Fedele, C.; Kurtoglu, S.; Tripathi, S.K.; Wang, K.; Hawkins, A.; et al. Prostate cancer sheds the alphavbeta3 integrin in vivo through exosomes. Matrix Biol. 2019, 77, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Boger, C.; Kalthoff, H.; Goodman, S.L.; Behrens, H.M.; Rocken, C. Integrins and their ligands are expressed in non-small cell lung cancer but not correlated with parameters of disease progression. Virchows Arch. 2014, 464, 69–78. [Google Scholar] [CrossRef]
- Wu, Y.J.; Pagel, M.A.; Muldoon, L.L.; Fu, R.; Neuwelt, E.A. High alphav Integrin Level of Cancer Cells Is Associated with Development of Brain Metastasis in Athymic Rats. Anticancer Res. 2017, 37, 4029–4040. [Google Scholar] [CrossRef]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Rodrigues, G.; Hoshino, A.; Kenific, C.M.; Matei, I.R.; Steiner, L.; Freitas, D.; Kim, H.S.; Oxley, P.R.; Scandariato, I.; Casanova-Salas, I.; et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat. Cell Biol. 2019, 21, 1403–1412. [Google Scholar] [CrossRef]
- Silva, J.; Garcia, V.; Zaballos, A.; Provencio, M.; Lombardia, L.; Almonacid, L.; Garcia, J.M.; Dominguez, G.; Pena, C.; Diaz, R.; et al. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur. Respir. J. 2011, 37, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Hamar, P.; Guo, C.; Basar, E.; Perdigao-Henriques, R.; Balaj, L.; Lieberman, J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Investig. 2014, 124, 5109–5128. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, Z. The roles of integrin alphavbeta6 in cancer. Cancer Lett. 2017, 403, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef]
- Aoyama, H.; Tago, M.; Shirato, H.; Japanese Radiation Oncology Study Group Investigators. Stereotactic Radiosurgery with or without Whole-Brain Radiotherapy for Brain Metastases: Secondary Analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015, 1, 457–464. [Google Scholar] [CrossRef]
- Farris, M.; McTyre, E.; Hughes, R.T.; Ayala-Peacock, D.N.; Randolph, D.M.; Bourland, J.D.; Tatter, S.B.; Laxton, A.W.; Watabe, K.; Ruiz, J.; et al. Brain Metastasis Velocity: A Novel Prognostic Metric Predictive of Overall Survival and Freedom from Whole-Brain Radiation Therapy After Upfront Radiosurgery Alone for Brain Metastases. Int. J. Radiat. Oncol. 2016, 96, S180. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Grubbs, F.E. Sample Criteria for Testing Outlying Observations. Ann. Math. Stat. 1950, 21, 27–58. [Google Scholar] [CrossRef]
- Lausen, B.; Schumacher, M. Maximally Selected Rank Statistics. Biometrics 1992, 48, 73. [Google Scholar] [CrossRef]
- Gray, R.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]


| Characteristics | Number | % |
|---|---|---|
| Number of Patients | 75 | 100% |
| Median age, years (range) | 61 (38–81) | |
| Gender | ||
| Male | 40 | 53.3% |
| Female | 35 | 46.7% |
| Prior SRS | 6 | 8.0% |
| Number of brain metastases | ||
| <5 | 18 | 24.0% |
| 5–10 | 17 | 22.7% |
| >10 | 40 | 53.3% |
| Leptomeningeal seeding | 45 | 60.0% |
| Histology | ||
| Adenocarcinoma | 61 | 81.3% |
| Squamous cell carcinoma | 4 | 5.3% |
| Non-small cell carcinoma, NOS | 5 | 6.7% |
| Small cell carcinoma | 4 | 5.3% |
| Adenosquamous | 1 | 1.3% |
| GPA | ||
| 0–1.0 | 12 | 16.0% |
| 1.5–2.0 | 33 | 44.0% |
| 2.5–3.0 | 27 | 36.0% |
| 3.5–4.0 | 3 | 4.0% |
| Median follow-up, months (range) | 8.0 (0.7–52.1) | |
| Death | ||
| Yes | 44 | 58.7% |
| No | 31 | 41.3% |
| Intracranial progression after WBRT | ||
| Yes | 37 | 49.3% |
| No | 31 | 41.3% |
| Unknown | 7 | 9.3% |
| Salvage treatment | ||
| Surgery + SRS | 1 | 1.3% |
| SRS | 3 | 4.0% |
| SRT | 2 | 2.7% |
| TKI | 2 | 2.7% |
| WBRT + Bev | 3 | 4.0% |
| Extracranial progression | ||
| Yes | 45 | 60.0% |
| No | 23 | 30.7% |
| Unknown | 7 | 9.3% |
| Prognostic Factors | HR | 95% CI | p-Value |
|---|---|---|---|
| Age (per 1-year increase) | 1.04 | 1.00–1.08 | 0.04 * |
| Sex (female vs. male) | 1.22 | 0.55–2.72 | 0.62 |
| Histology (adenocarcinoma vs. non-adenocarcinoma) | 0.38 | 0.18–0.83 | 0.02 * |
| GPA score (high vs. low) | 0.21 | 0.09–0.45 | <0.001 * |
| Integrin β3 (per 1-ng/mL increase) | 1.25 | 1.04–1.49 | 0.02 * |
| Integrin β6 (per 1-ng/mL increase) | 0.90 | 0.68–1.18 | 0.44 |
| Intracranial Failure | sHR | 95% CI | p-Value |
|---|---|---|---|
| Age (per 1-year increase) | 1.021 | 0.969–1.07 | 0.44 |
| Sex (female vs. male) | 0.817 | 0.438–1.52 | 0.52 |
| Histology (adenocarcinoma vs. non-adenocarcinoma) | 1.235 | 0.463–3.30 | 0.67 |
| GPA score (high vs. low) | 2.059 | 0.823–5.15 | 0.12 |
| Integrin β3 (per 1-ng/mL increase) | 1.216 | 1.012–1.46 | 0.037 * |
| Integrin β6 (per 1-ng/mL increase) | 0.868 | 0.679–1.11 | 0.26 |
| Intracranial failure (using cut-off points for integrin levels) | |||
| Age (per 1-year increase) | 1.033 | 0.983–1.09 | 0.2 |
| Sex (female vs. male) | 0.833 | 0.436–1.59 | 0.58 |
| Histology (adenocarcinoma vs. non-adenocarcinoma) | 1.274 | 0.417–3.89 | 0.67 |
| GPA score (high vs. low) | 1.939 | 0.758–4.96 | 0.17 |
| High integrin β3 (>1.818 ng/mL vs. ≤1.818 ng/mL) | 2.147 | 1.106–4.17 | 0.024 * |
| High integrin β6 (>1.99 ng/mL vs. ≤1.99 ng/mL) | 0.796 | 0.421–1.51 | 0.48 |
| Extracranial failure | |||
| Age (per 1-year increase) | 1.003 | 0.968–1.04 | 0.88 |
| Sex (female vs. male) | 0.813 | 0.437–1.51 | 0.51 |
| Histology (adenocarcinoma vs. non-adenocarcinoma) | 2.013 | 0.664–6.10 | 0.22 |
| GPA score (high vs. low) | 0.619 | 0.319–1.20 | 0.16 |
| Integrin β3 (per 1-ng/mL increase) | 1.052 | 0.830–1.33 | 0.68 |
| Integrin β6 (per 1-ng/mL increase) | 0.974 | 0.707–1.34 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-Y.; Cheng, J.C.-H.; Chen, Y.-F.; Yang, J.C.-H.; Hsu, F.-M. Circulating Exosomal Integrin β3 Is Associated with Intracranial Failure and Survival in Lung Cancer Patients Receiving Cranial Irradiation for Brain Metastases: A Prospective Observational Study. Cancers 2021, 13, 380. https://doi.org/10.3390/cancers13030380
Chen G-Y, Cheng JC-H, Chen Y-F, Yang JC-H, Hsu F-M. Circulating Exosomal Integrin β3 Is Associated with Intracranial Failure and Survival in Lung Cancer Patients Receiving Cranial Irradiation for Brain Metastases: A Prospective Observational Study. Cancers. 2021; 13(3):380. https://doi.org/10.3390/cancers13030380
Chicago/Turabian StyleChen, Guann-Yiing, Jason Chia-Hsien Cheng, Ya-Fang Chen, James Chih-Hsin Yang, and Feng-Ming Hsu. 2021. "Circulating Exosomal Integrin β3 Is Associated with Intracranial Failure and Survival in Lung Cancer Patients Receiving Cranial Irradiation for Brain Metastases: A Prospective Observational Study" Cancers 13, no. 3: 380. https://doi.org/10.3390/cancers13030380
APA StyleChen, G.-Y., Cheng, J. C.-H., Chen, Y.-F., Yang, J. C.-H., & Hsu, F.-M. (2021). Circulating Exosomal Integrin β3 Is Associated with Intracranial Failure and Survival in Lung Cancer Patients Receiving Cranial Irradiation for Brain Metastases: A Prospective Observational Study. Cancers, 13(3), 380. https://doi.org/10.3390/cancers13030380





