Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Survival Effects of NACT
2.1. Clinical Trials Evaluating NACT
2.2. Observational Studies Evaluating NACT
3. Potential Disadvantages of NACT
4. Optimal Cytoreduction
5. Potential Models to Guide Upfront Decision-Making
5.1. Clinical Factors
5.1.1. Models Based on Various Clinical Factors
5.1.2. Models Based on Radiology Studies
5.1.3. Models Based on Laparoscopic Triage
5.2. Molecular Markers
5.2.1. Models Based on Circulating Molecular Markers
5.2.2. Models Based on Tumor-Based Genetic Markers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Ovarian Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 21 December 2020).
- Khairuzzaman, M.Q. Cancer Facts & Figures. 2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf (accessed on 21 December 2020).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for ovarian cancer US preventive services task force recommendation statement. JAMA 2018, 319, 588–594. [Google Scholar]
- Ebell, M.H.; Culp, M.B.; Radke, T.J. A Systematic Review of Symptoms for the Diagnosis of Ovarian Cancer. Am. J. Prev. Med. 2016, 50, 384–394. [Google Scholar] [CrossRef]
- Melamed, A.; Hinchcliff, E.M.; Clemmer, J.T.; Bregar, A.J.; Uppal, S.; Bostock, I.; Schorge, J.O.; del Carmen, M.G.; Rauh-Hain, J.A. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol. Oncol. 2016, 143, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Knisely, A.T.; St. Clair, C.M.; Hou, J.Y.; Collado, F.K.; Hershman, D.L.; Wright, J.D.; Melamed, A. Trends in Primary Treatment and Median Survival Among Women with Advanced-Stage Epithelial Ovarian Cancer in the US From 2004 to 2016. JAMA Netw. Open 2020, 3, e2017517. [Google Scholar] [CrossRef]
- Wright, J.D.; Ananth, C.V.; Tsui, J.; Glied, S.A.; Burke, W.M.; Lu, Y.S.; Neugut, A.I.; Herzog, T.J.; Hershman, D.L. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer 2014, 120, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.A.; He, W.; Sun, C.C.; Zhao, H.; Wright, A.A.; Suidan, R.S.; Dottino, J.; Rauh-Hain, J.A.; Lu, K.H.; Giordano, S.H. Neoadjuvant chemotherapy in elderly women with ovarian cancer: Rates of use and effectiveness. Gynecol. Oncol. 2018, 150, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.A.; Cronin, A.M.; Sun, C.C.; Bixel, K.; Bookman, M.A.; Cristea, M.C.; Griggs, J.J.; Levenback, C.F.; Burger, R.A.; Mantia-Smaldone, G.; et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian cancer. J. Clin. Oncol. 2016, 34, 3854–3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, K.; Matsuzaki, S.; Nusbaum, D.J.; Maoz, A.; Oda, K.; Klar, M.; Roman, L.D.; Sood, A.K. Possible candidate population for neoadjuvant chemotherapy in women with advanced ovarian cancer. Gynecol. Oncol. 2021, 160, 32–39. [Google Scholar] [CrossRef]
- Machida, H.; Matsuo, K.; Enomoto, T.; Mikami, M. Neoadjuvant chemotherapy for epithelial ovarian cancer in Japan: A JSGO-JSOG joint study. J. Gynecol. Oncol. 2019, 30, 113. [Google Scholar] [CrossRef]
- Marchetti, C.; Muzii, L.; Romito, A.; Panici, P.B. First-line treatment of women with advanced ovarian cancer: Focus on bevacizumab. Oncol. Targets. Ther. 2019, 12, 1095–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Eoh, K.J.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T.; Lee, J.Y. A single-center, retrospective study of bevacizumab-containing neoadjuvant chemotherapy followed by interval debulking surgery for ovarian cancer. Yonsei Med. J. 2020, 61, 284–290. [Google Scholar] [CrossRef]
- Moore, K.N.; Pignata, S. Trials in progress: IMagyn050/GOG 3015/ENGOT-OV39. A Phase III, multicenter, randomized study of atezolizumab versus placebo administered in combination with paclitaxel, carboplatin, and bevacizumab to patients with newly-diagnosed stage III or stage IV ovarian, fallopian tube, or primary peritoneal cancer. Int. J. Gynecol. Cancer 2019, 29, 430–433. [Google Scholar] [CrossRef]
- Elies, A.; Rivière, S.; Pouget, N.; Becette, V.; Dubot, C.; Donnadieu, A.; Rouzier, R.; Bonneau, C. The role of neoadjuvant chemotherapy in ovarian cancer. Expert Rev. Anticancer Ther. 2018, 18, 555–566. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.C.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef] [Green Version]
- Onda, T.; Satoh, T.; Ogawa, G.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Mizutani, T.; Takehara, K.; Okamoto, A.; Ushijima, K.; et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur. J. Cancer 2020, 130, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Costantini, B.; Margariti, P.A.; Gueli Alletti, S.; Cosentino, F.; et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 2016, 59, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Onda, T.; Satoh, T.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Nakamura, K.; Wakabayashi, M.; Takehara, K.; Saito, M.; Ushijima, K.; et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group. Eur. J. Cancer 2016, 64, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, S. Neoadjuvant chemotherapy for ovarian cancer: Do we have enough evidence? Lancet 2015, 386, 223–224. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Xing, G.; Du, J.; Yang, B.; Yuan, Q.; Yang, Y. Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: A meta-analysis of peri-operative outcome. PLoS ONE 2017, 12, 0186725. [Google Scholar] [CrossRef] [Green Version]
- Mueller, J.J.; Zhou, Q.C.; Iasonos, A.; O’Cearbhaill, R.E.; Alvi, F.A.; El Haraki, A.; Eriksson, A.G.Z.; Gardner, G.J.; Sonoda, Y.; Levine, D.A.; et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol. Oncol. 2016, 140, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Karam, A.; Ledermann, J.A.; Kim, J.W.; Sehouli, J.; Lu, K.; Gourley, C.; Katsumata, N.; Burger, R.A.; Nam, B.H.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: First-line interventions. Ann. Oncol. 2017, 28, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Herzog, T.J.; Ison, G.; Alvarez, R.D.; Balasubramaniam, S.; Armstrong, D.K.; Beaver, J.A.; Ellis, A.; Tang, S.; Ford, P.; McKee, A.; et al. FDA ovarian cancer clinical trial endpoints workshop: A Society of Gynecologic Oncology White Paper. Gynecol. Oncol. 2017, 147, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessous, R.; Laskov, I.; Abitbol, J.; Bitharas, J.; Yasmeen, A.; Salvador, S.; Lau, S.; Gotlieb, W.H. Clinical outcome of neoadjuvant chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 2017, 144, 474–479. [Google Scholar] [CrossRef]
- Lyons, Y.A.; Reyes, H.D.; Mcdonald, M.E.; Newtson, A.; Devor, E.; Bender, D.P.; Goodheart, M.J.; Gonzalez Bosquet, J. Interval debulking surgery is not worth the wait: A National Cancer Database study comparing primary cytoreductive surgery versus neoadjuvant chemotherapy. Int. J. Gynecol. Cancer 2020, 30, 845–852. [Google Scholar] [CrossRef]
- Rauh-Hain, J.A.; Melamed, A.; Wright, A.; Gockley, A.; Clemmer, J.T.; Schorge, J.O.; Del Carmen, M.G.; Keating, N.L. Overall survival following neoadjuvant chemotherapy vs primary cytoreductive surgery in women with epithelial ovarian cancer: Analysis of the National Cancer Database. JAMA Oncol. 2017, 3, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Bartels, H.C.; Rogers, A.C.; McSharry, V.; McVey, R.; Walsh, T.; O’Brien, D.; Boyd, W.D.; Brennan, D.J. A meta-analysis of morbidity and mortality in primary cytoreductive surgery compared to neoadjuvant chemotherapy in advanced ovarian malignancy. Gynecol. Oncol. 2019, 154, 622–630. [Google Scholar] [CrossRef]
- Reuss, A.; Du Bois, A.; Harter, P.; Fotopoulou, C.; Sehouli, J.; Aletti, G.; Guyon, F.; Greggi, S.; Mosgaard, B.J.; Reinthaller, A.; et al. TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int. J. Gynecol. Cancer 2019, 29, 1327–1331. [Google Scholar] [CrossRef]
- Yoneoka, Y.; Ishikawa, M.; Uehara, T.; Shimizu, H.; Uno, M.; Murakami, T.; Kato, T. Treatment strategies for patients with advanced ovarian cancer undergoing neoadjuvant chemotherapy: Interval debulking surgery or additional chemotherapy? J. Gynecol. Oncol. 2019, 30. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhou, Q.C.; Iasonos, A.; Chi, D.S.; Zivanovic, O.; Sonoda, Y.; Gardner, G.; Broach, V.; O’Cearbhaill, R.; Konner, J.A.; et al. Pre-operative neoadjuvant chemotherapy cycles and survival in newly diagnosed ovarian cancer: What is the optimal number? A Memorial Sloan Kettering Cancer Center Team Ovary study. Int. J. Gynecol. Cancer 2020, 30, 1915–1921. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, C.; Han, J.; Liang, H.; Zhang, K.; Guo, H. Evaluating the benefits of neoadjuvant chemotherapy for advanced epithelial ovarian cancer: A retrospective study. J. Ovarian Res. 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauh-Hain, J.A.; Rodriguez, N.; Growdon, W.B.; Goodman, A.K.; Boruta, D.M.; Horowitz, N.S.; Del Carmen, M.G.; Schorge, J.O. Primary debulking surgery versus neoadjuvant chemotherapy in stage IV ovarian cancer. Ann. Surg. Oncol. 2012, 19, 959–965. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, X.; Gao, Q. Neoadjuvant chemotherapy-related platinum resistance in ovarian cancer. Drug Discov. Today 2020, 25, 1232–1238. [Google Scholar] [CrossRef]
- Ayub, T.H.; Keyver-Paik, M.D.; Debald, M.; Rostamzadeh, B.; Thiesler, T.; Schröder, L.; Barchet, W.; Abramian, A.; Kaiser, C.; Kristiansen, G.; et al. Accumulation of ALDH1-positive cells after neoadjuvant chemotherapy predicts treatment resistance and prognosticates poor outcome in ovarian cancer. Oncotarget 2015, 6, 16437–16448. [Google Scholar] [CrossRef]
- Matsuo, K.; Eno, M.L.; Im, D.D.; Rosenshein, N.B. Chemotherapy time interval and development of platinum and taxane resistance in ovarian, fallopian, and peritoneal carcinomas. Arch. Gynecol. Obstet. 2010, 281, 325–328. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Z.; Xu, M.; Liu, D.; Liu, T.; He, M.; Yao, S. Impact of the time interval from neoadjuvant chemotherapy to surgery in primary ovarian, tubal, and peritoneal cancer patients. J. Cancer 2018, 9, 4087–4091. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Zhou, Q.C.; Iasonos, A.; Filippova, O.T.; Chi, D.S.; Zivanovic, O.; Sonoda, Y.; Gardner, G.; Broach, V.; Ocearbhaill, R.; et al. Delays from neoadjuvant chemotherapy to interval debulking surgery and survival in ovarian cancer. Int. J. Gynecol. Cancer 2020, 30, 1554–1561. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.E.; Labaki, M.; Fabbro, M.; Bertrand, M.; Mourregot, A.; Gutowski, M.; Saint-Aubert, B.; Quenet, F.; Rouanet, P.; Mollevi, C. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecol. Oncol. 2014, 135, 223–230. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Bristow, R.E.; Ryu, H.S. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann. Surg. Oncol. 2012, 19, 4059–4067. [Google Scholar] [CrossRef]
- Jiang, Y.; He, W.; Yang, H.; Su, Z.; Sun, L. Analysis of clinical effects of neoadjuvant chemotherapy in advanced epithelial ovarian cancer. J. BU ON Off. J. Balk. Union Oncol. 2018, 23, 758–762. [Google Scholar]
- Manning-Geist, B.L.; Hicks-Courant, K.; Gockley, A.A.; Clark, R.M.; Del Carmen, M.G.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Muto, M.G.; Worley, M.J. A novel classification of residual disease after interval debulking surgery for advanced-stage ovarian cancer to better distinguish oncologic outcome. Am. J. Obstet. Gynecol. 2019, 221, 326.e1–326.e7. [Google Scholar] [CrossRef]
- Nick, A.M.; Coleman, R.L.; Ramirez, P.T.; Sood, A.K. A framework for a personalized surgical approach to ovarian cancer. Nat. Rev. Clin. Oncol. 2015, 12, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi24–vi32. [Google Scholar] [CrossRef]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, H.S.; Tajik, P.; Hof, M.H.P.; Vergote, I.; Kenter, G.G.; Mol, B.W.J.; Buist, M.R.; Bossuyt, P.M. Which patients benefit most from primary surgery or neoadjuvant chemotherapy in stage IIIC or IV ovarian cancer? An exploratory analysis of the European Organisation for Research and Treatment of Cancer 55971 randomised trial. Eur. J. Cancer 2013, 49, 3191–3201. [Google Scholar] [CrossRef]
- Feigenberg, T.; Clarke, B.; Virtanen, C.; Plotkin, A.; Letarte, M.; Rosen, B.; Bernardini, M.Q.; Kollara, A.; Brown, T.J.; Murphy, K.J. Molecular profiling and clinical outcome of high-grade serous ovarian cancer presenting with low- versus high-volume ascites. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Deng, F.; Lv, M.; Ren, B.; Guo, W.; Chen, X. Ascites regression following neoadjuvant chemotherapy in prediction of treatment outcome among stage IIIc to IV high-grade serous ovarian cancer. J. Ovarian Res. 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Muraji, M.; Sudo, T.; Iwasaki, S.I.; Ueno, S.; Wakahashi, S.; Yamaguchi, S.; Fujiwara, K.; Nishimura, R. Histopathology predicts clinical outcome in advanced epithelial ovarian cancer patients treated with neoadjuvant chemotherapy and debulking surgery. Gynecol. Oncol. 2013, 131, 531–534. [Google Scholar] [CrossRef]
- Leiserowitz, G.S.; Lin, J.F.; Tergas, A.I.; Cliby, W.A.; Bristow, R.E. Factors predicting use of neoadjuvant chemotherapy compared with primary debulking surgery in advanced stage ovarian cancer—A national cancer database study. Int. J. Gynecol. Cancer 2017, 27, 675–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morice, P.; Gouy, S.; Leary, A. Mucinous Ovarian Carcinoma. N. Engl. J. Med. 2019, 380, 1256–1266. [Google Scholar] [CrossRef]
- Oda, K.; Hamanishi, J.; Matsuo, K.; Hasegawa, K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecol. Oncol. 2018, 151, 381–389. [Google Scholar] [CrossRef]
- Matsuo, K.; Machida, H.; Matsuzaki, S.; Grubbs, B.H.; Klar, M.; Roman, L.D.; Sood, A.K.; Gershenson, D.M.; Wright, J.D. Evolving population-based statistics for rare epithelial ovarian cancers. Gynecol. Oncol. 2020, 157, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Fischerova, D.; Burgetova, A. Imaging techniques for the evaluation of ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 697–720. [Google Scholar] [CrossRef] [PubMed]
- Borley, J.; Wilhelm-Benartzi, C.; Yazbek, J.; Williamson, R.; Bharwani, N.; Stewart, V.; Carson, I.; Hird, E.; McIndoe, A.; Farthing, A.; et al. Radiological predictors of cytoreductive outcomes in patients with advanced ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.C.; Mullany, S.A.; Brandt, K.R.; Huppert, B.J.; Cliby, W.A. The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma. Cancer 2004, 101, 346–352. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, C.H.; Lee, Y.Y.; Kim, T.J.; Lee, J.W.; Bae, D.S.; Kim, B.G. Surgical outcome prediction in patients with advanced ovarian cancer using computed tomography scans and intraoperative findings. Taiwan. J. Obstet. Gynecol. 2014, 53, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Mironov, S.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Paul, H.; Hosaka, M.; et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol. Oncol. 2014, 134, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Denesopolis, J.; Zivanovic, O.; Long Roche, K.C.; et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Sheedy, S.; Kim, B.; Suidan, R.; Sarasohn, D.M.; Nikolovski, I.; Lakhman, Y.; McGree, M.E.; Weaver, A.L.; Chi, D.; et al. Models to predict outcomes after primary debulking surgery: Independent validation of models to predict suboptimal cytoreduction and gross residual disease. Gynecol. Oncol. 2019, 154, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Huang, H.; Chen, M.; Liang, Y.; Wang, H. Clinical study of a CT evaluation model combined with serum CA125 in predicting the treatment of newly diagnosed advanced epithelial ovarian cancer. J. Ovarian Res. 2018, 11. [Google Scholar] [CrossRef]
- Qayyum, A.; Coakley, F.V.; Westphalen, A.C.; Hricak, H.; Okuno, W.T.; Powell, B. Role of CT and MR imaging in predicting optimal cytoreduction of newly diagnosed primary epithelial ovarian cancer. Gynecol. Oncol. 2005, 96, 301–306. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Lucero, J. Comparison of MRI and CT for Predicting the Peritoneal Cancer Index (PCI) Preoperatively in Patients Being Considered for Cytoreductive Surgical Procedures. Ann. Surg. Oncol. 2015, 22, 1708–1715. [Google Scholar] [CrossRef]
- Rizzo, S.; De Piano, F.; Buscarino, V.; Pagan, E.; Bagnardi, V.; Zanagnolo, V.; Colombo, N.; Maggioni, A.; Del Grande, M.; Del Grande, F.; et al. Pre-operative evaluation of epithelial ovarian cancer patients: Role of whole body diffusion weighted imaging MR and CT scans in the selection of patients suitable for primary debulking surgery. A single-centre study. Eur. J. Radiol. 2020, 123, 108786. [Google Scholar] [CrossRef] [PubMed]
- Alessi, A.; Martinelli, F.; Padovano, B.; Serafini, G.; Lorusso, D.; Lorenzoni, A.; Ditto, A.; Lecce, F.; Mira, M.; Donfrancesco, C.; et al. FDG-PET/CT to predict optimal primary cytoreductive surgery in patients with advanced ovarian cancer: Preliminary results. Tumori 2016, 102, 103–107. [Google Scholar] [CrossRef]
- Chong, G.O.; Jeong, S.Y.; Lee, Y.H.; Lee, H.J.; Lee, S.W.; Han, H.S.; Hong, D.G.; Lee, Y.S. The ability of whole-body SUVmax in F-18 FDG PET/CT to predict suboptimal cytoreduction during primary debulking surgery for advanced ovarian cancer. J. Ovarian Res. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Ercoli, A.; Lorusso, D.; Rossi, M.; Scambia, G. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: A pilot study. Ann. Surg. Oncol. 2006, 13, 1156–1161. [Google Scholar] [CrossRef]
- Fleming, N.D.; Nick, A.M.; Coleman, R.L.; Westin, S.N.; Ramirez, P.T.; Soliman, P.T.; Fellman, B.; Meyer, L.A.; Schmeler, K.M.; Lu, K.H.; et al. Laparoscopic Surgical Algorithm to Triage the Timing of Tumor Reductive Surgery in Advanced Ovarian Cancer. Obstet. Gynecol. 2018, 132, 545–554. [Google Scholar] [CrossRef]
- Fagotti, A.; Vizzielli, G.; De Iaco, P.; Surico, D.; Buda, A.; Mandato, V.D.; Petruzzelli, F.; Ghezzi, F.; Garzarelli, S.; Mereu, L.; et al. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am. J. Obstet. Gynecol. 2013, 209, 462.e1–462.e11. [Google Scholar] [CrossRef]
- Petrillo, M.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Cosentino, F.; Chiantera, V.; Legge, F.; Carbone, V.; Scambia, G.; Fagotti, A. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: Proof of a concept. Gynecol. Oncol. 2015, 139, 5–9. [Google Scholar] [CrossRef]
- Eoh, K.J.; Yoon, J.W.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. A novel algorithm for the treatment strategy for advanced epithelial ovarian cancer: Consecutive imaging, frailty assessment, and diagnostic laparoscopy. BMC Cancer 2017, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andikyan, V.; Kim, A.; Gretz, H.F.; Zakashansky, K.; Prasad-Hayes, M.; Beddoe, A.M.; Dottino, P.; Mandeli, J.; Chuang, L. Laparoscopic Assessment to Determine the Likelihood of Achieving Optimal Cytoreduction in Patients Undergoing Primary Debulking Surgery for Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. Am. J. Clin. Oncol. 2018, 41, 938–942. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Abou-Taleb, H.; Yehia, A.; El Malek, N.A.A.; Siefeldein, G.S.; Badary, D.M.; Jabir, M.A. The accuracy of multi-detector computed tomography and laparoscopy in the prediction of peritoneal carcinomatosis index score in primary ovarian cancer. Acad. Radiol. 2019, 26, 1650–1658. [Google Scholar] [CrossRef]
- Rodriguez, N.; Rauh-Hain, J.A.; Shoni, M.; Berkowitz, R.S.; Muto, M.G.; Feltmate, C.; Schorge, J.O.; Del Carmen, M.G.; Matulonis, U.A.; Horowitz, N.S. Changes in serum CA-125 can predict optimal cytoreduction to no gross residual disease in patients with advanced stage ovarian cancer treated with neoadjuvant chemotherapy. Gynecol. Oncol. 2012, 125, 362–366. [Google Scholar] [CrossRef]
- Pelissier, A.; Bonneau, C.; Chéreau, E.; La Motte Rouge, T.D.; Fourchotte, V.; Daraï, E.; Rouzier, R. Dynamic analysis of CA125 decline during neoadjuvant chemotherapy in patients with epithelial ovarian cancer as a predictor for platinum sensitivity. Anticancer Res. 2016, 36, 1865–1871. [Google Scholar] [PubMed]
- Matsuhashi, T.; Takeshita, T.; Yamamoto, A.; Kawase, R.; Yamada, T.; Kurose, K.; Doi, D.; Konnai, K.; Onose, R.; Kato, H. Serum ca 125 level after neoadjuvant chemotherapy is predictive of prognosis and debulking surgery outcomes in advanced epithelial ovarian cancer. J. Nippon Med. Sch. 2017, 84, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, M.H.; Lee, S.W.; Park, J.Y.; Rhim, C.C.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Preoperative Predictive Factors for Complete Cytoreduction and Survival Outcome in Epithelial Ovarian, Tubal, and Peritoneal Cancer After Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer 2017, 27, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Matte, I.; Garde-Granger, P.; Bessette, P.; Piché, A. Serum CA125 and ascites leptin level ratio predicts baseline clinical resistance to first-line platinum-based treatment and poor prognosis in patients with high grade serous ovarian cancer. Am. J. Cancer Res. 2019, 9, 160–170. [Google Scholar]
- Shen, Y.; Li, L. Serum HE4 superior to CA125 in predicting poorer surgical outcome of epithelial ovarian cancer. Tumor Biol. 2016, 37, 14765–14772. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Scaletta, G.; Capriglione, S.; Montera, R.; Luvero, D.; Lopez, S.; Gatti, A.; De Cicco Nardone, C.; Terranova, C.; Angioli, R. The role of HE4, a novel biomarker, in predicting optimal cytoreduction after neoadjuvant chemotherapy in advanced ovarian cancer. Int. J. Gynecol. Cancer 2017, 27, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.L.; Gharpure, K.; Herbrich, S.M.; Unruh, A.K.; Nick, A.M.; Crane, E.K.; Coleman, R.L.; Guenthoer, J.; Dalton, H.J.; Wu, S.Y.; et al. Molecular biomarkers of residual disease after surgical debulking of high-grade serous ovarian cancer. Clin. Cancer Res. 2014, 20, 3280–3288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrillo, M.; Zannoni, G.F.; Beltrame, L.; Martinelli, E.; DiFeo, A.; Paracchini, L.; Craparotta, I.; Mannarino, L.; Vizzielli, G.; Scambia, G.; et al. Identification of high-grade serous ovarian cancer miRNA species associated with survival and drug response in patients receiving neoadjuvant chemotherapy: A retrospective longitudinal analysis using matched tumor biopsies. Ann. Oncol. 2016, 27, 625–634. [Google Scholar] [CrossRef]
- Shah, J.S.; Gard, G.B.; Yang, J.; Maidens, J.; Valmadre, S.; Soon, P.S.; Marsh, D.J. Combining serum microRNA and CA-125 as prognostic indicators of preoperative surgical outcome in women with high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yunusova, N.V.; Villert, A.B.; Spirina, L.V.; Frolova, A.E.; Kolomiets, L.A.; Kondakova, I.V. Insulin-Like Growth Factors and Their Binding Proteins in Tumors and Ascites of Ovarian Cancer Patients: Association with Response To Neoadjuvant Chemotherapy. Asian Pac. J. Cancer Prev. 2016, 17, 5315–5320. [Google Scholar] [CrossRef]
- Link, T.; Passek, S.; Wimberger, P.; Frank, K.; Vassileva, Y.D.; Kramer, M.; Kuhlmann, J.D. Serum calretinin as an independent predictor for platinum resistance and prognosis in ovarian cancer. Int. J. Cancer 2020, 146, 2608–2618. [Google Scholar] [CrossRef] [Green Version]
- Petrillo, M.; Marchetti, C.; De Leo, R.; Musella, A.; Capoluongo, E.; Paris, I.; Benedetti Panici, P.; Scambia, G.; Fagotti, A. BRCA mutational status, initial disease presentation, and clinical outcome in high-grade serous advanced ovarian cancer: A multicenter study. Am. J. Obstet. Gynecol. 2017, 217, 334.e1–334.e9. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.S.; Rim, J.H.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Germline BRCA, chemotherapy response scores, and survival in the neoadjuvant treatment of ovarian cancer. BMC Cancer 2020, 20. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.W. Association of BRCA1/2 mutations with ovarian cancer prognosis. Medicine 2018, 97. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Kigawa, J.; Kanamori, Y.; Itamochi, H.; Oishi, T.; Simada, M.; Uegaki, K.; Naniwa, J.; Terakawa, N. Expression of the c-myc gene as a predictor of chemotherapy response and a prognostic factor in patients with ovarian cancer. Cancer Sci. 2004, 95, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author | [20] | [21] # | [17] | [18] | ||||
|---|---|---|---|---|---|---|---|---|
| Year | 2020 | 2016 | 2015 | 2010 | ||||
| Enrolled cases | NACT | pTRS | NACT | pTRS | NACT | pTRS | NACT | pTRS |
| 149 | 152 | 55 | 55 | 274 | 276 | 334 | 336 | |
| Stage IV | 49 (32.9) | 47 (30.9) | 4 (7.3) | 8 (14.5) | 68 (24.8) | 70 (25.4) | 81 (24.3) | 77 (22.9) |
| PS 0–1 | 131 (86.2) | 130 (87.2) | 50 (90.9) | 51 (92.7) | 221 (80.7) | 221 (80.1) | 290 (86.8) | 294 (87.5) |
| PS ≥ 2 | 21 (13.8) | 19 (12.8) | 5 (9.1) | 4 (7.3) | 53 (19.3) | 54 (19.6) | 44 (13.2) | 40 (11.9) |
| Surgical time (mins) | 302 | 240 | 275 | 451 | 120 | 120 | 180 | 165 |
| R0 ‡ | 83 (63.8) | 17 (11.6) | 30 (57.7) | 25 (45.5) | 79 (39.3) | 39 (16.7) | 151 (51.2) | 61 (19.4) |
| Periop mortality | 0 | 1 (0.7) | 0 | 2 (3.6) | 1 (0.5) | 14 (5.5) | 2 (0.7) | 8 (2.5) |
| G3-4 AE | 7 (5.4) | 25 (17.0) | 3 (5.8) | 27 (49.1) | 30 (14) | 60 (24) | 17 (5.3) * | 56 (18.1) * |
| DFS | HR 0.96 (0.75–1.23) | HR 1.06 (0.77–1.46) † | HR 0.91 (0.76–1.09) | HR 1.01 (0.89–1.15) ¶ | ||||
| OS | HR 1.05 (0.84–1.33) § | - | - | HR 0.87 (0.72–1.05) | HR 0.98 (0.84–1.13) ¶ | |||
| Subgroup analysis of overall survival in the NACT group as compared with the pTRS group | ||||||||
| Age > 70 | - | - | - | - | Comparable | - | Comparable | - |
| PS ≥ 2 | Comparable | - | - | - | Comparable | - | Comparable | - |
| Stage IIIC | Comparable | - | - | - | Comparable | - | Comparable | - |
| Stage IV | Comparable | - | - | - | Comparable | - | NACT better | - |
| CSS | - | - | - | - | - | - | - | - |
| Other cause of death | - | - | - | - | - | - | - | - |
| Non-serous $ | Comparable | - | - | - | - | - | Comparable | - |
| R0 | - | - | - | - | Comparable | - | Comparable | - |
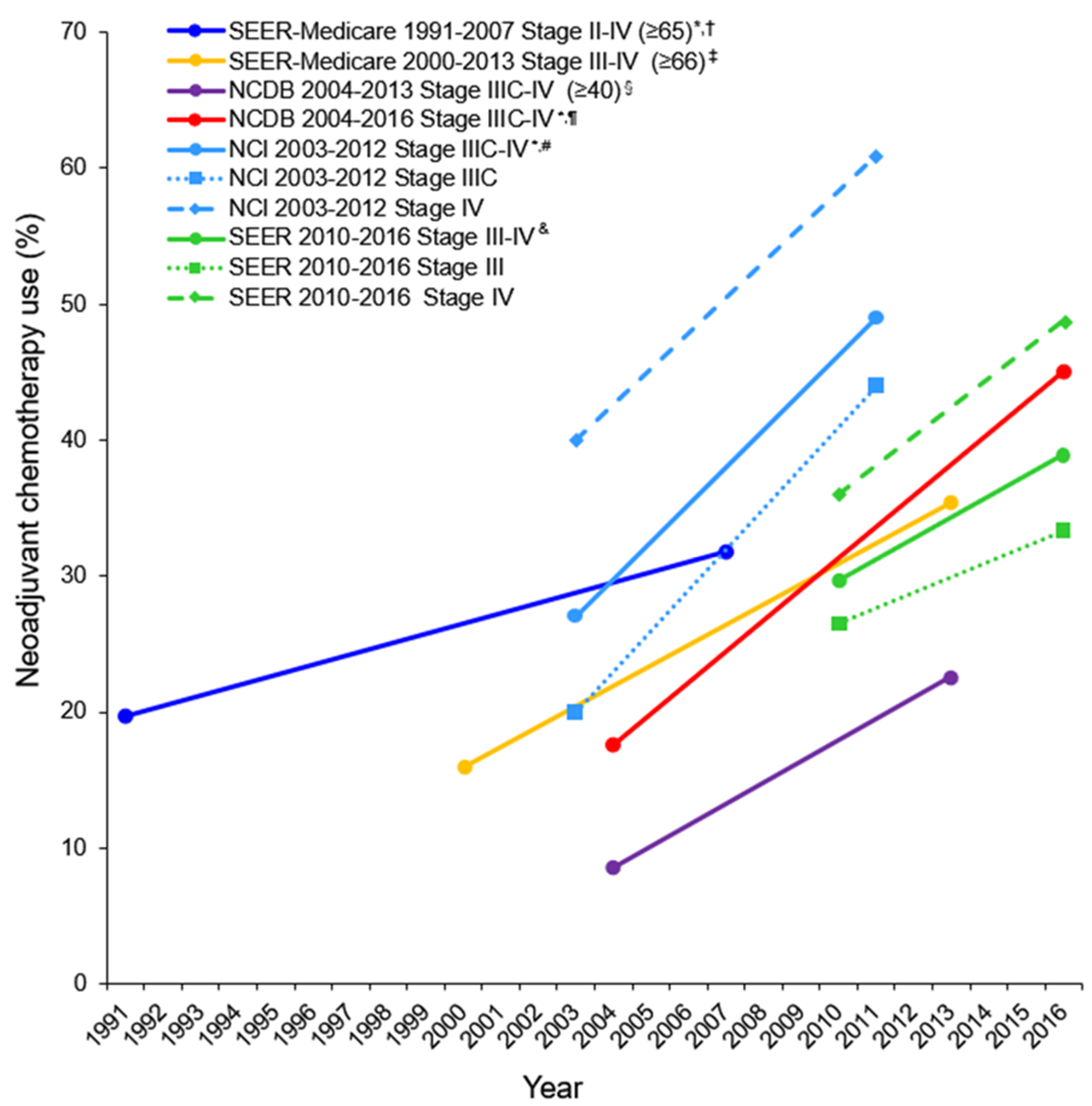
| Author | [11] | [7] | [29] | [9] | [30] | [6] | [10] | [8] |
|---|---|---|---|---|---|---|---|---|
| Year | 2021 | 2020 | 2020 | 2018 | 2017 | 2016 | 2016 | 2014 |
| Period | 2010–2016 | 2004–2016 | 2004–2015 | 2000–2013 | 2003–2011 | 2004–2013 | 2003–2012 | 1991–2007 |
| Data base | SEER | NCDB | NCDB | SEER-Medicare | NCDB | NCDB | NCCN ** | SEER-Medicare |
| No. | 4360 | 72171 | 36602 | 5417 | 22962 | 40694 | 1538 | 9587 |
| NACT | 1268 (29.1) | 19150 (26.5) | 9885 (27.0) | 1221 (22.5) | 3126 (13.6) | 5429 (13.3) | 416 (27.0) | 2238 (23.3) |
| Age | Any | Any | Any | ≥66 | ≤70 | ≥40 | Any | ≥65 |
| CCI | Any | Any | Any | Any | 0 | Any | Any | Any |
| Stage | III, IV | IIIC, IV | III, IV | III, IV | IIIC, IV | IIIC, IV | IIIC, IV | II-IV |
| R0 † | - | - | 65.4 vs. 56.1 # | - | - | - | 36.8 vs. 20.8 | - |
| Use of NACT (%) | 29.7 in2010 38.9 in 2016 | 17.6 in 2004 * 45.1 in 2016 * | - | 16 in 2000 35.4 in 2013 | - | 8.6 in 2004 22.6 in 2013 | 27 in 2003 * 49 in 2012 * | 19.7 in 1991 * 31.8 in 2007 * |
| p-trend | p < 0.001 | p < 0.001 ^ | - | p < 0.0001 | - | p < 0.001 | p < 0.01 | p < 0.0001 |
| OS ¶ | pTRS better | - | pTRS better | - | pTRS better | - | - | pTRS better |
| Subgroup analysis of overall survival in the NACT group as compared with the pTRS group | ||||||||
| Age > 70 | Comparable | - | - | pTRS better ‡ | - | - | - | - |
| Stage IIIC | pTRS better & | - | - | pTRS better | pTRS better | - | pTRS better | - |
| Stage IV | Comparable | - | - | Comparable | pTRS better | - | Comparable | - |
| Serous | - | - | - | - | pTRS better | - | - | - |
| HVC | - | - | - | - | pTRS better | - | - | - |
| R0 | - | - | pTRS better | - | - | - | Comparable | - |
| Biopsy-confirmed FIGO stage IV advanced epithelial ovarian, fallopian tube and peritoneal cancers Biopsy-confirmed FIGO stage IIIC advanced epithelial ovarian, fallopian tube and peritoneal cancers who are not fit for surgery |
| High-grade serous type of advanced epithelial ovarian, fallopian tube and peritoneal cancers |
| Higher perioperative morbidity or mortality: Poor performance status, advanced age, higher body mass index, poor nutritional status, low albumin, high-volume ascites, multiple comorbidities |
| Extensive intraperitoneal or extraperitoneal metastases such as large metastatic tumors (>45 mm), nonresectable parenchymal liver metastasis, metastasis to the lungs or mediastinum, mesenteric retraction, bulky periportal lymph nodes or unresectable extra abdominal lymph nodes, pleural effusion |
| Absence of acute intestinal obstruction or other symptoms of emergency surgery |
| Low possibility of optimal cytoreduction (<1 cm of residual disease) |
CT findings:
|
| Social factors: Distance of patients’ residence from the treating hospital, academic medical institutes or comprehensive community cancer centers. |
| Author (Year) | Criteria |
|---|---|
| [63] (2014) | 9 criteria: 3 clinical criteria (age ≥ 60 years, CA-125 ≥ 500 U/mL, American Society of Anesthesiologists [ASA] class ≥3) and 6 radiologic criteria (>1 cm lesions in the small bowel mesentery; >1 cm lesions in the root of the superior mesenteric artery; >1 cm lesions in the perisplenic area; >1 cm lesions in the lesser sac; >1 cm suprarenal retroperitoneal lymph nodes; and diffuse small bowel adhesions/thickening). |
| [64] (2017) | 11 criteria: 3 clinical criteria (age ≥ 60 years, CA-125 ≥ 600 U/mL, American Society of Anesthesiologists (ASA) class-≥3) and 8 radiologic criteria (>1 cm lesions in the root of the superior mesenteric artery; >1 cm lesions in the splenic hilum/ligaments; >1 cm retroperitoneal lymph nodes above the renal hilum including supradiaphragmatic lymph nodes; >1 cm lesser sac lesions; diffuse small bowel adhesions/thickening; moderate-severe abdominal ascites; lesions on gastrohepatic ligament/porta hepatis; and gallbladder fossa/intersegmental fissure lesions). |
| [66] (2018) | Scoring parameters: CA-125 level (≥500 U/mL); performance status of ≥2; large-volume ascites; omentum disease extension to the stomach, spleen or lesser sac; tumor extension to the pelvic sidewall, parametria or hydroureter; peritoneal thickening; ≥2 cm peritoneal implants; ≥1 cm suprarenal paraaortic lymph nodes; ≥2 cm diaphragm or lung base disease or confluent plaques; ≥2 cm inguinal canal disease or lymph nodes; ≥2 cm liver lesion on the surface or any size parenchymal lesion; porta hepatis or ≥1 cm gallbladder fossa disease; ≥2 cm infrarenal paraaortic lymph nodes; and ≥2 cm small or large bowel mesentery disease |
| [67] (2005) | Preoperative inoperable cancer sites: >2 cm of peritoneal implants in lesser sac, gall bladder fossa, gastrosplenic ligament, gastrohepatic ligament, root of the small bowel mesentery, subphrenic space, intersegmental fissure or porta hepatis; >2 cm of retroperitoneal adenopathy above the renal hilum; abdominal wall incursion; or hepatic metastases |
| Cancer Parameter | Score |
|---|---|
| Stomach infiltration (obvious cancer dissemination into gastric wall) | Absent = 0 Present = 2 |
| Diaphragmatic carcinomatosis (confluent nodules and/or extensive infiltration to diaphragmatic surface) | Absent = 0 Present = 2 |
| Mesenteric retraction (involvement of the root of the mesentery and/or large infiltrating nodules) | Absent = 0 Present = 2 |
| Omental cake (tumor dissemination of omentum to the small and large curvatures of the stomach) | Absent = 0 Present = 2 |
| Peritoneal carcinomatosis (enormous peritoneal diffusion and/or disease spread with miliary distribution pattern) | Absent = 0 Present = 2 |
| Bowel infiltration (tumor dissemination to small or large bowel necessitating colon resection (except rectosigmoid colon) | Absent = 0 Present = 2 |
| Liver metastases (superficial lesions >2 cm) | Absent = 0 Present = 2 |
| Biomarker | Summary |
|---|---|
| CA-125 | Serum CA-125 is the most common tumor marker used at diagnosis and to observe treatment response. A >80% decrease in serum CA-125 level after NACT is found to be associated with optimal cytoreduction. Cut-off level to measure response/progression is still debatable. |
| Leptin | Higher serum CA-125 to ascites leptin ratio is found to be suggestive of baseline chemoresistance. |
| HE4 | Serum HE4 level is found to be more valuable tumor marker in estimating surgery outcome. A >70% decrease in serum HE4 level after NACT is found to be associated with optimal cytoreduction. |
| ADLH1 | Higher ALDH1 level after NACT is found to be associated with poor outcome and higher risk of death. |
| ADH1B | Higher preoperative ADH1B level is found be associated with higher chances of RD after tumor reductive surgery. |
| FABP4 | Higher preoperative FABP4 level is found be associated with higher chances of RD after tumor reductive surgery. |
| MicroRNA | Higher level of specific MicroRNAs (Smad2 phosphorylation (P-Smad2), miR-181a-5p, miR-199a-5p and miR-199a-3p) is found to be associated with higher chances of RD after iTRS, decreased platinum-free interval and poor survival. |
| IGF-I | The presence of IGF-I in ascitic fluid is found to be an independent predictor of objective clinical response. |
| Calretinin | Higher serum CRT level is found to be associated with higher chances of suboptimal cytoreduction. |
| BRCA1/2 | The presence of BRCA1/2 is found to be associated with higher chances of optimal cytoreduction and better survival. |
| c-Myc | c-Myc expression of >200 is found to be associated with better 5-year survival rate. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Iyer, P.; Matsuzaki, S.; Matsuo, K.; Sood, A.K.; Fleming, N.D. Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer. Cancers 2021, 13, 626. https://doi.org/10.3390/cancers13040626
Patel A, Iyer P, Matsuzaki S, Matsuo K, Sood AK, Fleming ND. Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer. Cancers. 2021; 13(4):626. https://doi.org/10.3390/cancers13040626
Chicago/Turabian StylePatel, Ami, Puja Iyer, Shinya Matsuzaki, Koji Matsuo, Anil K. Sood, and Nicole D. Fleming. 2021. "Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer" Cancers 13, no. 4: 626. https://doi.org/10.3390/cancers13040626
APA StylePatel, A., Iyer, P., Matsuzaki, S., Matsuo, K., Sood, A. K., & Fleming, N. D. (2021). Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer. Cancers, 13(4), 626. https://doi.org/10.3390/cancers13040626








