Desmoplastic Small Round Cell Tumor: A Review of Main Molecular Abnormalities and Emerging Therapy
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Demographics of DSRCT
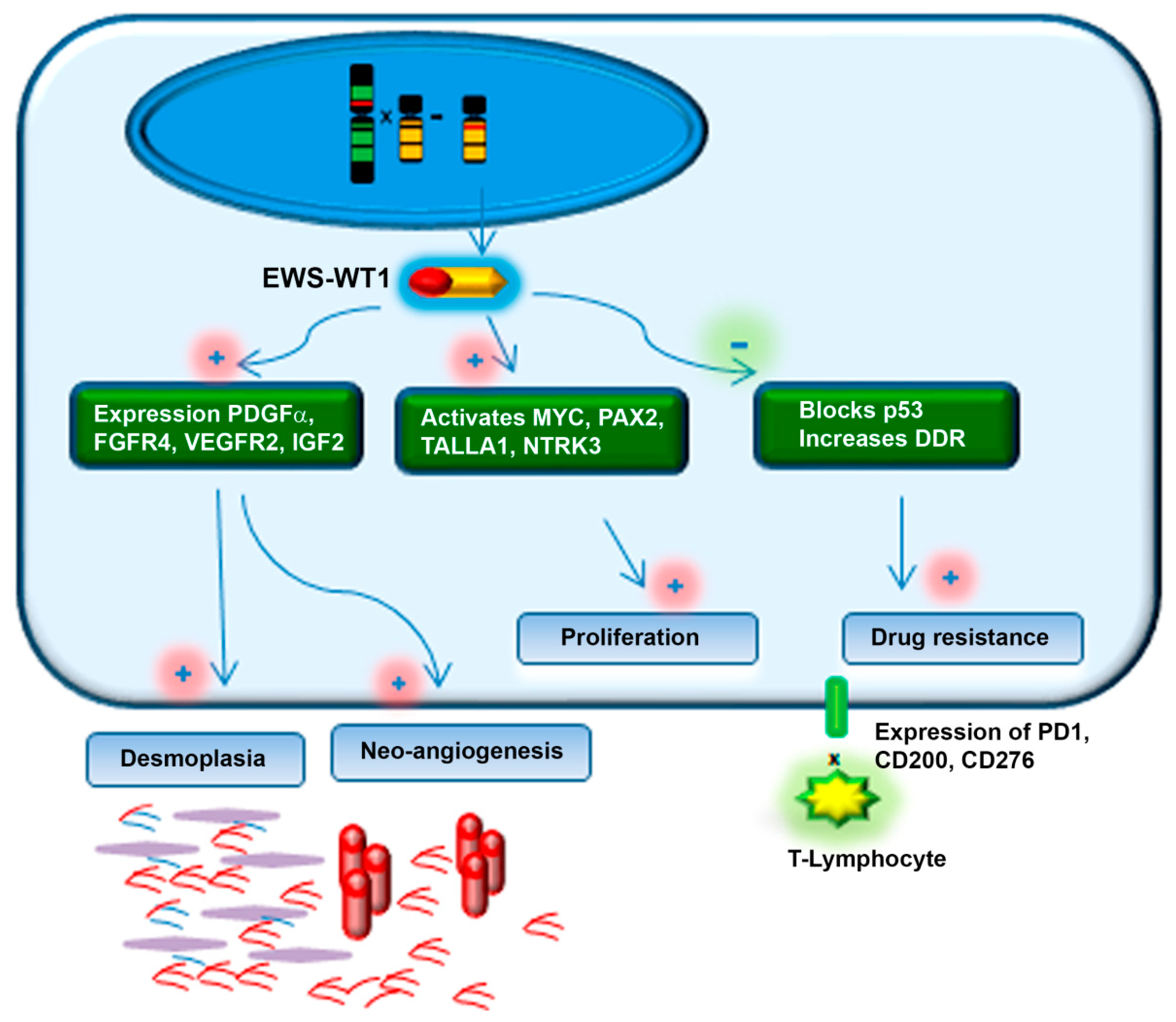
1.2. Molecular Profile of DSRCT
2. Clinical Presentation and Diagnosis
3. Differential Diagnosis
4. Treatment
4.1. Therapeutic Approach for Newly Diagnosed Patients
4.1.1. Radiation Therapy
4.1.2. HIPEC
4.2. Prognosis
4.3. Current and Emerging Therapy for Relapsed or Progressive Disease
4.3.1. The Importance of Pre-Clinical Models to Drug Development for Rare Sarcoma
4.3.2. Targeting Angiogenesis and Other TKR
4.3.3. Targeting Androgen Receptor Pathway
4.3.4. Targeting PI3K/AKT/mTOR Pathway
4.3.5. Targeting DNA Damage Repair (DDR) Proteins
4.3.6. Targeting c-MET and Insulin Growth Factor Pathway
4.3.7. Cancer Vaccines
4.3.8. Perspectives with Novel Targets (Immune Checkpoint and NTRK Inhibitors)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerald, W.L.; Rosai, J. Case 2 Desmoplastic small cell tumor with divergent differentiation. Pediatr. Pathol. 1989, 9, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Cassier, P.; Couraud, L.; Marec-Bérard, P.; Meeus, P.; Alberti, L.; Blay, J.-Y. Desmoplastic small round cell tumor: Current management and recent findings. Sarcoma 2012, 2012, 714986. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, A.; Fahy, B.N.; Xiu, J.; Rashad, S.; Mustafa, A.; Husain, H.; Hayes-Jordan, A. Desmoplastic Small Round Blue Cell Tumor: A Review of Treatment and Potential Therapeutic Genomic Alterations. Sarcoma 2017, 2017, 1278268. [Google Scholar] [CrossRef] [PubMed]
- Hayes-Jordan, A.; Laquaglia, M.P.; Modak, S. Management of Desmoplastic Small Round Cell Tumor. Semin Pediatr. Surg. 2016, 25, 299–304. [Google Scholar] [CrossRef]
- Honoré, C.; Delhorme, J.; Nassif, E.; Faron, M.; Ferron, G.; Bompas, E.; Glehen, O.; Italiano, A.; Bertucci, F.; Orbach, D.; et al. Can we cure patients with abdominal Desmoplastic Small Round Cell Tumor? Results of a retrospective multicentric study on 100 patients. Surg. Oncol. 2019, 29, 107–112. [Google Scholar]
- US Food and Drug Administration. Orphan Drug Act—Relevant Excerpts; US Food and Drug Administration: Washington, DC, USA, 2013.
- Greenlee, R.T.; Goodman, M.T.; Lynch, C.F.; Platz, C.E.; Havener, L.A.; Howe, H.L. The occurrence of rare cancers in U.S. adults, 1995–2004. Public Health Rep. 2010, 125, 28–43. [Google Scholar] [CrossRef]
- Mallone, S.; De Angelis, R.; Van Der Zwan, J.M.; Trama, A.; Siesling, S.; Gatta, G.; Capocaccia, R. Methodological aspects of estimating rare cancer prevalence in Europe: The experience of the RARECARE project. Cancer Epidemiol. 2013, 37, 850–856. [Google Scholar] [CrossRef]
- Lettieri, C.K.; Garcia-Filion, P.; Hingorani, P. Incidence and Outcomes of Desmoplastic Small Round Cell Tumor: Results from the Surveillance, Epidemiology, and End Results Database. J. Cancer Epidemiol. 2014, 2014, 680126. [Google Scholar] [CrossRef]
- Sawyer, J.R.; Tryka, A.F.; Lewis, J.M. A Novel Reciprocal Chromosome Translocation t(11;22)(p13;q12) in an Intraabdominal Desmoplastic Small Round-Cell Tumor. Am. J. Surg. Pathol. 1992, 16, 411–416. [Google Scholar] [CrossRef]
- Rodriguez, E.; Sreekantaiah, C.; Gerald, W.; Reuter, V.E.; Motzer, R.J.; Chaganti, R. A Recurring Translocation, t(11;22)(p13;q11.2), Characterizes Intra-Abdominal Desmoplastic Small Round-Cell Tumors. Cancer Genet Cytogent. 1993, 21, 17–21. [Google Scholar] [CrossRef]
- Gedminas, J.M.; Chasse, M.H.; McBrairty, M.; Beddows, I.; Kitchen-Goosen, S.M.; Grohar, P.J. Desmoplastic small round cell tumor is dependent on the EWS-WT1 transcription factor. Oncogenesis 2020, 9, 1–8. [Google Scholar] [CrossRef]
- Scharnhorst, V.; van der Eb, A.J.; Jochemsen, A.G. WT1 proteins: Functions in growth and differentiation. Gene 2001, 273, 141–161. [Google Scholar] [CrossRef]
- Lee, S.B.; Kolquist, K.A.; Nichols, K.; Englert, C.; Maheswaran, S.; Ladanyi, M.; Gerald, W.L.; Haber, D.A. The EWS-WT1 translocation product induces PDGFA in desmoplastic small round-cell tumour. Nat. Genet. 1997, 17, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nau, M.M.; Yeh, J.C.; Allegra, C.J.; Chu, E.; Wright, J.J. Molecular heterogeneity and function of EWS-WT1 fusion transcripts in desmoplastic small round cell tumors. Clin. Cancer Res. 2000, 6, 3522–3529. [Google Scholar]
- Vignaud, J.M.; Marie, B.; Klein, N.; Plénat, F.; Pech, M.; Borrelly, J.; Martinet, N.; Duprez, A.; Martinet, Y. The role of platelet-derived growth factor production by tumor-associated macrophages in tumor stroma formation in lung cancer. Cancer Res. 1994, 15, 5455–5463. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7923179 (accessed on 1 September 2020).
- Ferreira, E.N.; Barros, B.D.F.; De Souza, J.E.; Valieris, R.; Torrezan, G.; Garcia-Rosa, S.; Krepischi, A.C.V.; De Mello, C.A.L.; Da Cunha, I.W.; Pinto, C.A.L.; et al. A genomic case study of desmoplastic small round cell tumor: Comprehensive analysis reveals insights into potential therapeutic targets and development of a monitoring tool for a rare and aggressive disease. Hum. Genom. 2016, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.; Daniels, G.; Wang, D.; Deng, F.-M.; Lee, P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am. J. Cancer Res. 2017, 7, 1389–1406. [Google Scholar]
- Devecchi, A.; De Cecco, L.; Dugo, M.; Penso, D.; Dagrada, G.P.; Brich, S.; Stacchiotti, S.; Sensi, M.; Canevari, S.; Pilotti, S. The genomics of desmoplastic small round cell tumor reveals the deregulation of genes related to DNA damage response, epithelial-mesenchymal transition, and immune response 06 Biological Sciences 0604 Genetics. Cancer Commun. 2018, 38, 1–14. [Google Scholar] [CrossRef]
- Bulbul, A.; Shen, J.P.; Xiu, J.; Tamayo, P.; Husain, H. Genomic and Proteomic Alterations in Desmoplastic Small Round Blue-Cell Tumors. JCO Precis Oncol. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Negri, T.; Brich, S.; Bozzi, F.; Volpi, C.V.; Gualeni, A.V.; Stacchiotti, S.; De Cecco, L.; Canevari, S.; Gloghini, A.; Pilotti, S. New transcriptional-based insights into the pathogenesis of desmoplastic small round cell tumors (DSRCTs). Oncotarget 2017, 8, 32492–32504. [Google Scholar] [CrossRef]
- Lal, D.R.; Su, W.T.; Wolden, S.L.; Loh, K.C.; Modak, S.; La Quaglia, M.P. Results of multimodal treatment for desmoplastic small round cell tumors. J. Pediatr. Surg. 2005, 40, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Stiles, Z.E.; Dickson, P.V.; Glazer, E.S.; Murphy, A.J.; Davidoff, A.M.; Behrman, S.W.; Bishop, M.W.; Martin, M.G.; Deneve, J.L. Desmoplastic small round cell tumor: A nationwide study of a rare sarcoma. J. Surg. Oncol. 2018, 117, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Al-Ibraheemi, A.; Broehm, C.; Tanas, M.R.; Horvai, A.E.; Rubin, B.P.; Cheah, A.L.; Thway, K.; Fisher, C.; Bahrami, A.; Folpe, A.L.; et al. Desmoplastic Small Round Cell Tumors With Atypical Presentations: A Report of 34 Cases. Int. J. Surg. Pathol. 2018, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thondam, S.K.; Du Plessis, D.; Cuthbertson, D.J.; Das, K.S.V.; Javadpour, M.; Macfarlane, I.A.; Leggate, J.; Haylock, B.; Daousi, C. Intracranial desmoplastic small round cell tumor presenting as a suprasellar mass. J. Neurosurg. 2015, 122, 773–777. [Google Scholar] [CrossRef]
- Faras, F.; Abo-Alhassan, F.; Hussain, A.; Sebire, N.; Al-Terki, A. Primary desmoplastic small round cell tumor of upper cervical lymph nodes. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2015, 120, 4–10. [Google Scholar] [CrossRef]
- Xu, J.; Yao, M.; Yang, X.; Liu, T.; Wang, S.; Ma, D.; Li, X. Desmoplastic small round cell tumor of the middle ear. Med. Baltim. 2018, 97, 1–5. [Google Scholar] [CrossRef]
- Nakayama, J.; Nassau, S.; Atkins, K.; Modesitt, S.C. Desmoplastic small round cell tumor of the ovary: A rare but devastating disease in young women. Gynecol. Oncol. Rep. 2014, 7, 16–18. [Google Scholar] [CrossRef]
- Honoré, C.; Amroun, K.; Vilcot, L.; Mir, O.; Domont, J.; Terrier, P.; Le Cesne, A.; Le Pechoux, C.; Bonvalot, S. Abdominal Desmoplastic Small Round Cell Tumor: Multimodal Treatment Combining Chemotherapy, Surgery, and Radiotherapy is the Best Option. Ann. Surg. Oncol. 2015, 22, 1073–1079. [Google Scholar] [CrossRef]
- Gani, F.; Goel, U.; Canner, J.K.; Meyer, C.F.; Johnston, F.M. A national analysis of patterns of care and outcomes for adults diagnosed with desmoplastic small round cell tumors in the United States. J. Surg. Oncol. 2019, 119, 880–886. [Google Scholar] [CrossRef]
- Hayes-Jordan, A.; Anderson, P.M. The diagnosis and management of desmoplastic small round cell tumor: A review. Curr. Opin. Oncol. 2011, 23, 385–389. [Google Scholar] [CrossRef]
- Bellah, R.; Suzuki-Bordalo, L.; Brecher, E.; Ginsberg, J.P.; Maris, J.; Pawel, B.R. Desmoplastic Small Round Cell Tumor in the Abdomen and Pelvis: Report of CT Findings in 11 Affected Children and Young Adults. Am. J. Roentgenol. 2013, 184, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.C.; Price, A.P.; Fleming, S.; Sohn, M.J.; Magnan, H.; Laquaglia, M.P.; Abramson, S. Characteristic imaging features of desmoplastic small round cell tumour. Pediatr. Radiol. 2013, 43, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Fisher, A.J.; Balfe, D.M.; Dehner, L.P.; Huettner, P.C. Desmoplastic small round cell tumor of the abdomen: Radiologic-histopathologic correlation. Radiology 1999, 210, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; O’Regan, K.N.; Agoston, A.; Javery, O.; Jagannathan, J.; Ramaiya, N.H. Imaging of desmoplastic small round cell tumour in adults. Br. J. Radiol. 2012, 85, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-D.; Li, C.-X.; Liu, Q.-Y.; Hu, Y.-Y.; Cao, Y.; Huang, J.-H. CT, MRI, and FDG-PET/CT imaging findings of abdominopelvic desmoplastic small round cell tumors: Correlation with histopathologic findings. Eur. J. Radiol. 2011, 80, 269–273. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Kurth, T.; Pressman, A. FDG PET/CT Imaging of Desmoplastic Small Round Cell Tumor: Findings at Staging, During Treatment and at Follow Up. Pediatr. Radiol. 2016, 35, 1252–1260. [Google Scholar]
- Hayes-Jordan, A.; Green, H.; E Fitzgerald, N.; Xiao, L.; Anderson, P.M. Novel treatment for desmoplastic small round cell tumor: Hyperthermic intraperitoneal perfusion. J. Pediatr. Surg. 2010, 45, 1000–1006. [Google Scholar] [CrossRef]
- Lae, M.E.; Roche, P.C.; Jin, L.; Lloyd, R.V.; Nascimento, A.G. Desmoplastic Small Round Cell Tumor: A Clinicopathologic, Immunohistochemical, and Molecular Study of 32 Tumors. Am. J. Surg. Pathol. 2002, 26, 823–835. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Desmoplastic Small Round Cell Tumor: II: An Ultrastructural and Immunohistochemical Study with Emphasis on New Immunohistochemical Markers. Am. J. Surg. Pathol. 1998, 22, 1314–1327. [Google Scholar] [CrossRef]
- Gerald, W.L.; Ladanyi, M.; De Álava, E.; Cuatrecasas, M.; Kushner, B.H.; Laquaglia, M.P.; Rosai, J. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): Desmoplastic small round-cell tumor and its variants. J. Clin. Oncol. 1998, 16, 3028–3036. [Google Scholar] [CrossRef]
- Thway, K.; Noujaim, J.; Zaidi, S.; Miah, A.B.; Benson, C.; Messiou, C.; Jones, R.L.; Fisher, C. Desmoplastic Small Round Cell Tumor: Pathology, Genetics, and Potential Therapeutic Strategies. Int. J. Surg. Pathol. 2016, 24, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Navarro, L.; Pellin, A.; Navarro, S.; Agaimy, A.; Tardío, J.C.; Karseladze, A.; Petrov, S.; Scotlandi, K.; Picci, P.; et al. Defining Ewing and Ewing-like small round cell tumors (SRCT): The need for molecular techniques in their categorization and differential diagnosis. A study of 200 cases. Ann. Diagn Pathol. 2016, 22, 25–32. [Google Scholar] [CrossRef]
- Chicago Consensus Working Group; Izquierdo, F.J.; Plana, A.; Schuitevoerder, D.; Hayes-Jordan, A.; DeNeve, J.L.; Al-Kasspooles, M.; Bowne, W.B.; Brown, C.K.; Kane, J.M.; et al. The Chicago Consensus on peritoneal surface malignancies: Management of desmoplastic small round cell tumor, breast, and gastrointestinal stromal tumors. Cancer 2020, 126, 2566–2570. [Google Scholar]
- Casali, P.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Broto, J.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29 (Suppl. 4), iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lamhamedi-Cherradi, S.-E.; Cuglievan, B.; Menegaz, B.A.; Camacho, P.; Huh, W.W.; Ramamoorthy, V.; Anderson, P.M.; Pollock, R.E.; Lev, D.C.; et al. Multimodality treatment of desmoplastic small round cell tumor: Chemotherapy and complete cytoreductive surgery improve patient survival. Clin. Cancer Res. 2018, 24, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Shyyan, R.; Donohue, J.H.; Edmonson, J.H.; Gunderson, L.L.; Moir, C.R.; Arndt, C.A.; Nascimento, A.G.; Que, F.G. Intraabdominal desmoplastic small round cell tumors: A diagnostic and therapeutic challenge. Cancer 2005, 104, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.H.; Hatcher, H.M.; Benson, C.; Al-Muderis, O.; Horan, G.; Fisher, C.; Earl, H.; Judson, I. Desmoplastic small round cell tumour: Characteristics and prognostic factors of 41 patients and review of the literature. Clin. Sarcoma Res. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Farhat, F.; Culine, S.; Lhommé, C.; Duvillard, P.; Soulié, P.; Michel, G.; Terrier-Lacombe, M.-J.; Théodore, C.; Schreinerova, M.; Droz, J.P. Desmoplastic small round cell tumors: Results of a four-drug chemotherapy regimen in five adult patients. Cancer 1996, 77, 1363–1366. [Google Scholar] [CrossRef]
- Kushner, B.H.; Laquaglia, M.P.; Wollner, N.; A Meyers, P.; Lindsley, K.L.; Ghavimi, F.; E Merchant, T.; Boulad, F.; Cheung, N.K.; A Bonilla, M.; et al. Desmoplastic small round-cell tumor: Prolonged progression-free survival with aggressive multimodality therapy. J. Clin. Oncol. 1996, 14, 1526–1531. [Google Scholar] [CrossRef]
- Bertuzzi, A.F.; Castagna, L.; Nozza, A.; Quagliuolo, V.; Siracusano, L.; Balzarotti, M.; Compasso, S.; Alloisio, M.; Parra, H.S.; Santoro, A. High-dose chemotherapy in poor-prognosis adult small round-cell tumors: Clinical and molecular results from a prospective study. J. Clin. Oncol. 2002, 20, 2181–2188. [Google Scholar] [CrossRef]
- Scheer, M.; Vokuhl, C.; Blank, B.; Hallmen, E.; von Kalle, T.; Münter, M.; Wessalowski, R.; Hartwig, M.; Sparber-Sauer, M.; Schlegel, P.G.; et al. Desmoplastic small round cell tumors: Multimodality treatment and new risk factors. Cancer Med. 2019, 8, 527–542. [Google Scholar] [CrossRef]
- Liu, K.X.; Collins, N.B.; Greenzang, K.A.; Furutani, E.; Campbell, K.; Groves, A.; Mullen, E.A.; Shusterman, S.; Spidle, J.; Marcus, K.J.; et al. The use of interval-compressed chemotherapy with the addition of vincristine, irinotecan, and temozolomide for pediatric patients with newly diagnosed desmoplastic small round cell tumor. Pediatr. Blood Cancer 2020, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Atallah, V.; Honore, C.; Orbach, D.; Helfre, S.; Ducassou, A.; Thomas, L.; Levitchi, M.-B.; Mervoyer, A.; Naji, S.; Dupin, C.; et al. Role of adjuvant radiation therapy after surgery for abdominal desmoplastic small round cell tumors. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.B.; Stein, N.F.; Laquaglia, M.P.; Alektiar, K.M.; Kushner, B.H.; Modak, S.; Magnan, H.; Goodman, K.; Wolden, S. Reduced toxicity with intensity modulated radiation therapy (IMRT) for desmoplastic small round cell tumor (DSRCT): An update on the whole abdominopelvic radiation therapy (WAP-RT) experience. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, e67–e72. [Google Scholar] [CrossRef]
- Forlenza, C.J.; Kushner, B.H.; Kernan, N.; Boulad, F.; Magnan, H.; Wexler, L.; Wolden, S.L.; Laquaglia, M.P.; Modak, S. Myeloablative Chemotherapy with Autologous Stem Cell Transplant for Desmoplastic Small Round Cell Tumor. Sarcoma 2015, 2015, 269197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campos, F.; Coutinho, D.L.; Silva, M.L.G.; Lopes, A.; Nascimento, A.; Júnior, S.A.; Nicolau, U.R.; Formiga, M.N.; Costa, F.D.; Mello, C. Clinical Characteristics, Management, and Outcomes of 19 Nonpediatric Patients with Desmoplastic Small Round Cell Tumor: A Cohort of Brazilian Patients. Sarcoma 2020, 2020, 8713165. [Google Scholar] [CrossRef]
- Hayes-Jordan, A.; Coakley, B.A.; Green, H.L.; Xiao, L.; Fournier, K.F.; Herzog, C.E.; Ludwig, J.A.; McAleer, M.F.; Anderson, P.M.; Huh, W.W. Desmoplastic Small Round Cell Tumor Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial. Ann. Surg. Oncol. 2018, 25, 872–877. [Google Scholar] [CrossRef]
- Hayes-Jordan, A.; Green, H.L.; Lin, H.; Owusu-Agyemang, P.; Fitzgerald, N.; Arunkumar, R.; Mejia, R.; Okhuysen-Cawley, R.; Mauricio, R.; Fournier, K.; et al. Complete cytoreduction and HIPEC improves survival in desmoplastic small round cell tumor. Ann. Surg. Oncol. 2014, 21, 220–224. [Google Scholar] [CrossRef]
- Osborne, E.M.; Briere, T.M.; Hayes-Jordan, A.; Levy, L.B.; Huh, W.W.; Mahajan, A.; Anderson, P.M.; McAleer, M.F. Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol. 2016, 119, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Honoré, C.; Atallah, V.; Mir, O.; Orbach, D.; Ferron, G.; LePéchoux, C.; Delhorme, J.B.; Philippe-Chomette, P.; Sarnacki, S.; Msika, S.; et al. Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS ONE 2017, 12, e0171639. [Google Scholar] [CrossRef]
- Van Der Graaf, W.T.A.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Bertuzzi, A.; Castagna, L.; Quagliuolo, V.; Ginanni, V.; Compasso, S.; Magagnoli, M.; Balzarotti, M.; Nozza, A.; Siracusano, L.; Timofeeva, I.; et al. Prospective study of high-dose chemotherapy and autologous peripheral stem cell transplantation in adult patients with advanced desmoplastic small round-cell tumour. Br. J. Cancer 2003, 89, 1159–1161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magnan, H.; Price, A.; Chou, A.J.; Riedel, E.; Wexler, L.H.; Ambati, S.R.; Slotkin, E.K.; Ulaner, G.; Modak, S.; La Quaglia, M.P.; et al. A pilot trial of irinotecan, temozolomide and bevacizumab (ITB) for treatment of newly diagnosed patients with desmoplastic small round cell tumor (DSRCT). J. Clin. Oncol. 2017, 35 (Suppl. 15), 11050. [Google Scholar] [CrossRef]
- Bond, M.; Bernstein, M.L.; Pappo, A.; Schultz, K.R.; Krailo, M.; Blaney, S.M.; Adamson, P.C. A Phase II Study of Imatinib Mesylate in Children With Refractory or Relapsed Solid Tumors: A Children’s Oncology Group Study. Pediatr. Blood Cancer 2008, 50, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Demetri, G.; Barnette, P.; Desai, J.; Kavan, P.; Tozer, R.; Benedetto, P.W.; Friberg, G.; Deng, H.; McCaffery, I.; et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic ewing family tumors or desmoplastic small round cell tumors. J. Clin. Oncol. 2012, 30, 1849–1856. [Google Scholar] [CrossRef]
- Casanova, M.; Ferrari, A.; Bisogno, G.; Merks, J.H.; De Salvo, G.L.; Meazza, C.; Tettoni, K.; Provenzi, M.; Mazzarino, I.; Carli, M. Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: Pilot study for the upcoming European rhabdomyosarcoma protocol. Cancer 2004, 101, 1664–1671. [Google Scholar] [CrossRef]
- Bisogno, G.; Riccardi, R.; Ruggiero, A.; Arcamone, G.; Prete, A.; Surico, G.; Provenzi, M.; Bertolini, P.; Paolucci, P.; Carli, M. Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer 2006, 106, 703–707. [Google Scholar] [CrossRef]
- Italiano, A.; Kind, M.; Cioffi, A.; Maki, R.G.; Bui, B. Clinical activity of sunitinib in patients with advanced desmoplastic round cell tumor: A case series. Target Oncol. 2013, 8, 211–213. [Google Scholar] [CrossRef]
- Verret, B.; Honore, C.; Dumont, S.; Terrier, P.; Adam, J.; Cavalcanti, A.; Sourrouille, I.; Klausner, G.; Ahlenc-Gelas, M.; Kiavue, N.; et al. Trabectedin in advanced desmoplastic round cell tumors: A retrospective single-center series. Anticancer Drugs 2017, 28, 116–119. [Google Scholar] [CrossRef]
- Menegaz, B.A.; Cuglievan, B.; Benson, J.; Camacho, P.; Lamhamedi-Cherradi, S.; Leung, C.H.; Warneke, C.L.; Huh, W.; Subbiah, V.; Benjamin, R.S.; et al. Clinical Activity of Pazopanib in Patients with Advanced Desmoplastic Small Round Cell Tumor. Oncologist 2018, 23, 360–366. Available online: https://pubmed.ncbi.nlm.nih.gov/29212731 (accessed on 6 December 2020). [CrossRef]
- Tarek, N.; Hayes-Jordan, A.; Salvador, L.; McAleer, M.F.; Herzog, C.E.; Huh, W.W. Recurrent desmoplastic small round cell tumor responding to an mTOR inhibitor containing regimen. Pediatr. Blood Cancer 2018, 65, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Thijs, A.M.J.; van der Graaf, W.T.A. Temsirolimus for Metastatic Desmoplastic Small Round Cell Tumor. Pediatr. Blood Cancer 2010, 55, 1431–1432. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.; Shipley, J.M. Desmoplastic small round cell tumor (DSRCT): Emerging therapeutic targets and future directions for potential therapies. Expert Opin. Ther. Targets 2020, 24, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Nishio, J.; Iwasaki, H.; Ishiguro, M.; Ohjimi, Y.; Fujita, C.; Yanai, F.; Nibu, K.; Mitsudome, A.; Kaneko, Y.; Kikuchi, M. Establishment and characterization of a novel human desmoplastic small round cell tumor cell line, JN-DSRCT-1. Lab. Investig. 2002, 82, 1175–1182. [Google Scholar] [CrossRef]
- Van Erp, A.E.M.; Van Houdt, L.; Hillebrandt-Roeffen, M.H.S.; Van Bree, N.F.H.N.; Flucke, U.E.; Mentzel, T.; Shipley, J.; Desar, I.M.E.; Fleuren, E.D.G.; Versleijen-Jonkers, Y.M.H.; et al. Olaparib and temozolomide in desmoplastic small round cell tumors: A promising combination in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Gerald, W.L.; Rosai, J.; Ladanyi, M. Characterization of the genomic breakpoint and chimeric transcripts in the EWS-WT1 gene fusion of desmoplastic small round cell tumor. Proc. Natl. Acad. Sci. USA 2006, 92, 1028–1032. [Google Scholar] [CrossRef]
- Ito, E.; Honma, R.; Imai, J.-I.; Azuma, S.; Kanno, T.; Mori, S.; Yoshie, O.; Nishio, J.; Iwasaki, H.; Yoshida, K.; et al. A Tetraspanin-Family Protein, T-Cell Acute Lymphoblastic Leukemia-Associated Antigen 1, Is Induced by the Ewing’s Sarcoma-Wilms’ Tumor 1 Fusion Protein of Desmoplastic Small Round-Cell Tumor. Am. J. Pathol. 2003, 163, 2165–2172. [Google Scholar] [CrossRef]
- Li, H.; Smolen, G.A.; Beers, L.F.; Xia, L.; Gerald, W.; Wang, J.; Haber, D.A.; Lee, S.B. Adenosine Transporter ENT4 Is a Direct Target of EWS/WT1 Translocation Product and Is Highly Expressed in Desmoplastic Small Round Cell Tumor. PLoS ONE 2008, 3, e2353. [Google Scholar] [CrossRef]
- Kang, H.-J.; Park, J.H.; Chen, W.; Kang, S.I.; Moroz, K.; Ladanyi, M.; Lee, S.B. EWS-WT1 Oncoprotein Activates Neuronal Reprogramming Factor ASCL1 and Promotes Neural Differentiation. Cancer Res. 2014, 74, 4526–4535. [Google Scholar] [CrossRef]
- Hingorani, P.; Dinu, V.; Zhang, X.; Lei, H.; Shern, J.F.; Park, J.; Steel, J.; Rauf, F.; Parham, D.; Gastier-Foster, J.; et al. Transcriptome analysis of desmoplastic small round cell tumors identifies actionable therapeutic targets: A report from the Children’s Oncology Group. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Tirado, O.M.; Mateo-Lozano, S.; Notario, V. Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax: Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene 2005, 24, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Hayes-Jordan, A.A.; Ma, X.; Menegaz, B.A.; Lamhamedi-Cherradi, S.-E.; Kingsley, C.V.; Benson, J.A.; Camacho, P.E.; Ludwig, J.A.; Lockworth, C.R.; Garcia, G.E.; et al. Efficacy of ONC201 in Desmoplastic Small Round Cell Tumor. Neoplasia 2018, 20, 524–532. [Google Scholar] [CrossRef]
- Shi, C.; Feng, Y.; Zhang, L.C.; Ding, D.Y.; Yan, M.Y.; Pan, L. Effective treatment of apatinib in desmoplastic small round cell tumor: A case report and literature review. BMC Cancer 2018, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Uboldi, S.; Craparotta, I.; Colella, G.; Ronchetti, E.; Beltrame, L.; Vicario, S.; Marchini, S.; Panini, N.; Dagrada, G.P.; Bozzi, F.; et al. Mechanism of action of trabectedin in desmoplastic small round cell tumor cells. BMC Cancer 2017, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Minuzzo, M.; Virdis, E.; Tamborini, E.; Simone, M.; Tavecchio, M.; Erba, E.; Grosso, F.; Gronchi, A.; Aman, P.; et al. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol. Cancer Ther. 2009, 8, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Markides, C.S.; Coil, D.R.; Luong, L.H.; Mendoza, J.; Kozielski, T.; Vardeman, D.; Giovanella, B.C. Desmoplastic small round cell tumor (DSRCT) xenografts and tissue culture lines: Establishment and initial characterization. Oncol. Lett. 2013, 5, 1453–1456. [Google Scholar] [CrossRef]
- Magnan, H.D.; Chou, T.; LaQuaglia, M.P.; Gerald, W.; Ladanyi, M.; Merchant, M.S. Elevated expression of VEGFR-2 and VEGFA in desmoplastic small round cell tumor (DSRCT) and activity of bevacizumab and irinotecan in a xenograft model of DSRCT. J. Clin. Oncol. 2009, 27 (Suppl. 15), 10016. [Google Scholar] [CrossRef]
- Frezza, A.M.; Benson, C.; Judson, I.; Litière, S.; Marréaud, S.; Sleijfer, S.; Blay, J.-Y.; Dewji, R.; Fisher, C.; Van Der Graaf, W.; et al. Pazopanib in advanced desmoplastic small round cell tumours: A multi-institutional experience. Clin. Sarcoma Res. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Bétrian, S.; Bergeron, C.; Blay, J.-Y.; Bompas, E.; Cassier, P.A.; Chevallier, L.; Fayette, J.; Girodet, M.; Guillemet, C.; Le Cesne, A.; et al. Antiangiogenic effects in patients with progressive desmoplastic small round cell tumor: Data from the French national registry dedicated to the use of off-labeled targeted therapy in sarcoma (OUTC’s). Clin. Sarcoma Res. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Eckhardt, S.G. Sunitinib: From rational design to clinical efficacy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 884–896. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Negri, T.; Zaffaroni, N.; Palassini, E.; Morosi, C.; Brich, S.; Conca, E.; Bozzi, F.; Cassinelli, G.; Gronchi, A.; et al. Sunitinib in advanced alveolar soft part sarcoma: Evidence of a direct antitumor effect. Ann. Oncol. 2011, 22, 1682–1690. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0923753419384418 (accessed on 1 September 2020). [CrossRef] [PubMed]
- Scott, A.J.; Messersmith, W.A.; Jimeno, A. Apatinib: A promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today 2015, 51, 223. Available online: http://journals.prous.com/journals/servlet/xmlxsl/pk_journals.xml_summary_pr?p_JournalId=4&p_RefId=2320599&p_IsPs=N (accessed on 1 September 2020). [CrossRef] [PubMed]
- Tian, Y.; Cheng, X.; Li, Y. Chemotherapy combined with apatinib for the treatment of desmoplastic small round cell tumors: A case report. J. Cancer Res. Ther. 2020, 16, 1177. Available online: http://www.cancerjournal.net/text.asp?2020/16/5/1177/296439 (accessed on 1 September 2020). [PubMed]
- Fine, R.L.; Shah, S.S.; Moulton, T.A.; Yu, I.-R.; Fogelman, D.R.; Richardson, M.; Burris, H.A.; Samuels, B.L.; Assanasen, C.; Gorroochurn, P.; et al. Androgen and c-Kit receptors in desmoplastic small round cell tumors resistant to chemotherapy: Novel targets for therapy. Cancer Chemother Pharmacol. 2007, 59, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Helman, L.J. The biology behind mTOR inhibition in sarcoma. Oncologist 2007, 12, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Helman, L.J.; Meltzer, P. Mechanisms of sarcoma development. Nat. Rev. Cancer 2003, 3, 685–694. [Google Scholar] [CrossRef]
- Felkai, L.; Krencz, I.; Kiss, D.J.; Nagy, N.; Petővári, G.; Dankó, T.; Micsik, T.; Khoor, A.; Tornóczky, T.; Sápi, Z.; et al. Characterization of mTOR Activity and Metabolic Profile in Pediatric Rhabdomyosarcoma. Cancers 2020, 12, 1947. [Google Scholar] [CrossRef]
- Subbiah, V.; Brown, R.E.; Jiang, Y.; Buryanek, J.; Hayes-Jordan, A.; Kurzrock, R.; Anderson, P.M. Morphoproteomic Profiling of the Mammalian Target of Rapamycin (mTOR) Signaling Pathway in Desmoplastic Small Round Cell Tumor (EWS/WT1), Ewing’s Sarcoma (EWS/FLI1) and Wilms’ Tumor(WT1). PLoS ONE 2013, 8, e68985. [Google Scholar] [CrossRef]
- Jiang, Y.; Subbiah, V.; Janku, F.; Ludwig, J.A.; Naing, A.; Benjamin, R.S.; Brown, R.E.; Anderson, P.; Kurzrock, R. Novel secondary somatic mutations in Ewing’s sarcoma and desmoplastic small round cell tumors. PLoS ONE 2014, 9, e93676. [Google Scholar] [CrossRef]
- Naing, A.; Lorusso, P.; Fu, S.; Hong, D.S.; Anderson, P.; Benjamin, R.S.; Ludwig, J.; Chen, H.X.; Doyle, L.A.; Kurzrock, R. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mtor inhibitor temsirolimus in patients with refractory ewing’s sarcoma family tumors. Clin. Cancer Res. 2012, 18, 2625–2631. [Google Scholar] [CrossRef]
- Katz, D.; Azraq, Y.; Eleyan, F.; Gill, S.; Peretz, T.; Merimsky, O. Pazolimus: Pazopanib plus sirolimus following progression on pazopanib, a retrospective case series analysis. BMC Cancer 2016, 16, 616. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.A.; Yee, J.-K.; Tsark, W.; Wu, X.; Qin, H.; Guan, M.; Ross, J.S.; Ali, S.M.; Millis, S.Z. Recurrent secondary genomic alterations in desmoplastic small round cell tumors. BMC Med. Genet 2020, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Min, A.; Im, S.A. PARP Inhibitors as Therapeutics: Beyond Modulation of PARylation. Cancers 2020, 12, 394. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.-Y.N.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Lagarde, M.; Federico, S.M.; Tinkle, C.; Shelat, A.; Stewart, E. PARP inhibitor combination therapy in desmoplastic small round cell tumors. J. Clin. Oncol. 2017, 35 (Suppl. 15), e23212. [Google Scholar] [CrossRef]
- Aune, G.J.; Takagi, K.; Sordet, O.; Guirouilh-Barbat, J.; Antony, S.; Bohr, V.A.; Pommier, Y. Von hippel-lindau-coupled and transcription-coupled nucleotide excision repair-dependent degradation of RNA polymerase II in response to trabectedin. Clin. Cancer Res. 2008, 14, 6449–6455. [Google Scholar] [CrossRef]
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F.; et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013, 23, 249–262. [Google Scholar] [CrossRef]
- Pignochino, Y.; Capozzi, F.; D’Ambrosio, L.; Dell’Aglio, C.; Basiricò, M.; Canta, M.; Lorenzato, A.; Lutati, F.V.; Aliberti, S.; Palesandro, E.; et al. PARP1 expression drives the synergistic antitumor activity of trabectedin and PARP1 inhibitors in sarcoma preclinical models. Mol. Cancer 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Grignani, G.; D’Ambrosio, L.; Pignochino, Y.; Palmerini, E.; Zucchetti, M.; Boccone, P.; Aliberti, S.; Stacchiotti, S.; Bertulli, R.; Piana, R.; et al. Trabectedin and olaparib in patients with advanced and non-resectable bone and soft-tissue sarcomas (TOMAS): An open-label, phase 1b study from the Italian Sarcoma Group. Lancet Oncol. 2018, 19, 1360–1371. [Google Scholar] [CrossRef]
- Ferracini, R.; Di Renzo, M.F.; Scotlandi, K.; Baldini, N.; Olivero, M.; Lollini, P.; Cremona, O.; Campanacci, M.; Comoglio, P.M. The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene 1995, 10, 739–749. [Google Scholar] [PubMed]
- Davis, I.J.; McFadden, A.W.; Zhang, Y.; Coxon, A.; Burgess, T.L.; Wagner, A.J.; Fisher, D.E. Identification of the Receptor Tyrosine Kinase c-Met and Its Ligand, Hepatocyte Growth Factor, as Therapeutic Targets in Clear Cell Sarcoma. Cancer Res. 2010, 70, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.B.; Lee, Y.S.; Lee, H.; Park, J.B.; Park, H.; Choi, Y.-L.; Hong, D.; Kim, S.J. Combination therapy with c-met inhibitor and TRAIL enhances apoptosis in dedifferentiated liposarcoma patient-derived cells. BMC Cancer 2019, 19, 496. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Olivero, M.; Di Renzo, M.F.; Martano, M.; De Giovanni, C.; Nanni, P.; Basso, G.; Scotlandi, K.; Lollini, P.L.; Comoglio, P.M. Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene 1996, 12, 1697–1705. [Google Scholar] [PubMed]
- Chen, H.M.; Feng, G. Use of anlotinib in intra-abdominal desmoplastic small round cell tumors: A case report and literature review. Oncol. Targets Ther. 2019, 12, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Finkeltov, I.; Kuhn, S.; Glaser, T.; Idelman, G.; Wright, J.J.; Roberts, C.T.; Werner, H. Transcriptional regulation of IGF-I receptor gene expression by novel isoforms of the EWS-WT1 fusion protein. Oncogene 2002, 21, 1890–1898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Pender, A.; Jones, R.L.P.S. Optimising Cancer Vaccine Design in Sarcoma. Cancers 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.D.; Hutchinson, R.J.; Hohenkirk, L.F.; A McKenna, E.; A Yanik, G.; E Levine, J.; E Chang, A.; Braun, T.M.; Mulé, J.J. Vaccination Of Pediatric Solid Tumor Patients with Tumor Lysate-pulsed Dendritic Cells Can Expand Specific T Cells and Mediate Tumor Regression. Cancer Res. 2001, 61, 8513–8519. [Google Scholar] [PubMed]
- Merchant, M.S.; Bernstein, D.; Amoako, M.; Baird, K.; Fleisher, T.A.; Morre, M.; Steinberg, S.M.; Sabatino, M.; Stroncek, D.F.; Venkatasan, A.M.; et al. Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas. Clin. Cancer Res. 2016, 22, 3182–3191. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 2020, 20, 75–76. Available online: http://www.nature.com/articles/s41577-020-0275-8 (accessed on 1 September 2020). [CrossRef] [PubMed]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. Available online: http://www.nature.com/articles/nature12213 (accessed on 23 September 2020). [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; A Van Tine, B.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; A Priebat, D.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; A Van Tine, B.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1470204518300068 (accessed on 10 July 2020). [CrossRef]
- Petitprez, F.; De Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; E Rosenberg, A.; A Kerr, D.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Van Erp, A.; Versleijen-Jonkers, Y.M.H.; Hillebrandt-Roeffen, M.H.; Van Houdt, L.; Gorris, M.A.; Van Dam, L.S.; Mentzel, T.; Weidema, M.; Savci-Heijink, C.D.; Desar, I.M.; et al. Expression and clinical association of programmed cell death-1, programmed death-ligand-1 and CD8+ lymphocytes in primary sarcomas is subtype dependent. Oncotarget 2017, 8, 71371–71384. [Google Scholar] [CrossRef]
- Chen, J.L.; Mahoney, M.R.; George, S.; Antonescu, C.R.; Liebner, D.A.; Van Tine, B.A.; Milhem, M.M.; Tap, W.D.; Streicher, H.; Schwartz, G.K.; et al. A multicenter phase II study of nivolumab +/- ipilimumab for patients with metastatic sarcoma (Alliance A091401): Results of expansion cohorts. J. Clin. Oncol. 2020, 38 (Suppl. 15), 11511. [Google Scholar] [CrossRef]
- Modak, S.; Zanzonico, P.; Grkovski, M.; Slotkin, E.K.; Carrasquillo, J.A.; Lyashchenko, S.K.; Lewis, J.S.; Cheung, I.Y.; Heaton, T.E.; Laquaglia, M.P.; et al. B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Demetri, G.; Antonescu, C.; Bjerkehagen, B.; Bovée, J.; Boye, K.; Chacón, M.; Tos, A.D.; Desai, J.; Fletcher, J.; Gelderblom, H.; et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 2020, 31, 1506–1517. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0923753420422975 (accessed on 23 September 2020). [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1470204519308563 (accessed on 23 September 2020). [CrossRef]
- Ogura, K.; Somwar, R.; Hmeljak, J.; Magnan, H.; Benayed, R.; Momeni-Boroujeni, A.; Bowman, A.S.; Mattar, M.S.; Khodos, I.; De Stanchina, E.; et al. Therapeutic potential of NTRK3 inhibition in desmoplastic small round cell tumor. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | N | % Surgery + Radiation (N) | RdT Dose (Gy) | Comparison Group (RdT × no-RdT) | Outcome with Addition of Radiation |
|---|---|---|---|---|---|---|
| Lal et al. [22] | 2005 | 66 | 43% (29) | 30 | yes | Improved 3 year OS (55 × 27%, p < 0.02) |
| Forlenza et al. [56] | 2015 | 19 | 89% (17) | 30 | no | 5-year OS 16% |
| Stiles et al. [23] | 2018 | 102 | 20% (21) | NR | yes | No difference in median OS (27.5 × 28.8 mo, p = 0.32) |
| Subbiah et al. [46] | 2018 | 165 | 42% (69) | NR | yes | No improvement in OS (3.6 × 2.9, p = 0.38) |
| Honore et al. [5] | 2019 | 100 | 26% (26) | 30 | yes | Improved survival and cure rate (HR = 0.36, p = 0.00013) |
| Atallah et al. [54] | 2016 | 49 * | 55% (27) | 20–33 | yes | Improved median PPFS (22.5 × 14.2 mo, p = 0.024), no significant difference in OS (p = 0.40) |
| Campos et al. [57] | 2020 | 19 | 21% (4) | 30 | no | 5-year OS 12% |
| Author | Year | N | Study Design/Region | Therapy | Overall Survival | Relapse |
|---|---|---|---|---|---|---|
| Lal et al. [22] | 2005 | 66 | Retrospective, single center, USA | ChT, CRS, RdT | 5-year 15% | NR |
| Forlenza et al. [56] | 2015 | 19 | Prospective, single center, USA | ChT, CRS, BMT, RdT | 5-year 16% | 3-year EFS 11.0% |
| Osborne et al. [60] | 2015 | 32 | Retrospective, Single center USA | ChT, CRS, RdT | 5-year 38% | 3-year EFS 9.9% |
| Honore et al. [61] | 2017 | 48 | Retrospective | ChT, CRS, HIPEC, RdT | 5-year 19% | 5-year DFS 12% |
| Scheer et al. [52] | 2018 | 60 | Prospective, multicenter, Germany, Poland, Austria, Sweden, Switzerland | ChT, CRS, BMT, RdT HIPEC | 3-year 30% | 3-year EFS 11.0% |
| Stiles et al. [23] | 2018 | 125 | Retrospective, multicenter, USA | ChT, CRS, BMT HIPEC RdT | 5-year 10% | NR |
| Subbiah et al. [46] | 2018 | 165 | Retrospective, single center, USA | ChT, CRS, BMT, HIPEC, RdT | 5-year 25% | NR |
| Honore et al. [5] | 2019 | 100 | Retrospective, multicenter, France | ChT, CRS, HIPEC, RdT | 5-year 5% | 3-year DFS 7.0% |
| Campos et al. [57] | 2020 | 19 | Retrospective, single center, Brazil | ChT, CRS, HIPEC RdT | 5-year 12% | Median DFS 10 months |
| Author, Year | Line of Treatment | N | Median Age/Range (Years) | Systemic Therapy | Response | Survival |
|---|---|---|---|---|---|---|
| Farhat et al., 1996 [49] | 1st line | 5 | 22/16–26 | PA(E)VEP * | 80% SD 20% CR | mRFS: 6 mo |
| Bertuzzi et al., 2003 [63] | 1st line | 10 | 29/NA | IVE * | 50% PR 20% SD 30% PD | NA |
| Forlenza et al., 2015 [56] | 1st line | 19 | 18.5/10–42 | P6-protocol * | 78% SD 11% MR 11% PD | mPFS = 12.8 mo 3-year OS = 26 ± 10% |
| Magnan et al., 2017 [64] | 1st line | 15 | NA | ITB | RR = 27% | mTTP = 18.1 mo 3-year OS = 61% |
| Scheer et al., 2019 [52] | 1st line | 60 | 14.5/6–38 | P6-protocol * VAIA * CEVAIE * | NA ** | P6-protocol: EFS = 12.9 mo VAIA: EFS = 29.4 mo CEVAIE: EFS = 12 mo |
| Kushner et al., 1996 [50] | 1st and 2nd line | 12 | 14/7–22 | P6-protocol * | 83% PR | mPFS > 12 mo |
| Bond et al., 2008 [65] | 1st line and beyond | 10 | 16/3–29 | Imatinib Mesylate | 100% PD | NA |
| Tap et al., 2012 [66] | 1st and beyond | 16 | 33/19–63 | Ganitumab | 6% PR 63% SD | mPFS = 19 mo |
| Casanova et al., 2004 [67] | 2nd line | 1 | 17 | Vinorelbine/ Cyclophosphamide | PR | mPFS > 6 mo |
| Bisogno et al., 2006 [68] | 2nd line and beyond | 3 | 10.6/1–18.5 | Irinotecan | 100% PD | NA |
| Italiano et al., 2013 [69] | 2nd line and beyond | 8 | 23/14–58 | Sunitinib | 25% PR 37.5% SD 37.5% PD | mPFS > 4 mo |
| Verret, B. et al., 2017 [70] | 2nd line and beyond | 6 | 25/19–52 | Trabectedin | 33% SD 67% PD | mPFS = 3.2 mo mOS = 4 mo |
| Menegaz et al., 2018 [71] | 2nd line and beyond | 38 | 25/5–48 | Pazopanib | 3% CR 3% PR 55% SD 38% PD | mPFS = 5.6 mo mOS = 15.7 mo |
| Tarek et al., 2018 [72] | 3rd line and beyond | 5 | 15/11–28 | VCT | 80% PR 20% SD | mTTP = 8.5 mo |
| Thijs et al., 2010 [73] | 4th line | 1 | 21 | Temsirolimus | SD | PFS = 10 mo OS = 13 mo |
| Phase of Trial | Design | Primary Outcome | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Phase 1/2 | Ramucirumab IV + Cyclophosphamide p.o. + Vinorelbine IV (experimental arm), versus Cyclophosphamide p.o. + Vinorelbine IV | 1. Progression-fFree survival | NCT04145349 |
| Phase 1 | 2 cycles of the investigational combination irinotecan, temozolomide and bevacizumab, will be given followed by conventional chemotherapy with a modified P6 approach and surgical local control. Completion of modified P6 chemotherapy will be followed by a second-look surgery. | 1. Tolerability 2. Adverse event profile | NCT01189643 |
| Phase 2 | Experimental arm A: Single dose of IP RIT administered through an IP catheter with 131 I-omburtamab at 80 mCi/m2, followed by WA-IMRT approximately 2–4 weeks after completing IP RIT Experimental arm B: Single dose of IP RIT administered through an IP catheter with 131 I-omburtamab at 80 mCi/m2 Experimental arm C: Single dose of IP RIT administered through an IP catheter with 131 I-omburtamab at 80 mCi/m2 | 1. Progression-free survival | NCT04022213 |
| Phase 1/2 | Dose Escalation/Dose Expansion Study of Prexasertib in Combination with Irinotecan 15 mg/m2 IV daily × 10 days in 21 day cycles | 1. Recommended phase II does of Prexasertib 2. Response | NCT04095221 |
| Phase 2 | Nab-paclitaxel will be administered as follows: Age ≥ 21: 125 mg/m2 days 1, 8 and 15 in cycles of 28 days Age ≥ 6 months and ≤ 20 years: 240 mg/m2 (for patients weighing > 10 kg) and 11.5 mg/kg (for patients weighing ≤ 10 kg) on days 1, 8 and 15 in cycles of 28 days | 1. Overall response rate 2. Objective response rate | NCT03275818 |
| Phase 2 | Participants will receive vincristine, doxorubicin, cyclophosphamide, ifosfamide, etoposide, irinotecan, temozolomide, temsirolimus, bevacizumab, and sorafenib. Depending on the size and location of the participant’s tumor, they will have surgery alone, radiation alone or surgery followed by radiation. | Participants with DSRCT will not be included in the analysis of primary outcome | NCT01946529 |
| Phase 2 | Allogeneic Hematopoietic Stem Cell Transplantation | 1. Transplant-related mortality 2. Rate of grade III or higher organ toxicity attributable to conditioning | NCT04530487 |
| Phase 1 | Patients undergo cytoreduction and HIPEC over 60 min consisting of doxorubicin and cisplatin. Patients then receive sodium thiosulfate IV over 12 h. | 1. To assess the feasibility of HIPEC with doxorubicin and cisplatin after surgical resection. 2. To assess morbidity, hospital length of stay and peri-operative mortality outcome. | NCT04213794 |
| Phase 1 | Experimental arm A: participants will receive B7H3-specific CAR T cells only Experimental arm B: participants will receive CAR T cells directed at B7H3 and CD19 | 1. Safety and tolerability 2. Determine the MTD 3. Assess the DLT and describe the full toxicity profile 4. Assess the feasibility of manufacturing B7H3 and B7H3xCD19 specific CARs | NCT04483778 |
| Phase 1 | Experimental arm A: participants will receive EGFR-specific CAR T cells only. Experimental arm B: participants will receive CAR T cells directed at EGFR and CD19 | 1. Estimate the MTD and DLT 2. Assess the number of successfully manufactured EGFR806 and EGFR806xCD19 CAR T cell products 3. Safety | NCT03618381 |
| Phase 2 | Nivolumab 240 mg IV every 2 weeks plus Ipilimumab 1 mg/m2 IV every 6 weeks | 1. Response to therapy as evaluated by RECIST 1.1 | NCT02982486 |
| Phase 2 | Reduced-intensity chemotherapy, haploidentical bone marrow, post-transplant cyclophosphamide and shortened duration tacrolimus | 1. Safety | NCT01804634 |
| Phase 1 | CLR 131 intravenous administration | 1. Number of participants with DLT | NCT03478462 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mello, C.A.; Campos, F.A.B.; Santos, T.G.; Silva, M.L.G.; Torrezan, G.T.; Costa, F.D.; Formiga, M.N.; Nicolau, U.; Nascimento, A.G.; Silva, C.; et al. Desmoplastic Small Round Cell Tumor: A Review of Main Molecular Abnormalities and Emerging Therapy. Cancers 2021, 13, 498. https://doi.org/10.3390/cancers13030498
Mello CA, Campos FAB, Santos TG, Silva MLG, Torrezan GT, Costa FD, Formiga MN, Nicolau U, Nascimento AG, Silva C, et al. Desmoplastic Small Round Cell Tumor: A Review of Main Molecular Abnormalities and Emerging Therapy. Cancers. 2021; 13(3):498. https://doi.org/10.3390/cancers13030498
Chicago/Turabian StyleMello, Celso Abdon, Fernando Augusto Batista Campos, Tiago Goss Santos, Maria Leticia Gobo Silva, Giovana Tardin Torrezan, Felipe D’Almeida Costa, Maria Nirvana Formiga, Ulisses Nicolau, Antonio Geraldo Nascimento, Cassia Silva, and et al. 2021. "Desmoplastic Small Round Cell Tumor: A Review of Main Molecular Abnormalities and Emerging Therapy" Cancers 13, no. 3: 498. https://doi.org/10.3390/cancers13030498
APA StyleMello, C. A., Campos, F. A. B., Santos, T. G., Silva, M. L. G., Torrezan, G. T., Costa, F. D., Formiga, M. N., Nicolau, U., Nascimento, A. G., Silva, C., Curado, M. P., Nakagawa, S. A., Lopes, A., & Aguiar, S., Jr. (2021). Desmoplastic Small Round Cell Tumor: A Review of Main Molecular Abnormalities and Emerging Therapy. Cancers, 13(3), 498. https://doi.org/10.3390/cancers13030498





