Tumor-Infiltrating CD20+ B Lymphocytes: Significance and Prognostic Implications in Oral Cancer Microenvironment
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
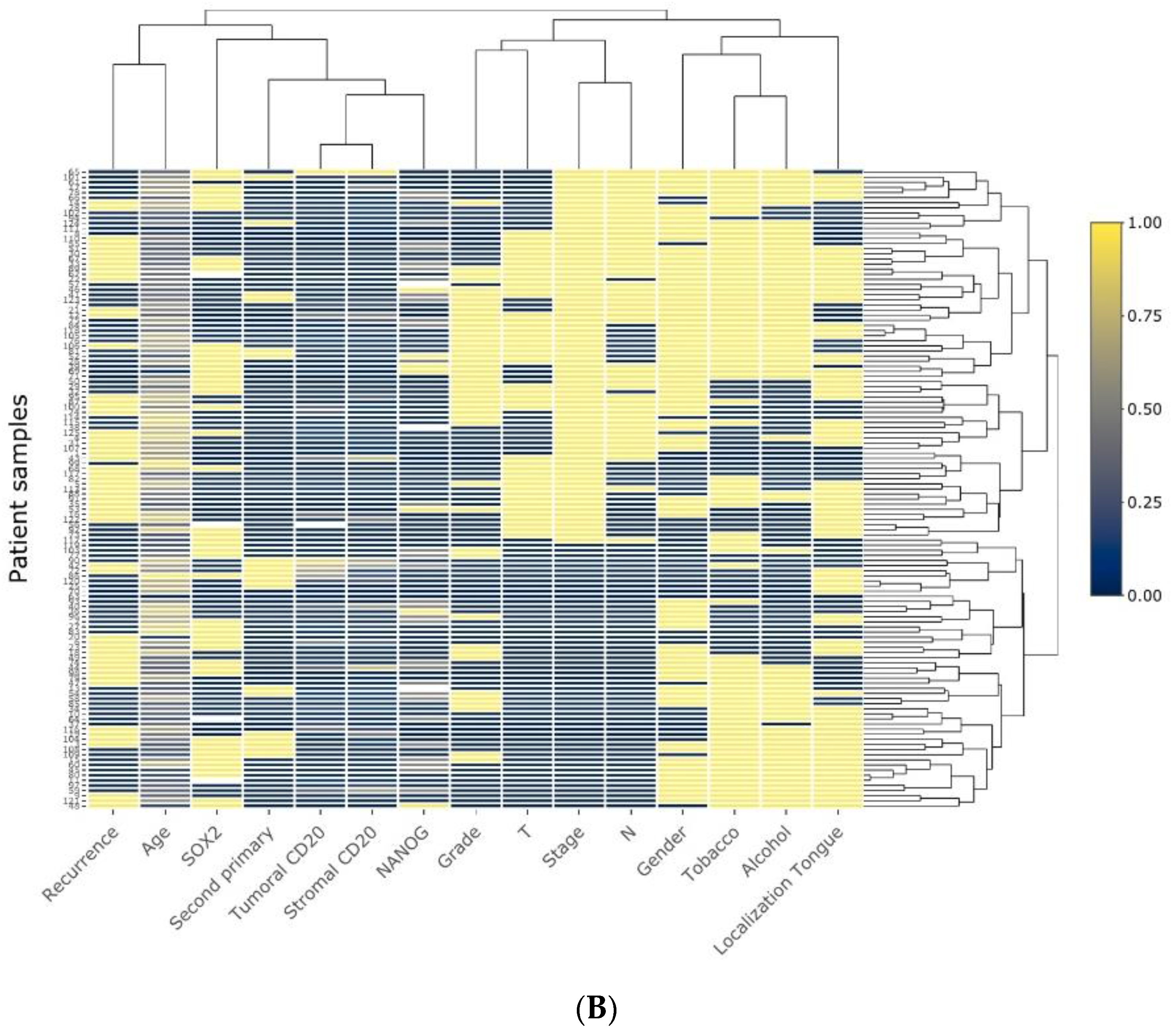
2.1. Immunohistochemical Analysis of CD20+ TILs in OSCC Tissue Specimens and Associations with Other Immune Subtypes
2.2. Associations between CD20+ TILs and Clinicopathological Variables
2.3. Associations between CD20+ TILs, CSC Markers, and PD-L1
2.4. Impact of CD20+ TIL Infiltration on the Survival of OSCC Patients
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Specimens
4.2. Immunohistochemistry (IHC)
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 3, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, C.Y. Targeting cancer stem cells in squamous cell carcinoma. Precis Clin. Med. 2019, 2, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Böhm, D.; von Törne, C.; Steiner, E.; Puhl, A.; Pilch, H.; Lehr, H.A.; Hengstler, J.G.; Kölbl, H.; Gehrmann, M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008, 68, 5405–5413. [Google Scholar] [CrossRef]
- Hussein, M.R.; Elsers, D.A.H.; Fadel, S.A.; Omar, A.E.M. Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J. Clin. Pathol. 2006, 59, 316–324. [Google Scholar] [CrossRef]
- Evrard, D.; Szturz, P.; Tijeras-Raballand, A.; Astorgues-Xerri, L.; Abitbol, C.; Paradis, V.; Raymond, E.; Albert, S.; Barry, B.; Faivre, S. Macrophages in the microenvironment of head and neck cancer: Potential targets for cancer therapy. Oral Oncol. 2019, 88, 29–38. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Wirsing, A.M. Tissue-infiltrating immune cells as prognostic markers in oral squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer 2019, 120, 714–727. [Google Scholar] [CrossRef]
- Wang, T.; Niu, G.; Kortylewski, M.; Burdelya, L.; Shain, K.; Zhang, S.; Bhattacharya, R.; Gabrilovich, D.; Heller, R.; Coppola, D.; et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 2004, 10, 48–54. [Google Scholar] [CrossRef]
- da Silveira, E.J.; da Costa Miguel, M.C.; Lima, K.C.; de Almeida Freitas, R.; Queiroz, L.M.G. Analysis of local immunity in squamous cell carcinoma of the tongue and lower lip. Exp. Mol. Pathol. 2010, 88, 171–175. [Google Scholar] [CrossRef]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. Immunohistochemical analysis of Foxp3+, CD4+, CD8+ cell infiltrates and PD-L1 in oral squamous cell carcinoma. Pathol. Oncol. Res. 2018, 24, 497–505. [Google Scholar] [CrossRef]
- Wirsing, A.M.; Ervik, I.K.; Seppola, M.; Uhlin-Hansen, L.; Steigen, S.E.; Hadler-Olsen, E. Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma. Mod. Pathol. 2018, 31, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E. Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Ho, W.J.; Mohan, A.; Shah, Y.; Vithayathil, T.; Leatherman, J.; Dennison, L.; Zaidi, N.; Ganguly, S.; Woolman, S.; et al. Effects of B cell-activating factor on tumor immunity. JCI Insight 2020, 5, e136417. [Google Scholar] [CrossRef]
- Disis, M.L. Immune regulation of cancer. J. Clin. Oncol. 2010, 28, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Yang, J.M.; Kim, H.; Chung, J.H.; Ahn, S.H.; Jeong, W.J.; Paik, J.H. Clinicopathologic implications of the miR-197/PD-L1 axis in oral squamous cell carcinoma. Oncotarget 2017, 8, 66178–66194. [Google Scholar] [CrossRef]
- Mizoguchi, A.M.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19 (+) CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef]
- Morrison, B.J.; Steel, J.C.; Morris, J.C. Reduction of MHC-I expression limits T-lymphocyte-mediated killing of cancer initiating cells. BMC Cancer 2018, 18, 469. [Google Scholar] [CrossRef]
- Baniebrahimi, G.; Mir, F.; Khanmohammadi, R. Cancer stem cells and oral cancer: Insights into molecular mechanisms and therapeutic approaches. Cancer Cell Int. 2020, 20, 113. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Alikhani, M.; Yazdanian, A.; Yazdanian, M.; Tebyanian, H.; Seifalian, A. The current markers of cancer stem cell in oral cancers. Life Sci. 2020, 249, 117483. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.J.; Divi, V.; Owen, J.H.; Bradford, C.R.; Carey, T.E.; Papagerakis, S.; Prince, M.E.P. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Major, A.G.; Pitty, L.P.; Farah, C.S. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013, 2013, 319489. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Allonca, E.; Vallina, A.; Singhania, A.; Donate-Pérez Del Molino, P.; García-Pedrero, J.M. The emerging role of NANOG as an early cancer risk biomarker in patients with oral potentially malignant disorders. J. Clin. Med. 2019, 8, 1376. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Donate-Pérez Del Molino, P.; Rodrigo, J.P.; Allonca, E.; Hermida-Prado, F.; Granda-Díaz, R.; Rodríguez Santamarta, T.; García-Pedrero, J.M. SOX2 Expression is an independent predictor of oral cancer progression. J. Clin. Med. 2019, 8, 1744. [Google Scholar] [CrossRef]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef]
- Tsai, L.L.; Yu, C.C.; Chang, Y.C.; Yu, C.H.; Chou, M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med. 2011, 40, 621–628. [Google Scholar] [CrossRef]
- Ren, Z.H.; Zhang, C.P.; Ji, T. Expression of SOX2 in oral squamous cell carcinoma and the association with lymph node metastasis. Oncol. Lett. 2016, 11, 1973–1979. [Google Scholar] [CrossRef]
- Miao, Y.; Yang, Y.; Levorse, J.; Yuan, S.; Polak, L.; Sribour, M.; Singh, B.; Rosenblum, M.D.; Fuchs, E. Adaptive immune resistance emerges from tumor-initiating stem cells. Cell 2019, 177, 1172–1186.e1114. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Takahashi, G.; Sakakura, K.; Ferrone, S.; Masuyama, K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck 2011, 33, 208–215. [Google Scholar] [CrossRef]
- Piersma, S.J.; Jordanova, E.S.; van Poelgeest, M.I.; Kitty, M.C.; Kwappenberg, J.M.; van der Hulst, M.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G.; Fleuren, G.J.; et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res 2007, 67, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, C.; Li, Q.; Dong, J.; Liu, Y.; Huang, Y.; Jiang, T.; Wu, A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br. J. Cancer 2014, 110, 2560–2568. [Google Scholar] [CrossRef]
- Hladíková, K.; Koucký, V.; Bouček, J.; Laco, J.; Grega, M.; Hodek, M.; Zábrodský, M.; Vošmik, M.; Rozkošová, K.; Vošmiková, H.; et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J. Immunother. Cancer 2019, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Blanco-Lorenzo, V.; Allonca, E.; García-Pedrero, J.M. PD-L1 expression in tumor cells is an independent unfavorable prognostic factor in oral squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Pretscher, D.; Distel, L.V.; Grabenbauer, G.G.; Wittlinger, M.; Buettner, M.; Niedobitek, G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 2009, 9, 292. [Google Scholar] [CrossRef]
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirström, K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J. Ovarian Res. 2016, 9, 21. [Google Scholar] [CrossRef]
- Distel, L.V.; Fickenscher, R.; Dietel, K.; Hung, A.; Iro, H.; Zenk, J.; Nkenke, E.; Büttner, M.; Niedobitek, G.; Grabenbauer, G.G. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: Prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009, 45, e167–e174. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L., Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012, 72, 1070–1080. [Google Scholar] [CrossRef]
- Garg, K.; Maurer, M.; Griss, J.; Brüggen, M.C.; Wolf, I.H.; Wagner, C.; Willi, N.; Mertz, K.D.; Wagner, S.N. Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome. Hum. Pathol. 2016, 54, 157–164. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, M.; Thompson, A.K.; Monteagudo, C. A significant percentage of CD20-positive TILs correlates with poor prognosis in patients with primary cutaneous malignant melanoma. Histopathology 2014, 65, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, N.; Mohsenifar, Z.; Baghban, A.A.; Arjomandkhah, A. CD20+ Tumor Infiltrating B Lymphocyte in Oral Squamous Cell Carcinoma: Correlation with Clinicopathologic Characteristics and Heat Shock Protein 70 Expression. Pathol. Res. Int. 2018, 2018, 4810751. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.M.; Liang, Y.J.; Su, Y.X.; Zhang, S.E.; Zhou, X.I.; Liao, G.Q. Distribution and significance of interstitial fibrosis and stroma-infiltrating B cells in tongue squamous cell carcinoma. Oncol. Lett. 2016, 11, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ tumor-infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Li, Q.; Grover, A.C.; Donald, E.J.; Carr, A.; Yu, J.; Whitfield, J.; Nelson, M.; Takeshita, N.; Chang, A.E.J. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. J. Immunol. 2005, 175, 1424–1432. [Google Scholar] [CrossRef]
- Zhang, Y.; Morgan, R.; Chen, C.; Cai, Y.; Clark, E.; Khan, W.; Shin, S.; Cho, H.; Bayati, A.A.; Pimentel, A.; et al. Mammary-tumor-educated B cells acquire LAP/TGF-β and PD-L1 expression and suppress anti-tumor immune responses. Int. Immunol. 2016, 28, 423–433. [Google Scholar] [CrossRef]
- Schrama, D.; thor Straten, P.; Fischer, W.H.; McLellan, A.D.; Bröcker, E.B.; Reisfeld, R.A.; Becker, J.C. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001, 14, 111–121. [Google Scholar] [CrossRef]
- Pimenta, E.M.; Barnes, B.J. Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: Potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers 2014, 6, 969–997. [Google Scholar] [CrossRef]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Siliņa, K.; Rulle, U.; Kalniņa, Z.; Linē, A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: A novel anti-cancer treatment avenue? Cancer Immunol. Immunother. 2014, 63, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Ito, M.; Ohmura, H.; Hanamura, F.; Nakano, M.; Tsuchihashi, K.; Nagai, S.; Ariyama, H.; Kusaba, H.; Yamamoto, H.; et al. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal cancer. Oncoimmunology 2020, 9, 1724763. [Google Scholar] [CrossRef] [PubMed]
- Wirsing, A.M.; Rikardsen, O.G.; Steigen, S.E.; Uhlin-Hansen, L.; Hadler-Olsen, E. Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC Clin. Pathol. 2014, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Colbeck, E.J.; Ager, A.; Gallimore, A.; Jones, G.W. Tertiary lymphoid structures in cancer: Drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front. Immunol. 2017, 9, 1830. [Google Scholar] [CrossRef]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef]
- Li, K.; Guo, Q.; Zhang, X.; Dong, X.; Liu, W.; Zhang, A.; Li, Y.; Yan, J.; Jia, G.; Zheng, Z.; et al. Oral cancer-associated tertiary lymphoid structures: Gene expression profile and prognostic value. Clin. Exp. Immunol. 2020, 199, 172–181. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.C.; Goc, J.; Giraldo, N.A.; Sautès-Fridman, C.; Fridman, W.H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014, 35, 571–580. [Google Scholar] [CrossRef]
- Sakimura, C.; Tanaka, H.; Okuno, T.; Hiramatsu, S.; Muguruma, K.; Hirakawa, K.; Wanibuchi, H.; Ohira, M. B cells in tertiary lymphoid structures are associated with favorable prognosis in gastric cancer. J. Surg. Res. 2017, 215, 74–82. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, W.; Ly, D.; Xu, H.; Qu, L.; Zhang, L. Tumor-infiltrating B cells: Their role and application in anti-tumor immunity in lung cancer. Cell Mol. Immunol. 2019, 16, 6–18. [Google Scholar] [CrossRef]
- Tobón, G.J.; Izquierdo, J.H.; Cañas, C.A. B lymphocytes: Development, tolerance, and their role in autoimmunity-focus on systemic lupus erythematosus. Autoimmune Dis. 2013, 827254. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 controls follicular T helper cell positioning and function. Immunity 2018, 49, 264–274.e4. [Google Scholar] [CrossRef] [PubMed]
- Mehla, K.; Singh, P.K. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer 2019, 5, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.S.; Roy, A.; Rajpoot, S.; Liu, D.; Savai, R.; Banerjee, S.; Kawada, M.; Faisal, S.M.; Saluja, R.; Saqib, U.; et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 2020, 69, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Suárez-Canto, J.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Macrophages in oral carcinomas: Relationship with cancer stem cell markers and PD-L1 expression. Cancers 2020, 12, 1764. [Google Scholar] [CrossRef]
- Ravindran, S.; Rasool, S.; Maccalli, C. The cross talk between cancer stem cells/cancer initiating cells and tumor microenvironment: The missing piece of the puzzle for the efficient targeting of these cells with immunotherapy. Cancer Microenviron. 2019, 12, 133–148. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; Ridge, J.A.; O’Sullivan, B.; Shah, J.P. Staging head and neck cancers. In AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing AG: New York, NY, USA, 2017; pp. 55–181. [Google Scholar]



| Variable | Stromal CD20+ (Mean) | Tumoral CD20+ (Mean) |
|---|---|---|
| Stromal CD8+ (mean) | 0.772 <0.001 | 0.624 <0.001 |
| Tumoral CD8+ (mean) | 0.404 <0.001 | 0.358 <0.001 |
| Stromal CD4+ (mean) | 0.662 <0.001 | 0.488 <0.001 |
| Tumoral CD4+ (mean) | 0.353 <0.001 | 0.362 <0.001 |
| Stromal FOXP3+ (mean) | 0.326 <0.001 | 0.328 <0.001 |
| Tumoral FOXP3+ (mean) | 0.253 0.005 | 0.246 0.006 |
| Stromal CD68+ (mean) | 0.354 <0.001 | 0.377 <0.001 |
| Tumoral CD68+ (mean) | 0.259 0.004 | 0.261 0.004 |
| Stromal CD163+ (mean) | 0.418 <0.001 | 0.373 <0.001 |
| Tumoral CD163+ (mean) | 0.190 0.03 | 0.228 0.01 |
| Variable | Number | Stromal CD20+ Mean (SD) | p | Tumoral CD20+ Mean (SD) | p |
|---|---|---|---|---|---|
| Age (years) | |||||
| <65 | 77 | 35.06 (74.34) | 0.35 | 1.26 (2.90) | 0.14 |
| ≥65 | 48 | 54.36 (84.50) | 2.21 (3.92) | ||
| Gender | |||||
| Female | 43 | 46.61 (82.46) | 0.81 | 2.42 (4.31) | 0.27 |
| Male | 82 | 40.30 (76.97) | 1.22 (2.66) | ||
| Tobacco consumption | |||||
| No | 41 | 48.43 (77.72) | 0.25 | 2.40 (3.99) | 0.06 |
| Yes | 84 | 39.56 (79.37) | 1.26 (2.95) | ||
| Alcohol consumption | |||||
| No | 56 | 42.01 (74.87) | 0.90 | 2.14 (3.88) | 0.09 |
| Yes | 69 | 42.84 (82.09) | 1.11 (2.74) | ||
| pT classification | |||||
| T1 + T2 | 81 | 48.91 (83.98) | 0.02 | 1.95 (3.76) | 0.10 |
| T3 + T4 | 44 | 30.62 (66.97) | 1.02 (2.29) | ||
| pN classification | |||||
| N0 | 76 | 41.03 (74.05) | 0.63 | 1.73 (3.42) | 0.70 |
| N+ | 49 | 44.70 (86.00) | 1.47 (3.26) | ||
| Stage | |||||
| I + II | 52 | 51.18 (83.82) | 0.31 | 2.22 (3.90) | 0.15 |
| III + IV | 73 | 36.26 (74.68) | 1.20 (2.84) | 0.15 | |
| Grade | |||||
| Well | 80 | 52.93 (91.88) | 0.06 | 1.99 (3.94) | 0.31 |
| Moderate + Poor | 45 | 23.88 (41.41) | 1.00 (1.78) | ||
| Site | |||||
| Tongue | 51 | 57.11 (103.48) | 0.37 | 2.09 (4.26) | 0.74 |
| Other | 74 | 32.38 (53.99) | 1.30 (2.51) | ||
| Recurrence | |||||
| No | 71 | 39.68 (76.77) | 0.36 | 1.44 (3.32) | 0.90 |
| Yes | 54 | 46.14 (81.59) | 1.87 (3.39) | ||
| Second primary tumor | |||||
| No | 106 | 37.61 (76.86) | 0.02 | 1.30 (2.71) | 0.24 |
| Yes | 19 | 69.56 (84.97) | 3.45 (5.46) |
| Variable Mean (SD) | Stromal CD20/CD8 Ratio | p | Tumoral CD20/CD8 Ratio | p | Stromal CD20/CD4 Ratio | p | Tumoral CD20/CD4 Ratio | p | Stromal CD20/FOXP3 Ratio | p | Tumoral CD20/FOXP3 Ratio | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||||||
| <65 | 0.50 (3.06) | 0.50 | 0.04 (0.10) | 0.35 | 0.71 (1.10) | 0.88 | 1.10 (3.97) | 0.92 | 3.50 (8.34) | 0.01 | 0.40 (0.75) | 0.01 |
| ≥65 | 0.20 (0.24) | 0.21 (0.60) | 0.78 (1.18) | 0.73 (2.25) | 16.38 (42.95) | 1.99 (4.30) | ||||||
| Gender | ||||||||||||
| Female | 0.16 (0.18) | 0.88 | 0.08 (0.23) | 0.46 | 0.66 (1.07) | 0.61 | 0.69 (2.30) | 0.91 | 14.03 (45.37) | 0.76 | 2.05 (4.57) | 0.55 |
| Male | 0.50 (2.97) | 0.13 (0.47) | 0.78 (1.16) | 1.08 (3.79) | 5.88 (12.30) | 0.46 (0.68) | ||||||
| Tobacco | ||||||||||||
| No | 0.17 (0.16) | 0.47 | 0.07 (0.23) | 0.50 | 0.69 (0.96) | 0.68 | 0.93 (2.51) | 0.57 | 17.25 (45.85) | 0.16 | 1.62 (4.12) | 0.20 |
| Yes | 0.48 (2.93) | 0.13 (0.46) | 0.76 (1.20) | 0.94 (3.70) | 4.34 (11.08) | 0.68 (1.72) | ||||||
| Alcohol | ||||||||||||
| No | 0.15 (0.16) | 0.81 | 0.12 (0.44) | 0.32 | 0.62 (1.00) | 0.50 | 0.65 (2.06) | 0.92 | 12.71 (39.44) | 0.46 | 1.50 (3.64) | 0.06 |
| Yes | 0.57 (3.23) | 0.10 (0.36) | 0.83 (1.21) | 1.22 (4.19) | 5.16 (12.28) | 0.60 (1.80) | ||||||
| pT | ||||||||||||
| T1 + T2 | 0.52 (2.98) | 0.03 | 0.15 (0.48) | 0.07 | 0.86 (1.26) | 0.13 | 0.87 (2.27) | 0.15 | 7.60 (15.79) | 0.21 | 1.25 (3.27) | 0.75 |
| T3 + T4 | 0.12 (0.15) | 0.03 (0.09) | 0.51 (0.79) | 1.08 (4.78) | 11.02 (46.03) | 0.40 (0.65) | ||||||
| pN | ||||||||||||
| N0 | 0.51 (3.08) | 0.47 | 0.07 (0.29) | 0.41 | 0.66 (1.02) | 0.53 | 0.62 (1.88) | 0.69 | 9.75 (34.76) | 0.29 | 1.25 (3.42) | 0.82 |
| N+ | 0.18 (0.23) | 0.17 (0.52) | 0.85 (1.28) | 1.42 (4.70) | 7.10 (14.57) | 0.56 (0.86) | ||||||
| Stage | ||||||||||||
| I + II | 0.70 (3.72) | 0.35 | 0.11 (0.35) | 0.93 | 0.76 (1.15) | 0.77 | 0.78 (2.23) | 0.14 | 6.86 (14.90) | 0.94 | 1.61 (3.93) | 0.64 |
| III + IV | 0.15 (0.20) | 0.11 (0.43) | 0.72 (1.11) | 1.04 (3.86) | 10.15 (35.83) | 0.46 (0.75) | ||||||
| Grade | ||||||||||||
| Well | 0.52 (3.00) | 0.29 | 0.08 (0.28) | 0.22 | 0.78 (1.14) | 0.19 | 0.73 (1.97) | 0.19 | 11.36 (34.96) | 0.09 | 1.35 (3.54) | 0.69 |
| Moderate + Poor | 0.14 (0.17) | 0.16 (0.55) | 0.67 (1.10) | 1.27 (4.72) | 4.20 (10.36) | 0.47 (0.56) | ||||||
| Site | ||||||||||||
| Tongue | 0.71 (3.76) | 0.23 | 0.08 (0.28) | 0.64 | 0.73 (1.17) | 0.74 | 1.11 (4.39) | 0.33 | 4.68 (10.99) | 0.93 | 0.94 (2.21) | 0.29 |
| Other | 0.15 (0.20) | 0.13 (0.47) | 0.75 (1.10) | 0.79 (2.07) | 11.38 (35.62) | 1.04 (3.15) | ||||||
| Recurrence | ||||||||||||
| No | 0.16 (0.22) | 0.46 | 0.14 (0.49) | 0.91 | 0.74 (1.15) | 0.64 | 0.53 (1.60) | 0.90 | 6.69 (16.84) | 0.01 | 0.66 (1.86) | 0.37 |
| Yes | 0.67 (3.65) | 0.07 (0.21) | 0.73 (1.10) | 1.43 (4.58) | 11.59 (39.79) | 1.56 (3.83) | ||||||
| Second primary tumor | ||||||||||||
| No | 0.40 (2.61) | 0.01 | 0.12 (0.43) | 0.38 | 0.66 (1.08) | 0.02 | 0.99 (3.58) | 0.23 | 7.88 (29.81) | 0.10 | 0.88 (2.65) | 0.57 |
| Yes | 0.26 (0.21) | 0.07 (0.13) | 1.16 (1.28) | 0.68 (1.01) | 12.87 (22.13) | 1.58 (3.42) |
| Factor | Stromal CD20+ (Mean, SD) | p | Tumoral CD20+ (Mean, SD) | p |
|---|---|---|---|---|
| SOX2 | ||||
| Negative (N = 72, 60%) | 48.47 (77.74) | 0.29 | 1.86 (3.38) | 0.008 |
| Positive (N = 49, 40%) | 36.23 (82.77) | 1.31 (3.39) | ||
| NANOG | ||||
| Negative (N = 83, 68%) | 44.10 (77.05) | 0.14 | 1.86 (3.67) | 0.36 |
| Positive (N = 39, 32%) | 40.23 (85.20) | 1.23 (2.65) | ||
| PD-L1 | ||||
| ≤10% (N = 104, 83%) | 43.96 (79.55) | 0.85 | 1.46 (3.30) | 0.21 |
| >10% (N = 18, 15%) | 39.27 (80.81) | 2.70 (3.67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Rodrigo, J.P.; Hermida-Prado, F.; Suárez-Canto, J.; Rodríguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Tumor-Infiltrating CD20+ B Lymphocytes: Significance and Prognostic Implications in Oral Cancer Microenvironment. Cancers 2021, 13, 395. https://doi.org/10.3390/cancers13030395
Suárez-Sánchez FJ, Lequerica-Fernández P, Rodrigo JP, Hermida-Prado F, Suárez-Canto J, Rodríguez-Santamarta T, Domínguez-Iglesias F, García-Pedrero JM, de Vicente JC. Tumor-Infiltrating CD20+ B Lymphocytes: Significance and Prognostic Implications in Oral Cancer Microenvironment. Cancers. 2021; 13(3):395. https://doi.org/10.3390/cancers13030395
Chicago/Turabian StyleSuárez-Sánchez, Faustino Julián, Paloma Lequerica-Fernández, Juan Pablo Rodrigo, Francisco Hermida-Prado, Julián Suárez-Canto, Tania Rodríguez-Santamarta, Francisco Domínguez-Iglesias, Juana M. García-Pedrero, and Juan Carlos de Vicente. 2021. "Tumor-Infiltrating CD20+ B Lymphocytes: Significance and Prognostic Implications in Oral Cancer Microenvironment" Cancers 13, no. 3: 395. https://doi.org/10.3390/cancers13030395
APA StyleSuárez-Sánchez, F. J., Lequerica-Fernández, P., Rodrigo, J. P., Hermida-Prado, F., Suárez-Canto, J., Rodríguez-Santamarta, T., Domínguez-Iglesias, F., García-Pedrero, J. M., & de Vicente, J. C. (2021). Tumor-Infiltrating CD20+ B Lymphocytes: Significance and Prognostic Implications in Oral Cancer Microenvironment. Cancers, 13(3), 395. https://doi.org/10.3390/cancers13030395






