TP53 Mutations as a Driver of Metastasis Signaling in Advanced Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Primary and Metastatic NGS Profiling of Advanced Cancer Patients
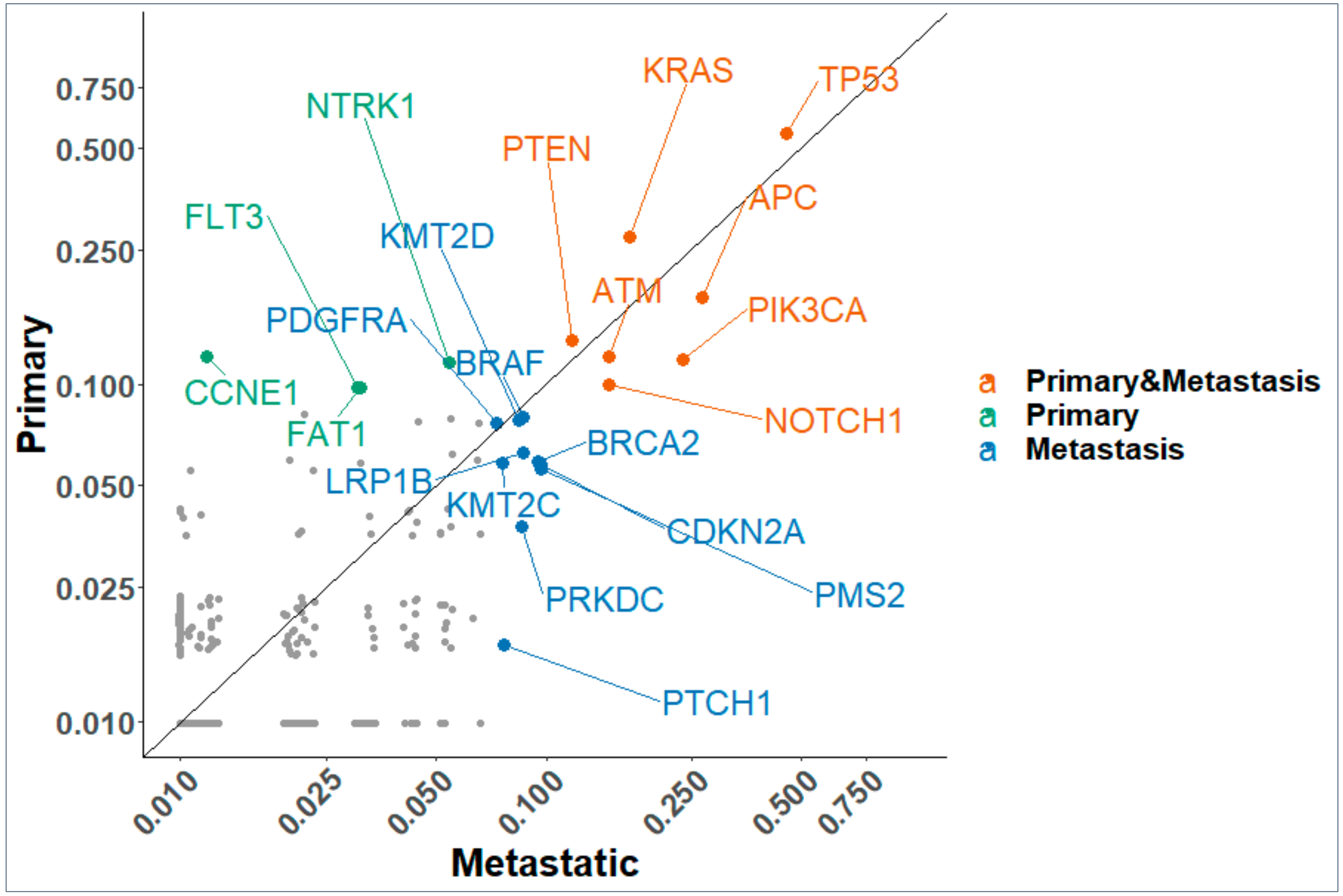
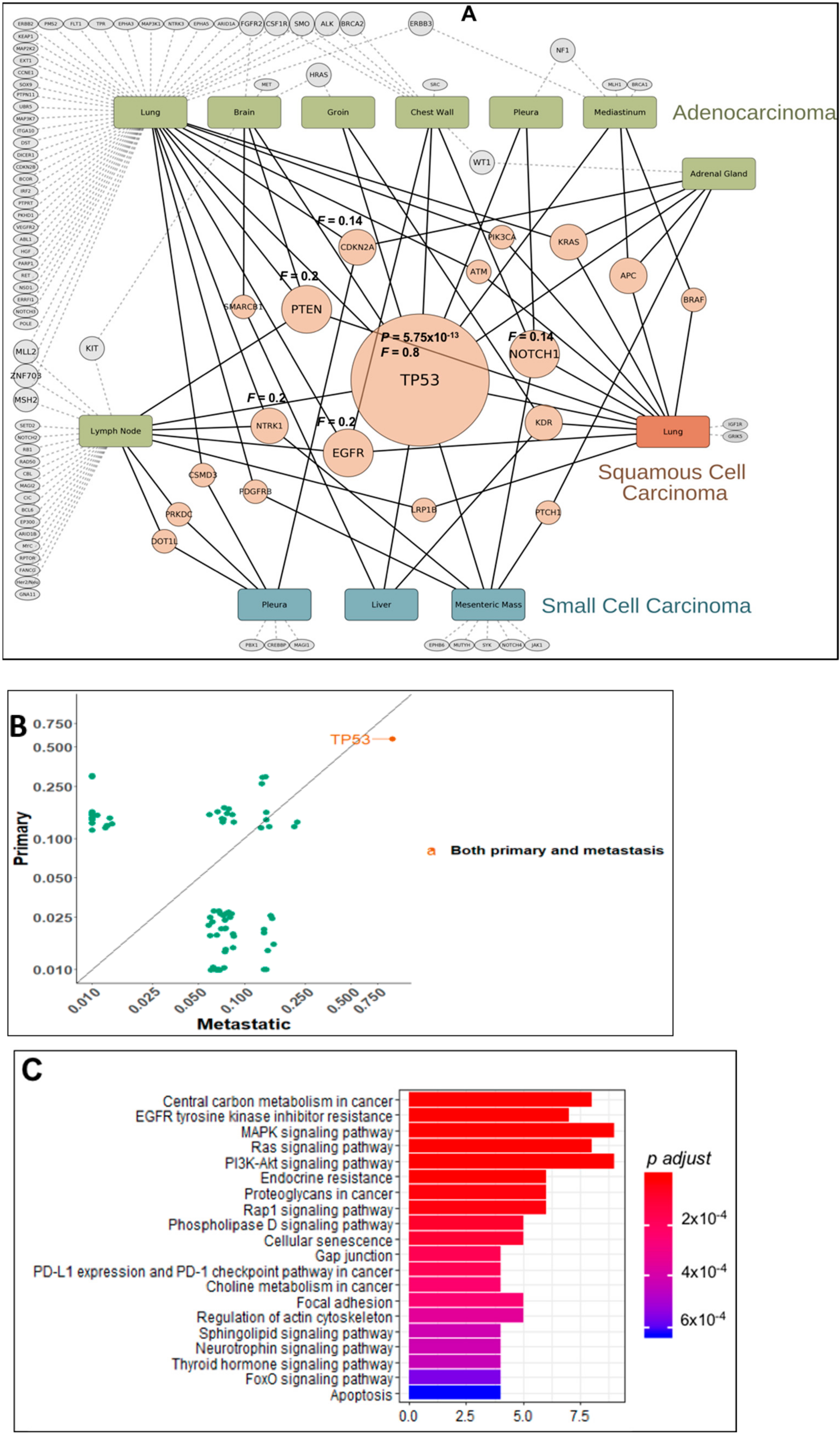
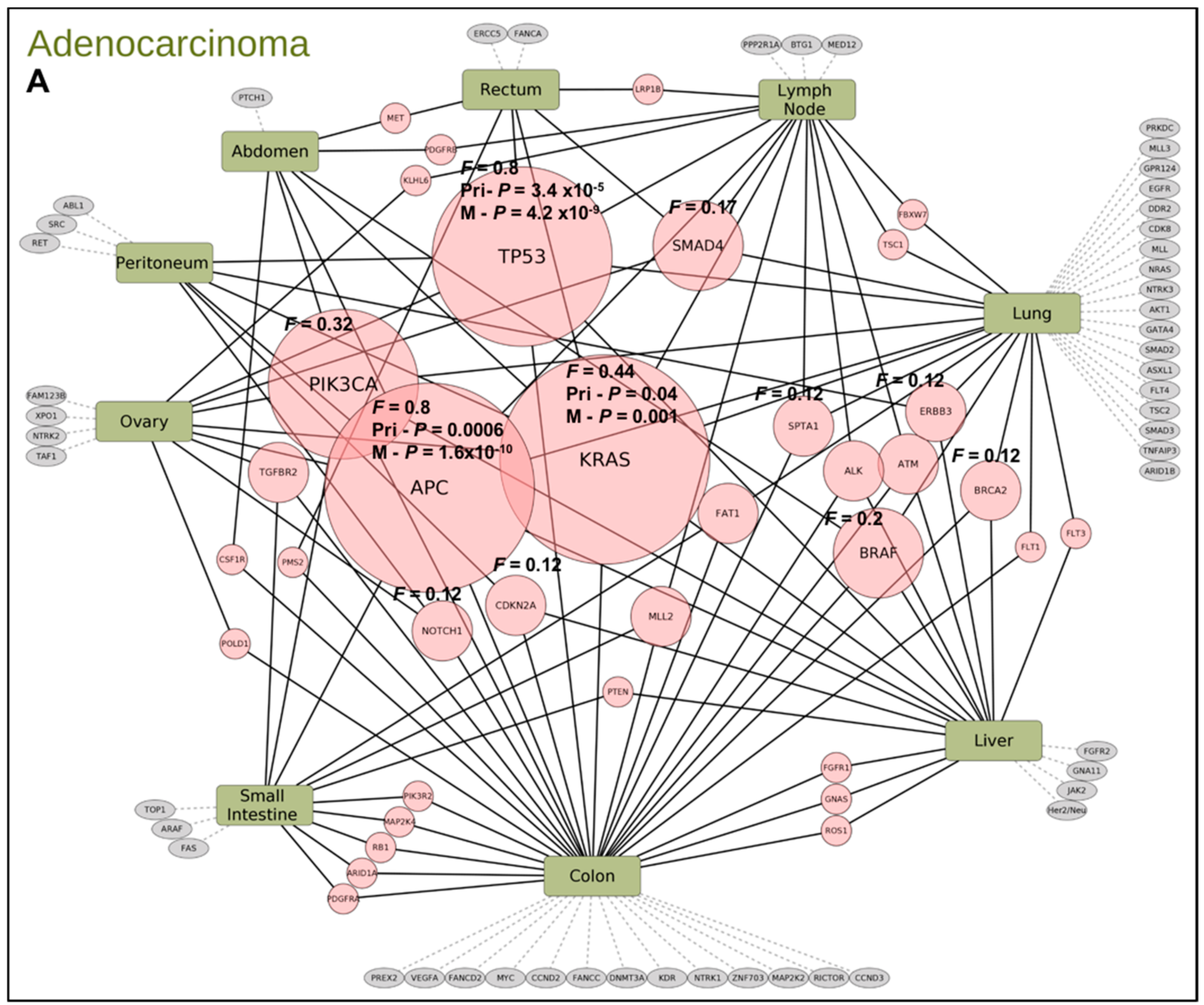
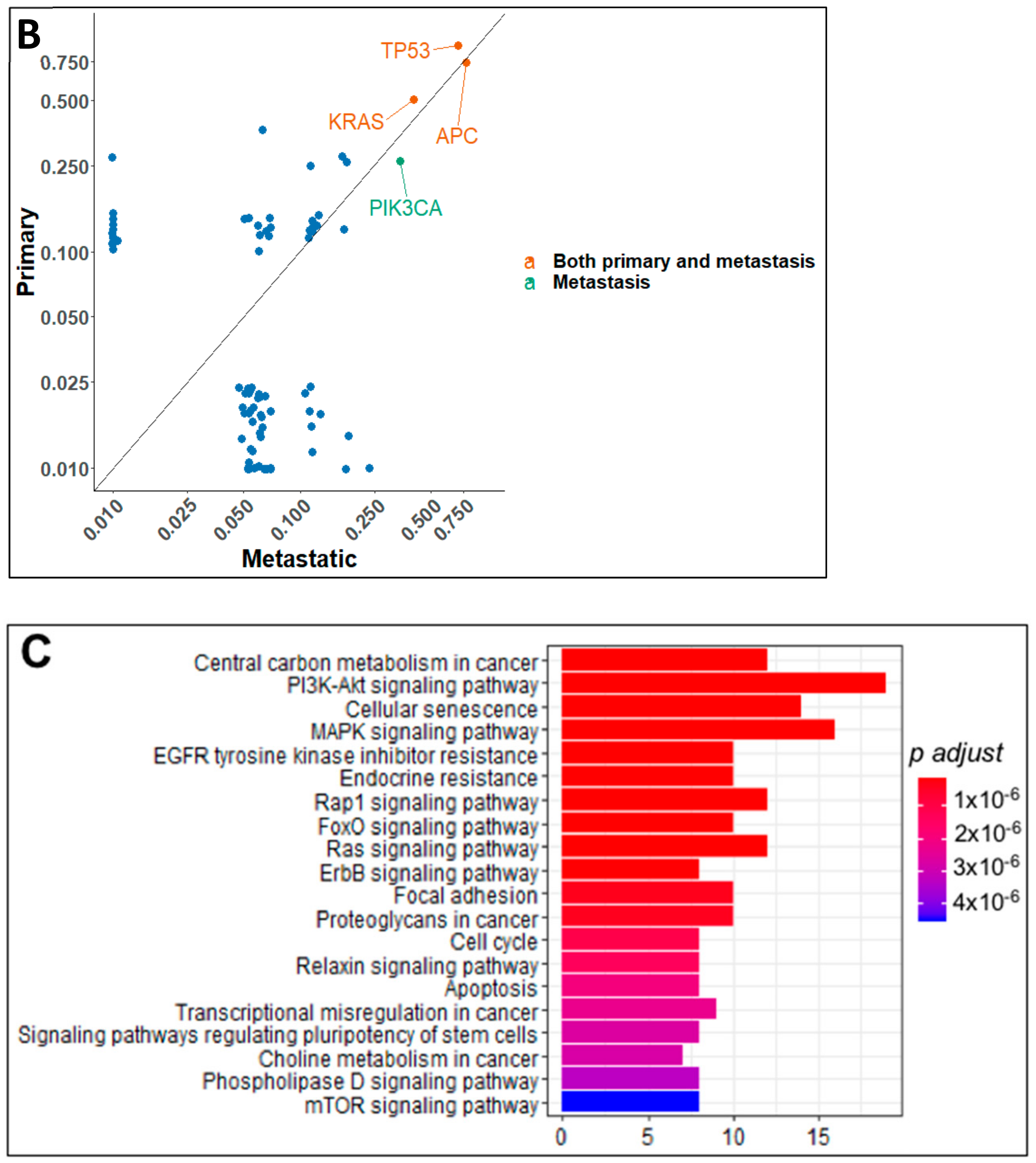
2.2. Gene Mutation Networks in Lung and Colon Cancer
2.3. Distribution of TP53 Mutations among Tumor Types
2.4. Pre- and Post-Targeted Therapy Response
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. Profiling Platforms
4.3. Mutation Analysis in Primary and Metastatic Tissue Samples
4.4. Gene Mutation Pathways and Protein Interaction
4.5. Three-Dimensional Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The International Cancer Genome Consortium. International network of cancer genome projects. Nature 2010, 464, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Garraway, L.A.; Lander, E.S. Lessons from the cancer genome. Cell 2013, 153, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R. Journeys into the genome of cancer cells. EMBO Mol. Med. 2013, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Horswell, S.; Larkin, J.; Rowan, A.J.; Salm, M.P.; Varela, I.; Fisher, R.; McGranahan, N.; Matthews, N.; Santos, C.R.; et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 2014, 46, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C. Cancer evolution: The final frontier of precision medicine? Ann. Oncol. 2014, 25, 549–551. [Google Scholar] [CrossRef] [PubMed]
- NCT01888601. Available online: http://clinicaltrials.gov/show/NCT01888601 (accessed on 1 December 2020).
- Cancer Research UK News. Available online: http://www.cancerresearchuk.org/about-us/cancer-news/press-release/new-research-to-revolutionise-understanding-of-lung-cancer (accessed on 18 July 2013).
- Larson, K.; Kannaiyan, R.; Pandey, R.; Chen, Y.; Babiker, H.M.; Mahadevan, D. A Comparative Analysis of Tumors and Plasma Circulating Tumor DNA in 145 Advanced Cancer Patients Annotated by 3 Core Cellular Processes. Cancers 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- MacConaill, L.E. Existing and emerging technologies for tumor genomic profiling. J. Clin. Oncol. 2013, 31, 1815–1824. [Google Scholar] [CrossRef]
- Schott, A.F.; Perou, C.M.; Hayes, D.F. Genome Medicine in Cancer: What’s in a name? Cancer Res. 2015, 75, 1930–1935. [Google Scholar] [CrossRef]
- Conley, B.A.; Doroshow, J.H. Molecular analysis of therapy choice: NCI MATCH. Semin. Oncol. 2014, 41, 297–299. [Google Scholar] [CrossRef]
- NCI-MPACT: Molecular Profiling-Based Assignment of Cancer Therapy for Patients with Advanced Tumors. 2014. Available online: http://clinicaltrials.gov/show/NCT01827384 (accessed on 1 December 2020).
- Schilsky, R.L. Implementing personalized cancer care. Nat. Rev. Clin. Oncol. 2014, 11, 432–438. [Google Scholar] [CrossRef]
- Garraway, L.A. Genomics-driven oncology: Framework for an emerging paradigm. J. Clin. Oncol. 2013, 31, 1806–1814. [Google Scholar] [CrossRef]
- Heuckmann, J.M.; Thomas, R.K. A new generation of cancer genome diagnostics for routine clinical use: Overcoming the roadblocks to personalized cancer medicine. Ann. Oncol. 2015, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Birkbak, N.J.; McGranahan, N. Cancer Genome Evolutionary Trajectories in Metastasis. Cancer Cell. 2020, 37, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.C.; Dou, Y. Hijacked in cancer: The KMT2 (MLL) family of methyltransferases. Nat. Rev. Cancer 2015, 15, 334–346. [Google Scholar] [CrossRef]
- Khan, Z.A.; Jonas, S.K.; Le-Marer, N.; Patel, H.; Wharton, R.Q.; Tarragona, A.; Ivison, A.; Allen-Mersh, T.G. P53 mutations in primary and metastatic tumors and circulating tumor cells from colorectal carcinoma patients. Clin. Cancer Res. 2000, 6, 3499–3504. [Google Scholar]
- Kitayner, M.; Rozenberg, H.; Rohs, R.; Suad, O.; Rabinovich, D.; Honig, B.; Shakked, Z. Diversity in DNA recognition by p53 revealed by crystal structures with Hoogsteen base pairs. Nat. Struct. Mol. Biol. 2010, 17, 423–429. [Google Scholar] [CrossRef]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef]
- Muller, P.A.J.; Vousden, K.H. Mutant p53 in Cancer: New Functions and Treatment Opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Giri, S.; Patel, J.K.; Mahadevan, D. Novel mutations in a patient with ALK-rearranged lung cancer. N. Engl. J. Med. 2014, 371, 1655–1656. [Google Scholar] [CrossRef]
- Mazumder, S.; DuPree, E.L.; Almasan, A. A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy. Curr. Cancer Drug Targets 2004, 4, 65–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, R.; Wilkerson, P. From integrative genomics to therapeutic targets. Cancer Res. 2013, 73, 3483–3488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, H.; Dhroso, A.; Johnson, N.; Korkin, D. The variation game: Cracking complex genetic disorders with NGS and omics data. Methods 2015, 79–80, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Bykov, V.J.; Wiman, K.G. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett. 2014, 588, 2622–2627. [Google Scholar] [CrossRef]
- Canon, J.; Osgood, T.; Olson, S.H.; Saiki, A.Y.; Robertson, R.; Yu, D.; Eksterowicz, J.; Ye, Q.; Jin, L.; Chen, A.; et al. The MDM2 Inhibitor AMG 232 Demonstrates Robust Antitumor Efficacy and Potentiates the Activity of p53-Inducing Cytotoxic Agents. Mol. Cancer Ther. 2015, 14, 649–658. [Google Scholar] [CrossRef]
- Senisterra, G.; Wu, H.; Allali-Hassani, A.; Wasney, G.A.; Barsyte-Lovejoy, D.; Dombrovski, L.; Dong, A.; Nguyen, K.T.; Smil, D.; Bolshan, Y.; et al. Small-molecule inhibition of MLL activity by disruption of its interaction with WDR5. Biochem. J. 2013, 449, 151–159. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]


| Primary | Number of Patients | Histology | Number of Mutated Genes | Mutated Gene ID′s |
|---|---|---|---|---|
| Anus | 2 | Squamous Cell Carcinoma | 27 | APC, ARID2, ASXL1, ATRX, CCND1, CDKN1B, CDKN2A, EPHA5, ERBB2, ERBB4, FANCA, FGF19, FGF4, FLT1, INHBA, MED12, KMT2D, MYCN, NKX2-1, PALB2, PIK3CA, POLE, RARA, RB1, SMARCA4, TERT, TSHR |
| Appendix | 2 | Adenocarcinoma | 13 | AR, CDK12, FGFR1, FLT3, GATA6, GNAS, KRAS, KMT2A, KMT2C, NOTCH1, PRKDC, SMAD4, SPEN |
| - | 1 | Mucinous Adenocarcinoma | 1 | KRAS |
| Bladder | 2 | Transitional Cell Carcinoma | 24 | ATM, CSMD3, EP400, EPHB4, FGFR3, FN1, LTF, MAF, KMT2A, MSH6, MTR, MYH9, NUMA1, PDE4DIP, PDGFB, PDGFRa, PIK3C2B, PIK3CA, PRKAR1A, PTEN, SF3B1, THBS1, TP53, WRN |
| Brain | 1 | Astrocytoma | 2 | IDH1, TP53 |
| - | 3 | Glioblastoma Multiforme | 16 | AKT3, BRAF, EGFR, EGFRvIII, ERBB2, ETV4, FLT3, KRAS, NF1, NOTCH2, PTEN, SMAD2, SMO, TNFAIP3, TRRAP, UBR5 |
| - | 1 | Medulloblastoma | 10 | BCOR, CARD11, FAM123B, GNAS, LZTR1, KMT2D, NRAS, RARA, SMO, TERT |
| - | 1 | Meningioma | 5 | FAT1, FGF19, LRP1B, KMT2D, NF2 |
| Breast | 17 | Adenocarcinoma | 79 | ABL1, ABL2, AKT1, AR, ARFRP1, ARID1A, ASXL1, ATM, BARD1, BCL2L2, BCL9, BRCA2, CCNE1, CDH1, CDK12, CDKN2A, CHD4, CHEK2, CIC, CREBBP, CSMD3, DAXX, DDR2, DST, EGFR, EPHA3, ERBB4, ESR1, FAM123B, FAT1, FGFR1, FGFR3, FH, FLT1, FLT3, GATA3, GRIN2A, HSP90AB1, Her2/Neu, IDH2, JAK1, JAK2, JAK3, KDM6A, KIT, KRAS, MAP2K4, MAPK8, MST1R, MYCL1, MYST3, NF1, NFKB1, NOTCH1, PARP1, PBRM1, PDGFRA, PDGFRB, PDGFRa, PIK3CA, PIK3R2, PMS2, PRKCI, PTCH1, PTEN, PTPN11, PTPRD, RET, RPS6KA2, RUNX1, SDHA, SGK1, TGM7, TLR4, TNK2, TOP2A, TP53, TPR, TSC1 |
| CUP | 8 | Carcinoma | 90 | ABL2, ACVR2A, AFF1, AKT2, AKT3, APC, AR, ARFRP1, ARID1A, ARID1B, ASXL1, ATM, ATR, ATRX, AXL, BCORL1, BCR, BRCA2, BTK, CCND2, CCNE1, CDH5, CDK12, CDK8, CDKN1B, CHD2, CHD4, CREBBP, CSMD3, CTNNB1, DNMT3A, EPHA3, EPHB1, ERCC1, EZH2, FANCA, FANCE, FAT1, FGF23, FGF6, FGFR2, GATA2, HGF, IL7R, IRS2, KDM5A, KEAP1, KEL, KRAS, LRP1B, MAP2K2, MAP3K1, MDM4, MET, KMT2C, MRE11A, MSH2, MSH6, MUTYH, MYCL1, MYST3, NOTCH1, NOTCH2, NTRK1, NTRK3, PAK3, PDCD1LG2, PIK3CG, PIK3R2, POLD1, PPP2R1A, PRKDC, PTCH1, PTEN, PTGS2, RALGDS, RANBP2, ROS1, RPTOR, SETD2, SMARCA4, SMARCB1, SNCAIP, STK36, SYK, TCF7L2, TP53, TSC2, ZNF217, ZNF703 |
| Cervix | 1 | Adenocarcinoma | 5 | APC, PIK3CA, PTEN, RB1, TP53 |
| - | 6 | Squamous Cell Carcinoma | 45 | ABL2, AKT1, ARID1A, ATM, ATRX, AURKB, BCORL1, CASP8, CCNE1, CDH5, CEBPA, CHEK2, CIC, CYLD, EPHA5, FGF23, FLCN, FLT3, GDNF, IDH1, IGF1R, LRP1B, MAP2K4, MAPK8, MED12, KMT2C, MYC, MYH9, MYST3, NOTCH1, NOTCH2, PALB2, PIK3CA, PIK3CG, PIK3R1, PMS2, PRKDC, RAD51, RANBP2, ROS1, SOCS1, TAL1, TCF7L1, TET2, TOP2A |
| Colon | 28 | Adenocarcinoma | 87 | ABL1, AKT1, ALK, APC, ARAF, ARID1A, ARID1B, ASXL1, ATM, BRAF, BRCA2, BTG1, CCND2, CCND3, CDK8, CDKN2A, CSF1R, DDR2, DNMT3A, EGFR, ERBB3, ERCC5, FAM123B, FANCA, FANCC, FANCD2, FAS, FAT1, FBXW7, FGFR1, FGFR2, FLT1, FLT3, FLT4, GATA4, GNA11, GNAS, GPR124, Her2/Neu, JAK2, KDR, KLHL6, KRAS, LRP1B, MAP2K2, MAP2K4, MED12, MET, KMT2A, KMT2D, KMT2C, MYC, NOTCH1, NRAS, NTRK1, NTRK2, NTRK3, PDGFRA, PDGFRB, PIK3CA, PIK3R2, PMS2, POLD1, PPP2R1A, PREX2, PRKDC, PTCH1, PTEN, RB1, RET, RICTOR, ROS1, SMAD2, SMAD3, SMAD4, SPTA1, SRC, TAF1, TGFBR2, TNFAIP3, TOP1, TP53, TSC1, TSC2, VEGFA, XPO1, ZNF703 |
| Endometrium | 6 | Adenocarcinoma | 24 | ABL1, ABL2, APC, AURKA, AURKB, BRCA1, CYLD, EPHA5, ERBB3, ERCC1, FGFR2, IDH1, JAK3, MAP2K4, NF1, NOTCH1, NRAS, NTRK1, PIK3CA, PTCH1, PTEN, TGM7, TP53, TSC2 |
| Esophagus | 3 | Adenocarcinoma | 54 | ACVR1B, ASXL1, ATM, ATR, AXIN1, BCL2L1, BRIP1, CARD11, CCND2, CCND3, CCNE1, CDKN2C, CEBPA, CREBBP, CTNNB1, DST, EGFR, EP300, EPHA3, FANCC, FANCL, FH, FLT1, GATA4, GATA6, GLI1, IKZF1, INHBA, JAK1, KDM5C, KEAP1, KRAS, LRP1B, MAGI1, KMT2C, MTOR, MYC, NKX2-1, NTRK3, PMS2, RNF43, RUNX1, RUNX1T1, SLIT2, SMARCA4, SPEN, STAG2, TAF1, TOP1, TP53, TSHR, VEGFA, WT1, XPO1 |
| Head & Neck | 1 | Carcinoma | 3 | ATM, BRCA2, CDKN2A |
| - | 1 | Mucoepidermoid Carcinoma | 11 | CDKN2A, CDKN2B, CJD2, CREBBP, EWSR1, KDM6A, KMT2D, NOTCH1, NOTCH3, SPTA1, TBX3 |
| - | 2 | Squamous Cell Carcinoma | 8 | BRCA2, CDK4, FLT3, KDR, MET, MSH2, NOTCH2, TP53 |
| Kidney | 1 | Clear Cell Adenocarcinoma | 3 | BRAF, MEK2, NF1 |
| - | 4 | Clear Cell Carcinoma | 24 | ACVR1B, ATM, AXL, CD79A, CDK4, EPHA3, EPHA5, FGFR1, FRS2, GLI1, HSP90AA1, KEAP1, MDM2, MSH2, PBRM1, PIK3CA, PMS2, POT1, PRKDC, SETD2, SMARCA4, SPEN, TET2, VHL |
| Liver | 1 | Adenocarcinoma | 4 | CDKN2A, Her2/Neu, KRAS, TP53 |
| - | 1 | Cholangiocarcinoma | 15 | APC, ATR, CDH2, CDKN2A, CSF1R, ERCC1, EZH2, FANCD2, FLT1, IL21R, KIT, NOTCH2, NOTCH4, SRC, XPC |
| Lung | 20 | Adenocarcinoma | 85 | ABL1, ALK, APC, ARID1A, ARID1B, ATM, BCL6, BCOR, BRAF, BRCA1, BRCA2, CBL, CCNE1, CDKN2A, CDKN2B, CIC, CSF1R, CSMD3, DICER1, DOT1L, DST, EGFR, EP300, EPHA3, EPHA5, ERBB2, ERBB3, ERRFI1, EXT1, FANCG, FGFR2, FLT1, GNA11, HGF, HRAS, Her2/Neu, IRF2, ITGA10, KDR, KEAP1, KIT, KRAS, LRP1B, MAGI2, MAP2K2, MAP3K1, MAP3K7, MET, MLH1, KMT2D, MSH2, MYC, NF1, NOTCH1, NOTCH2, NOTCH3, NSD1, NTRK1, NTRK3, PARP1, PDGFRB, PIK3CA, PKHD1, PMS2, POLE, PRKDC, PTCH1, PTEN, PTPN11, PTPRT, RAD50, RB1, RET, RPTOR, SETD2, SMARCB1, SMO, SOX9, SRC, TP53, TPR, UBR5, VEGFR2, WT1, ZNF703 |
| - | 1 | Neuroendocrine Carcinoma | 7 | CDKN2A, CREBBP, CSMD3, DOT1L, MAGI1, PBX1, PRKDC |
| - | 2 | Small Cell Carcinoma | 12 | EPHB6, JAK1, KDR, MUTYH, NOTCH1, NOTCH4, NTRK1, PDGFRB, PTCH1, SMARCB1, SYK, TP53 |
| - | 3 | Squamous Cell Carcinoma | 13 | APC, ATM, BRAF, EGFR, GRIK5, IGF1R, KDR, KRAS, LRP1B, NOTCH1, PIK3CA, PTEN, TP53 |
| Lymph Node | 1 | Diffuse Large B-Cell Lymphoma | 9 | ABL2, AFF1, DNMT3A, EZH2, PIK3R2, PRKDC, PTGS2, STK36, TP53 |
| - | 1 | Mantle Cell Lymphoma | 6 | ATM, NOTCH2, PTPRT, RPS6KA2, TP53, TSC2 |
| Not Specified | 1 | Adenocarcinoma | 1 | PDGFRA |
| - | 1 | Diffuse Large B-Cell Lymphoma | 15 | BCL2, CBL, DUSP2, GNA13, HIST1H1D, IGH, KDM4C, MAP3K1, KMT2D, MYC, PIM1, PLCG2, RAD50, RB1, TNGRSF14 |
| - | 2 | Melanoma | 17 | APC, BRCA2, CCND2, CSF1R, EPHA5, GNA13, IDH1, LRP1B, MAP3K1, KMT2C, MYC, NRAS, PMS2, PRKDC, PTEN, TSC1, TSHR |
| - | 1 | Sarcoma | 22 | APC, ARHGAP26, ATRX, C17orf39, CREBBP, ELP2, EP300, FANCG, FANCL, FBXW7, FGF10, GNA13, IL7R, KMT2A, KMT2D, NKX2-1, RB1, RICTOR, SDHB, SMARCA4, TP53, ZNF24 |
| - | 1 | Squamous Cell Carcinoma | 8 | ALK, ATM, BRAF, BRCA1, JAK2, NOTCH1, NTRK1, TP53 |
| Ovary | 14 | Adenocarcinoma | 51 | ABL1, ALK, APC, ARAF, ARID1A, ARID1B, ATM, ATRX, AXIN1, BAP1, BARD1, BCR, BLM, BRAF, BRCA1, BRIP1, C11orf30, CCNE1, CDH2, CRKL, CSF1R, CTNNB1, DNMT3A, EP300, ERBB2, ERBB3, ERBB4, FANCD2, FBXW7, FLT4, HRAS, KDM5A, KEAP1, KRAS, LRP1B, MAML2, NOTCH1, NOTCH2, NOTCH4, PALB2, PAX8, PDGFRA, PDGFRB, PMS2, PTEN, RET, SDHA, SLIT2, TAF1, TBX3, TP53 |
| Pancreas | 8 | Adenocarcinoma | 35 | AKT2, APC, ARID1B, BARD1, BRAF, BRCA1, CDH2, CDK6, CDKN2A, CDKN2B, CTNNB1, ERCC4, FBX27, GNAS, HGF, HNF1A, HRAS, KRAS, MEK2, MET, MUTYH, MYST3, PALB2, PDGFRA, PIK3CA, PIK3R3, PMS1, PMS2, RET, RICTOR, ROS1, SMO, STK11, TP53, TSC1 |
| Peritoneum | 1 | Carcinoma | 2 | EGFR, TP53 |
| Pharynx | 2 | Squamous Cell Carcinoma | 8 | CHEK2, EPHA7, KDR, MET, PALB2, RAF1, TP53, TSC1 |
| Pleura | 1 | Mesothelioma | 6 | BAP1, FOXL2, MYCN, NF2, POLD1, SETD2 |
| Prostate | 5 | Adenocarcinoma | 49 | ABL1, APC, ARID1A, ARID1B, BCL2L2, BCL6, BRCA2, CDKN1B, CIC, ERBB4, FANCC, FAS, FGF6, FGFR2, FGFR3, FLT1, FLT4, GABRA6, GNAS, IDH2, IRF2, LRP1B, LYN, MAGI2, MAP2K4, MAP3K1, KMT2D, KMT2C, NF1, NIN, NTRK1, PIK3C2B, PIK3CA, POLD1, POLE, PRDM1, PREX2, PTCH1, PTEN, RET, RUNX1T1, SDHD, SETD2, SMAD3, TAF1, TCF7L1, TMPRSS2, TP53, ZNF217 |
| Rectum | 2 | Adenocarcinoma | 20 | APC, ATM, ATRX, FAT1, FGF23, GPR124, IDH1, KLHL6, KRAS, KMT2D, KMT2C, MYST3, NRAS, PDGFRA, PIK3CA, PRKDC, PTEN, RANBP2, SMAD4, ZNF703 |
| - | 1 | Melanoma | 6 | DAXX, FANCA, NRAS, PMS2, SUFU, TRRAP |
| - | 1 | Squamous Cell Carcinoma | 1 | PIK3CA |
| Skin | 3 | Melanoma | 26 | ALK, ARID1A, ATM, ATR, BCL2, BRAF, BRCA2, CARD11, CYLD, DDR2, DNMT3A, FLT1, GNAS, IDH1, INPP4B, MAGI2, KMT2A, KMT2C, NRAS, PDK1, PRKCI, RPTOR, SOX10, SPTA1, TERT, TET2 |
| - | 1 | Sarcoma | 2 | FGFR1, NOTCH1 |
| Soft Tissue | 1 | Sarcoma | 23 | CCND2, CD36, CDKN2A, CDKN2B, DOT1L, EP300, FANCA, FANCE, FGFR2, GNA12, KIT, LRP1B, MKI67, KMT2A, KMT2C, PRKDC, RAD21, RUNX1T1, TCF3, TP53, TRAF5, TSC2, WDR90 |
| Uterus | 3 | Adenocarcinoma | 38 | APC, ATR, BCL11A, CATA6, CBL, CDKN2A, DOT1L, ERBB4, FANCD2, FANCF, FAT1, FBXW7, FGF19, FLT4, FRS2, GATA6, IGF1R, KDM5C, KDM6A, KRAS, LRP1B, MAP2K4, MED12, KMT2C, MST1R, MTOR, PDGFRa, PIK3CA, PMS2, PRDM1, PRKDC, PTCH1, RANBP2, RB1, RICTOR, RPTOR, SRC, TP53 |
| - | 1 | Leiomyosarcoma | 4 | EPHB6, GID4, TET2, TP53 |
| Primary | Histology | Number of Patients | Number of Patients with mutTP53 | Fraction Patients with mutTP53 |
|---|---|---|---|---|
| Breast | Adenocarcinoma | 17 | 7 | 0.41 |
| Colon | Adenocarcinoma | 28 | 21 | 0.75 |
| CUP | Carcinoma | 8 | 5 | 0.63 |
| Endometrium | Adenocarcinoma | 6 | 2 | 0.33 |
| Lung | Adenocarcinoma | 20 | 16 | 0.80 |
| Ovary | Adenocarcinoma | 14 | 7 | 0.50 |
| Pancreas | Adenocarcinoma | 8 | 6 | 0.75 |
| Protein Mutation | Exon/Intron | Residue Function | Domain Function | Structural Motif |
|---|---|---|---|---|
| P47S | 4 exon | na | Transactivation | N-terminal Transactivation |
| P58R | 4 exon | na | na | N-term |
| Q100 * | 4 exon | na | na | N-term |
| K132M | 5 exon | Buried | DNA binding | L1/S/H2 |
| C141Y | 5 exon | Buried | DNA binding | NDBL/beta-sheets |
| V143M | 5 exon | Buried | DNA binding | NDBL/beta-sheets |
| Y163N | 5 exon | Buried | DNA binding | NDBL/beta-sheets |
| Y163C | 5 exon | Buried | DNA binding | NDBL/beta-sheets |
| R174W | 5 exon | Partially exposed | DNA binding | L2/L3 |
| H179L | 5 exon | Zn binding | DNA bindin | L2/L3 |
| R181P | 5 exon | Exposed | DNA binding | L2/L3 |
| H193R | 6 exon | Buried | DNA binding | L2/L3 |
| R196G | 6 exon | Buried | DNA binding | NDBL/beta-sheets |
| S215I | 6 exon | Buried | DNA binding | NDBL/beta-sheets |
| Y220C | 6 exon | Buried | DNA binding | NDBL/beta-sheets |
| M237I | 7 exon | Buried | DNA binding | L2/L3 |
| M237I | 7 exon | Buried | DNA binding | L2/L3 |
| M237I | 7 exon | Buried | DNA binding | L2/L3 |
| R248W | 7 exon | DNA binding | DNA binding | L2/L3 |
| R248W | 7 exon | DNA binding | DNA binding | L2/L3 |
| E258G | 7 exon | Buried | DNA binding | NDBL/beta-sheets |
| R273L | 8 exon | DNA binding | DNA binding | L1/S/H2 |
| P278A | 8 exon | Buried | DNA binding | L1/S/H2 |
| R283P | 8 exon | DNA binding | DNA binding | L1/S/H2 |
| Protein Mutation | Exon/Intron | Residue Function | Domain Function | Structural Motif |
|---|---|---|---|---|
| C141Y | 5 exon | Buried | DNA binding | NDBL/beta-sheets |
| R175H | 5 exon | Buried | DNA binding | L2/L3 |
| R213L | 6 exon | Buried | DNA binding | NDBL/beta-sheets |
| I232S | 7 exon | Buried | DNA binding | NDBL/beta-sheets |
| M237K | 7 exon | Buried | DNA binding | L2/L3 |
| C238S | 7 exon | Zn binding | DNA binding | L2/L3 |
| C238S | 7 exon | Zn binding | DNA binding | L2/L3 |
| G244S | 7 exon | Exposed | DNA binding | L2/L3 |
| G245S | 7 exon | Buried | DNA binding | L2/L3 |
| R248Q | 7 exon | DNA binding | DNA binding | L2/L3 |
| R248Q | 7 exon | DNA binding | DNA binding | L2/L3 |
| R273C | 8 exon | DNA binding | DNA binding | L1/S/H2 |
| R273H | 8 exon | DNA binding | DNA binding | L1/S/H2 |
| E286K | 8 exon | Partially exposed | DNA binding | L1/S/H2 |
| E286K | 8 exon | Partially exposed | DNA binding | L1/S/H2 |
| E286G | 8 exon | Partially exposed | DNA binding | L1/S/H2 |
| E286G | 8 exon | Partially exposed | DNA binding | L1/S/H2 |
| Index Case | Prior Therapy | Targeted or Immunotherapy | Site of Biopsy | Mutational Signature | Pathway Analysis | Clinical Decisions |
|---|---|---|---|---|---|---|
| 64 y F with KRAS WT metastatic rectal adenoCA | FOLFIRI/Avastin; Panitumumab; FOLFOX/Avastin | CDK4/6 Inhibitor | B/L pulmonary metastasis → adenoCA of rectal origin | NRAS, FLT3, KMT2A, TP53, CDK8, BRCA2, DDR2, EGFR, FLT1, GPR124, KMT2C, PRKDC, SMAD4, SPTA1 | EGFR, HER2 & SMAD2/SMAD3: SMAD4 signaling Double-strand break repair | 1. NRAS mutation explains lack of response to EGFR therapy 2. FLT3 mutation → Regorafenib 3. PARP inhibitor or CDK8 inhibitor |
| 64 y F with metastatic uterine adenoCA | Carboplatin Taxol Doxil | PI3K inhibitor CDK4/6 Inhibitor | Uterus | ERBB2, BFBXW7, FLT3, NF1, PIK3CA, PTEN, TSC1, DNMT3A, SMARCB1, TET2, ARID1A, ESR1, MDM4, MSH6, ATRX, FGF3, RAD51 | HER2, PI3K/AKT & PI3K events in ERRB4 signaling PIP3 activates AKT signaling DNA Repair Aberrations | 1. ERBB2 mutation identified however IHC was negative and HER2 not amplified → deferred monoclonal antibody therapy 2. PI3K is as an active pathway 3. FLT3 mutation → off label Sorafenib recommended 4. Epigenetic therapy with PARP inhibitor or Aurora kinase inhibitor |
| 66 y F with hx of early stage breast cancer develops L supraclavicular LAD biopsy proven -Neuroendocrine Carcinoma, unknown primary | Carboplatin & Etoposide | CDK4/6 Inhibitor | Diffuse LAD | NTRK3, PTEN, TCF7L2, SMARCA4, AKT3, CCNE1, ERCC1, FANCE | FGFR, BCR, PI3K, ERBB2 and ERBB4 signaling Negative regulation of the PI3K/AKT network Active Cell Cycle | 1. Due to CCNE1 mutation → CDK4/6 inhibitor trial. Stable disease at C11. 2. PI3K pathway is active suggesting next therapy if patient progresses |
| 69 y F with stage IV (T4N0M1b) NSCLC | Carboplatin & Pemetrexed Anti PD-L1 | CKD4/6 inhibitor + anti-VEGFR2 | Lung Nodule | KRAS, TP53, CDKN2A, BRCA2, [cMET, EGFR, PD-1+, PD-LI-] IHC. | EGFR, ERBB2 & FGFR signaling Oncogene induced senescence Immune Checkpoint | 1. CDKN2A mutation → CDK4/6 inhibitor trial + anti-VEGFR2 2. Immune checkpoint therapy |
| 52 y F with metastatic EML4-ALK NSCLC | Crizotinib; Crizotinib + HSP90 Inhibitor ChemoRT to the R hilum | Crizotinib | L supraclavicular node | Persistent ALK + by IHC & FISH. No ALK mutation within EML4-ALK translocation; PD-1 and PD-L1 negative, BRCA2, FGFR1, NOTCH1 | FGFR and FGFR1 ligand binding, activation & signaling Receptor-ligand binding initiates second proteolytic cleavage of NOTCH receptor Double-strand break repair | 1. Continue Crizotinib as there is no new mutation acquired in the ALK domain. 2. Investigate FGFR1 mutation as an active driver of potential clinical relevance and laboratory focus. |
| 60 y M with metastatic squamous cell carcinoma of the lung | ChemoRT | Anti-PDL1 antibody | Lung | PD-1+, APC PTCH1, c-MET; TL3, TOPO1; TUBB3 | Beta-catenin phosphorylation cascadeTruncated APC mutants & deletions of AMER1 destabilize the destruction complex | 1. AMER1 mutation, a tumor suppressor gene resulting over-activity of the Wnt signaling pathway. 2. Immune checkpoint |
| 61 y F with metastatic lung adenocarcinoma with EGFR exon 19 deletion and HER2 amplification by CISH/IHC | Tarceva Afatinib | Monoclonal antibody to HER2 | Lung Pleural fluid | Pre-targeted therapy: EGFR Exon 19 Deletion (L747_S752 del) ERBB2 amplification (FISH/CISH 6.4) PIK3CA TOPO2A TP53 KEL intron 3 Rearrangement Post-targeted therapy: Loss of ERBB2 amplification by FISH and IHC EGFR Exon 19 deletion Loss of PIK3CA FLT3 (V194M) TP53 TOPO2A PD-1 negative PD-L1 negative | EGFR, ERBB2, FGFR & PI3K Signaling PI3K/AKT activation G1/S DNA Damage Checkpoints | 1.Tumor evolution across therapy—Loss of target 2. Network analysis reveals alternate activated pathways- PI3K and DNA repair |
| 60 y F with metastatic adenocarcinoma of the lung with EGFR INDEL (exon 19) mutation | Carboplatin Pemetrexed Bevacizumab | Tarceva Afatinib | Lung nodule | Pre-targeted therapy: EGFR (INDEL) exon 19 TP53 CSF-1R PMS2 ARID1A PKHD1 PTPRT TPR Post-targeted therapy: EGFR (INDEL) exon19 EGFR (T790M) TP53 CSF-1R PD-1 positive PD-L1 negative c-MET positive M237I | p-53 dependent G1/S DNA Damage Checkpoint EGFR & ERBB2 signaling | 1. Recommend AZD9291 ± Mab to PD-L1 or Mab to MET 2. Consider MEK inhibitor3. Consider Osimertinib |
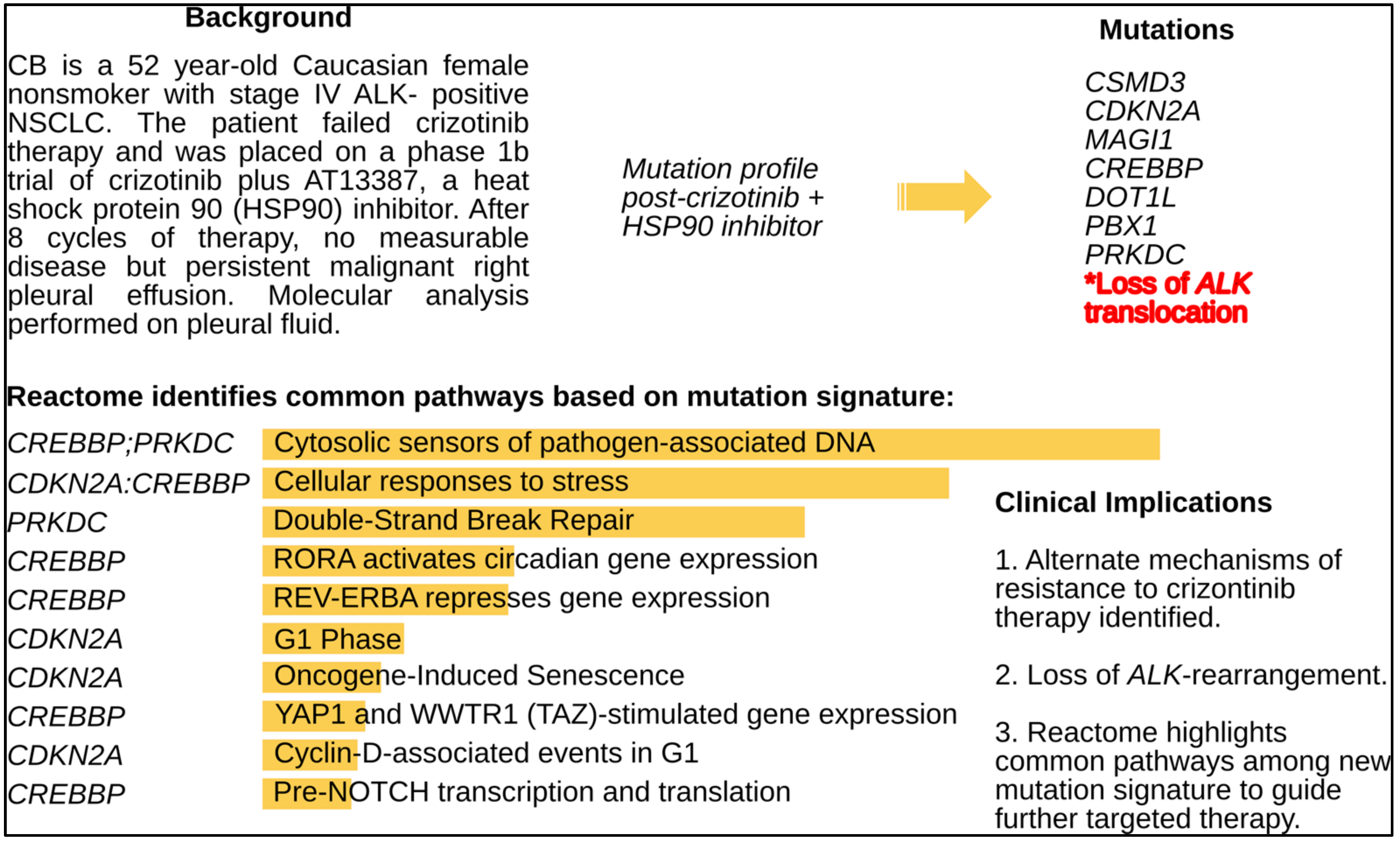
| 52 y F with Stage IV EML4-ALK NSCLC | Crizotinib | HSP90 inhibitor + crizotinib | Persistent R pleural effusion→ moderately differentiated adenocarcinoma | Loss of EML4-ALK by FISH, CDKN2A, CSMD3, MAGI1, CREBBP, DOT1L, PBX1, PRKDC | Pre-NOTCH Transcription and Translation Double Strand Break Repair Notch-HLH transcription pathway | 1. Loss of ALK (inversion) 2. Alternate activated pathways for future targeting with epigenetic therapy, DNA repair inhibitors and cell cycle inhibitors 3. Anti-PD-1 Mab |
| 10. 74 y F with stage IVA triple hit DLBCL | R-EPOCH X 6 cycles | IMid + BTK inhibitor + Rituximab | Axillary Lymph Node | TP53 PIK3R2 PTGS2 STK36 EZH2 DNMT3A PRKDC ABL2 AFF1 BCL-2 BCL-6 c-MYC | Epigenetic regulation of gene expression Double-strand Break Repair CD28 dependent PI3K/Akt signaling Pre-NOTCH Transcription and Translation TP53 Dependent G1 DNA Damage Response | 1. Epigenetic Therapy (e.g., EZH2 or DNMT3A inhibitor) 2. PI3K inhibitor + anti-CD20 Mab 3. STK36 Hedgehog pathway3. CAR-T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, R.; Johnson, N.; Cooke, L.; Johnson, B.; Chen, Y.; Pandey, M.; Chandler, J.; Mahadevan, D. TP53 Mutations as a Driver of Metastasis Signaling in Advanced Cancer Patients. Cancers 2021, 13, 597. https://doi.org/10.3390/cancers13040597
Pandey R, Johnson N, Cooke L, Johnson B, Chen Y, Pandey M, Chandler J, Mahadevan D. TP53 Mutations as a Driver of Metastasis Signaling in Advanced Cancer Patients. Cancers. 2021; 13(4):597. https://doi.org/10.3390/cancers13040597
Chicago/Turabian StylePandey, Ritu, Nathan Johnson, Laurence Cooke, Benny Johnson, Yuliang Chen, Manjari Pandey, Jason Chandler, and Daruka Mahadevan. 2021. "TP53 Mutations as a Driver of Metastasis Signaling in Advanced Cancer Patients" Cancers 13, no. 4: 597. https://doi.org/10.3390/cancers13040597
APA StylePandey, R., Johnson, N., Cooke, L., Johnson, B., Chen, Y., Pandey, M., Chandler, J., & Mahadevan, D. (2021). TP53 Mutations as a Driver of Metastasis Signaling in Advanced Cancer Patients. Cancers, 13(4), 597. https://doi.org/10.3390/cancers13040597







