Prognostic Value of Metabolic Imaging Data of 11C-choline PET/CT in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
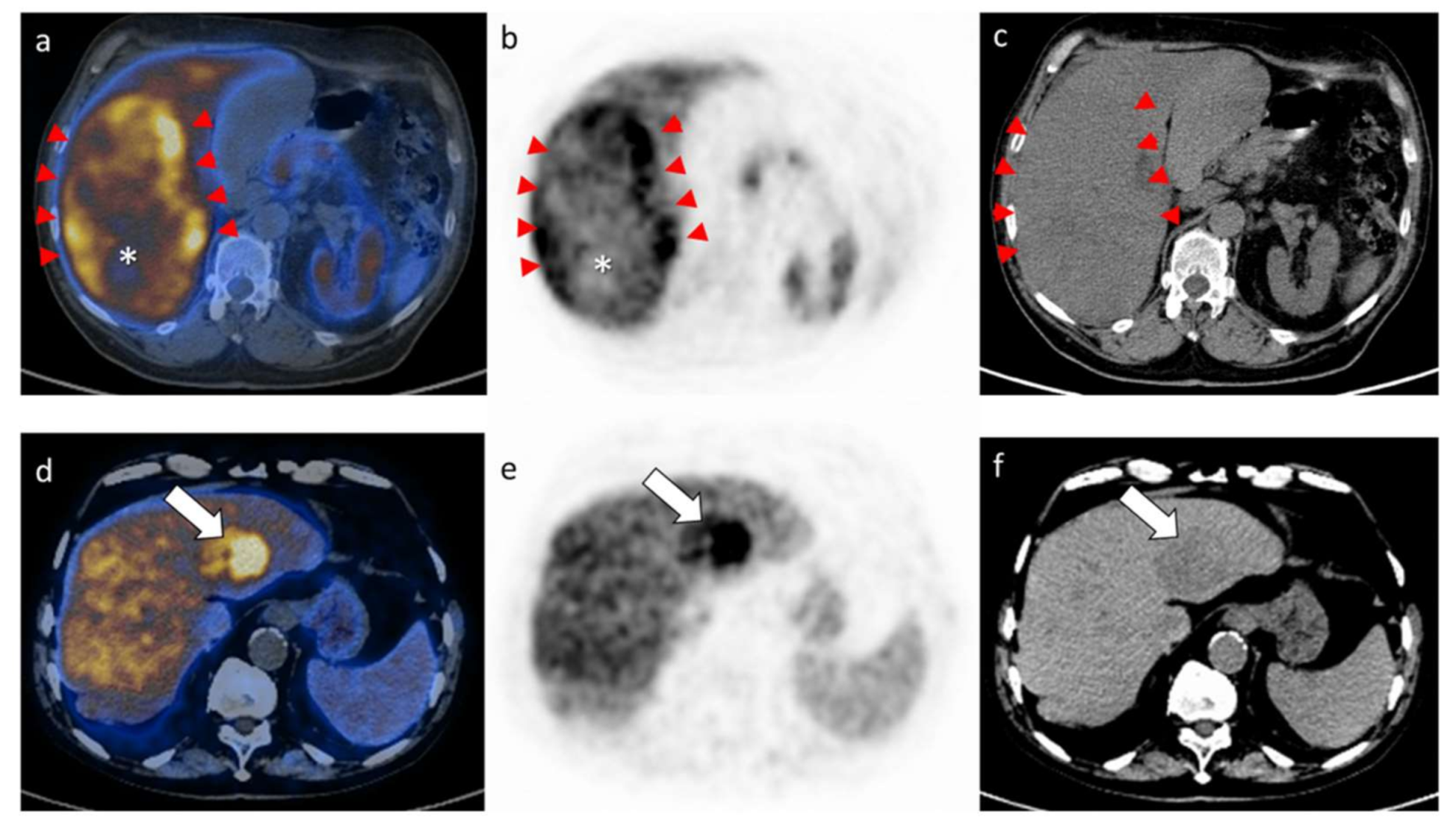
2.2. Results of the PET/CT Parameters
2.3. Results of the Survival Analysis
2.4. Association between MTV and Survival
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Collection
4.2. Study Endpoint
4.3. Predictors of Survival
4.4. Study Selection Criteria
4.5. Preoperative Workup and Selection Criteria for Hepatectomy
4.6. 11C-choline PET/CT
4.7. Follow-Up
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Erridge, S.; Pucher, P.H.; Markar, S.R.; Malietzis, G.; Athanasiou, T.; Darzi, A.; Sodergren, M.H.; Jiao, L.R. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West study group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Ferrero, A.; Nuzzo, G.; Vauthey, J.N.; Choti, M.A.; De Santibanes, E.; Makuuchi, M. Predicting Individual Survival After Hepatectomy for Hepatocellular Carcinoma: A Novel Nomogram from the “HCC East & West Study Group”. J. Gastrointest. Surg. 2016, 20, 1154–1162. [Google Scholar] [PubMed]
- Beyer, T.; Townsend, D.W.; Brun, T.; Kinahan, P.E.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 2000, 41, 1369–1379. [Google Scholar]
- Delbeke, D.; Martin, W.H.; Sandler, M.P.; Chapman, W.C.; Wright, J.K.; Pinson, C.W. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch. Surg. 1998, 133, 510–515. [Google Scholar] [CrossRef]
- Verhoef, C.; Valkema, R.; de Man, R.A.; Krenning, E.P.; Yzermans, J.N. Fluorine-18 FDG imaging in hepatocellular carcinoma using positron coincidence detection and single photon emission computed tomography. Liver 2002, 22, 51–56. [Google Scholar] [CrossRef]
- Khan, M.A.; Combs, C.S.; Brunt, E.M.; Lowe, V.J.; Wolverson, M.K.; Solomon, H.; Collins, B.T.; Di Bisceglie, A.M. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J. Hepatol. 2000, 32, 792–797. [Google Scholar] [CrossRef]
- Trojan, J.; Schroeder, O.; Raedle, J.; Baum, R.P.; Herrmann, G.; Jacobi, V.; Zeuzem, S. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am. J. Gastroenterol. 1999, 94, 3314–3319. [Google Scholar] [CrossRef]
- Talbot, J.N.; Gutman, F.; Fartoux, L.; Grange, J.D.; Ganne, N.; Kerrou, K.; Grahek, D.; Montravers, F.; Poupon, R.; Rosmorduc, O. PET/CT in patients with hepatocellular carcinoma using PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.N.; Fartoux, L.; Balogova, S.; Nataf, V.; Kerrou, K.; Gutman, F.; Huchet, V.; Ancel, D.; Grange, J.D.; Rosmorduc, O. Detection of hepatocellular carcinoma with PET/CT: A prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J. Nucl. Med. 2010, 51, 1699–1706. [Google Scholar] [CrossRef]
- Wu, H.B.; Wang, Q.S.; Li, B.Y.; Li, H.S.; Zhou, W.I.; Wang, Q.Y. F-18 FDG in conjunction with 11C-choline PET/CT in the diagnosis of hepatocellular carcinoma. Clin. Nucl. Med. 2011, 36, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Kuang, Y.; Wang, F.; Maclennan, G.T.; Lee, Z. PET imaging of hepatocellular carcinoma with 2-deoxy-2[18F]fluoro-D-glucose, 6-deoxy-6[18F]fluoro-D-glucose, [1-11C]-acetate and [N-methyl-11C]-choline. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 144–156. [Google Scholar] [PubMed]
- Kuang, Y.; Salem, N.; Tian, H.; Kolthammer, J.A.; Corn, D.J.; Wu, C.; Wang, F.; Wang, Y.; Lee, Z. Imaging lipid synthesis in hepatocellular carcinoma with [methyl-11C]choline: Correlation with in vivo metabolic studies. J. Nucl. Med. 2011, 52, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Torzilli, G.; Poretti, D.; de Neto, L.J.; Donadon, M.; Rimassa, L.; Lanza, E.; Sabongi, J.G.; Ceriani, R.; Personeni, N.; et al. Diagnostic accuracy of 11C-choline PET/CT in comparison with CT and/or MRI in patients with hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1399–1407. [Google Scholar] [CrossRef]
- Lanza, E.; Donadon, M.; Felisaz, P.; Mimmo, A.; Chiti, A.; Torzilli, G.; Balzarini, L.; Lopci, E. Refining the management of patients with hepatocellular carcinoma integrating 11C-choline PET/CT scan into the multidisciplinary team discussion. Nucl. Med. Commun. 2017, 38, 826–836. [Google Scholar] [CrossRef]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; Ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef]
- The, S.H.; Christein, J.; Donohue, J.; Que, F.; Kendrick, M.; Farnell, M.; Cha, S.; Kamath, P.; Kim, R.; Nagorney, D.M. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of end-stage liver disease (MELD) score predicts perioperative mortality. J. Gastrointest. Surg. 2005, 9, 1207–1215. [Google Scholar]
- Donadon, M.; Costa, G.; Cimino, M.; Procopio, F.; Del Fabbro, D.; Palmisano, A.; Torzilli, G. Safe hepatectomy selection criteria for hepatocellular carcinoma patients: A validation of 336 consecutive hepatectomies. The BILCHE score. World J. Surg. 2015, 39, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Fontana, A.; Palmisano, A.; Viganò, L.; Procopio, F.; Cimino, M.; Del Fabbro, D.; Torzilli, G. Individualized risk estimation for postoperative morbidity after hepatectomy: The Humanitas score. HPB 2017, 19, 910–918. [Google Scholar] [CrossRef]
- Torzilli, G. Ultrasound-Guided Liver Surgery: An Atlas; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Torzilli, G.; Montorsi, M.; Donadon, M.; Palmisano, A.; Del Fabbro, D.; Gambetti, A.; Olivari, N.; Makuuchi, M. “Radical but conservative” is the main goal for ultrasonography-guided liver resection: Prospective validation of this approach. J. Am. Coll. Surg. 2005, 201, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Yu, S.J. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin. Mol. Hepatol. 2016, 22, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Yu, S.C.; Yeung, D.W. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J. Nucl. Med. 2003, 44, 213–221. [Google Scholar]
- Park, J.W.; Kim, J.H.; Kim, S.K.; Kang, K.W.; Park, K.W.; Choi, J.I.; Lee, W.J.; Kim, C.M.; Nam, B.N. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J. Nucl. Med. 2008, 49, 1912–1921. [Google Scholar] [CrossRef]
- Torizuka, T.; Tamaki, N.; Inokuma, T.; Magata, Y.; Sasayama, S.; Yonekura, Y.; Tanaka, A.; Yamaoka, Y.; Yamamoto, K.; Konishi, J. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J. Nucl. Med. 1995, 36, 1811–1817. [Google Scholar]
- Hayakawa, N.; Nakamoto, Y.; Nakatani, K.; Hatano, E.; Seo, S.; Higashi, T.; Saga, T.; Uemoto, S.; Togashi, K. Clinical utility and limitations of FDG PET in detecting recurrent hepatocellular carcinoma in postoperative patients. Int. J. Clin. Oncol. 2014, 19, 1020–1028. [Google Scholar] [CrossRef]
- Filippi, L.; Schillaci, O.; Bagni, O. Recent advances in PET probes for hepatocellular carcinoma characterization. Expert Rev. Med. Devices 2019, 16, 341–350. [Google Scholar] [CrossRef]
- Bertagna, F.; Bertoli, M.; Bosio, G.; Biasiotto, G.; Sadeghi, R.; Giubbini, R.; Treglia, G. Diagnostic role of radiolabelled choline PET or PET/CT in hepatocellular carcinoma: A systematic review and meta-analysis. Hepatol. Int. 2014, 8, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Gougelet, A.; Sartor, C.; Senni, N.; Calderaro, J.; Fartoux, L.; Lequoy, M.; Wendum, D.; Talbot, J.N.; Prignon, A.; Chalaye, J.; et al. Hepatocellular Carcinomas With Mutational Activation of Beta-Catenin Require Choline and Can Be Detected by Positron Emission Tomography. Gastroenterology 2019, 157, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Park, M.S.; Kim, K.A.; Park, J.Y.; Kim, I.; Kang, W.J.; Lee, S.K.; Kim, M.J. 18F-FDG PET metabolic parameters and MRI perfusion and diffusion parameters in hepatocellular carcinoma: A preliminary study. PLoS ONE 2013, 8, e71571. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Erdi, Y.; Akhurst, T.; Mazumdar, M.; Macapinlac, H.A.; Finn, R.D.; Casilla, C.; Fazzari, M.; Srivastava, N.; Yeung, H.W.; et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging: The visual response score and the change in total lesion glycolysis. Clin. Positron Imaging 1999, 2, 159–171. [Google Scholar] [CrossRef]
- Lee, J.; Sato, M.M.; Coel, M.N.; Lee, K.-H.; Kwee, S.A. Prediction of PSA progression in castrate-resistant prostate cancer based on treatment-associated change in tumor burden quatified by 18F-fluorocholine PET/CT. J. Nucl. Med. 2016, 57, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Donadon, M.; Marconi, M.; Palmisano, A.; Del Fabbro, D.; Spinelli, A.; Botea, F.; Montorsi, M. Hepatectomy for stage B and stage C hepatocellular carcinoma in the Barcelona Clinic Liver Cancer classification: Results of a prospective analysis. Arch. Surg. 2008, 143, 1082–1090. [Google Scholar] [CrossRef]
- Makuuchi, M.; Thai, B.L.; Takayasu, K.; Takayama, T.; Kosuge, T.; Gunvén, P.; Yamazaki, S.; Hasegawa, H.; Ozaki, H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: A preliminary report. Surgery 1990, 107, 521–527. [Google Scholar]


| Characteristic | No. (%) |
|---|---|
| Age (years) | |
| Median, range | 72 (18–83) |
| Gender | |
| Men | 47 (78%) |
| Women | 13 (22%) |
| BILCHE score | |
| 0 | 35 (58%) |
| 1 | 8 (13%) |
| 2 | 10 (17%) |
| 3 | 7 (12%) |
| CPT score A | 60 (100%) |
| MELD score | |
| Median, range | 8 (6–11) |
| BCLC stage | |
| 0–A | 14 (23%) |
| B | 37 (62%) |
| C | 9 (15%) |
| Tumor number | |
| Median, range | 1 (1–8) |
| >1 | 22 (37%) |
| 1 | 38 (63%) |
| 2 | 17 |
| 3 | 1 |
| 4 | 2 |
| 6 | 1 |
| 8 | 1 |
| Tumor size (cm) | |
| Median, range | 7.5 (1.4–26) |
| Milan criteria | |
| In | 12 (20%) |
| Out | 48 (80%) |
| Alpha-fetoprotein (ng/mL) | |
| Median, range | 17 (2–45,667) |
| >7 * | 37 (62%) |
| Tumor grading | |
| 1–2 | 34 (57%) |
| 3–4 | 26 (43%) |
| Microvascular invasion | |
| No | 20 (33%) |
| Yes | 40 (67%) |
| Cirrhosis | |
| No | 47 (78%) |
| Yes | 10 (17%) |
| Unknown | 3 (5%) |
| Data | No. (%) |
|---|---|
| Type of hepatectomy | |
| Minor resection | 51 (85%) |
| Major resection (>3 segments) | 9 (15%) |
| Thoracoabdominal approach | 22 (37%) |
| Blood loss (mL) | |
| Median, range | 350 (20–3000) |
| Blood transfusions | 11 (18%) |
| Complications | |
| Overall | 24 (40%) |
| Minor (Dindo-Clavien 1–2) | 19 (79%) |
| Major (Dindo-Clavien 3–4) | 5 (21%) |
| Mortality | |
| 30-day | 0 |
| Parameter | Value |
|---|---|
| SUV max | |
| Median; range | 15.2 (6.2–28.2) |
| Mean; SD | 16.4 (±5.6) |
| SUV mean | |
| Mean; SD | 11 (±3) |
| SUV liver | |
| Median; range | 10.8 (5.6–16.6) |
| Mean; SD | 10.9 (±2.4) |
| MTV | |
| Median; range | 33.4 (1.7–1665.8) |
| Mean; SD | 185.9 (±375.9) |
| Photopenic areas (yes) | 34 (57%) |
| MTB | |
| Median; range | 447.8 (13–12,141) |
| Mean; SD | 1593.9 (±2697.6) |
| SUV ratio | |
| Median; range | 1.5 (0.63–3.12) |
| Mean; SD | 1.5 (±0.6) |
| Factor | HR | 95% CI | p-Value |
|---|---|---|---|
| MELD score | 1.03 | 0.58–2.12 | 0.138 |
| BILCHE score | 1.22 | 0.91–2.91 | 0.235 |
| BCLC classification | 2.94 | 1.41–4.51 | 0.003 |
| Number of tumor | 1.26 | 0.81–1.96 | 0.087 |
| Size of tumor | 1.18 | 0.91–1.99 | 0.094 |
| AFP | 0.91 | 0.49–1.91 | 0.814 |
| Tumor grading | 1.12 | 0.81–1.71 | 0.331 |
| Microvascular invasion | 1.33 | 0.32–1.51 | 0.393 |
| Cirrhosis | 0.81 | 0.39–1.81 | 0.941 |
| SUVmax | 0.86 | 0.39–1.61 | 0.569 |
| SUVmean | 0.87 | 0.70–1.07 | 0.190 |
| SUVliver | 0.84 | 0.41–1.12 | 0.640 |
| MTV | 2.11 | 1.51–3.45 | 0.026 |
| Photopenic areas | 0.52 | 0.06–1.39 | 0.527 |
| MTB | 0.99 | 0.89–1.79 | 0.213 |
| SUVratio | 1.18 | 0.91–2.96 | 0.336 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadon, M.; Lopci, E.; Galvanin, J.; Giudici, S.; Del Fabbro, D.; Lanza, E.; Pedicini, V.; Chiti, A.; Torzilli, G. Prognostic Value of Metabolic Imaging Data of 11C-choline PET/CT in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Cancers 2021, 13, 472. https://doi.org/10.3390/cancers13030472
Donadon M, Lopci E, Galvanin J, Giudici S, Del Fabbro D, Lanza E, Pedicini V, Chiti A, Torzilli G. Prognostic Value of Metabolic Imaging Data of 11C-choline PET/CT in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Cancers. 2021; 13(3):472. https://doi.org/10.3390/cancers13030472
Chicago/Turabian StyleDonadon, Matteo, Egesta Lopci, Jacopo Galvanin, Simone Giudici, Daniele Del Fabbro, Ezio Lanza, Vittorio Pedicini, Arturo Chiti, and Guido Torzilli. 2021. "Prognostic Value of Metabolic Imaging Data of 11C-choline PET/CT in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma" Cancers 13, no. 3: 472. https://doi.org/10.3390/cancers13030472
APA StyleDonadon, M., Lopci, E., Galvanin, J., Giudici, S., Del Fabbro, D., Lanza, E., Pedicini, V., Chiti, A., & Torzilli, G. (2021). Prognostic Value of Metabolic Imaging Data of 11C-choline PET/CT in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Cancers, 13(3), 472. https://doi.org/10.3390/cancers13030472









