Tuberculous Fibrosis Enhances Tumorigenic Potential via the NOX4–Autophagy Axis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
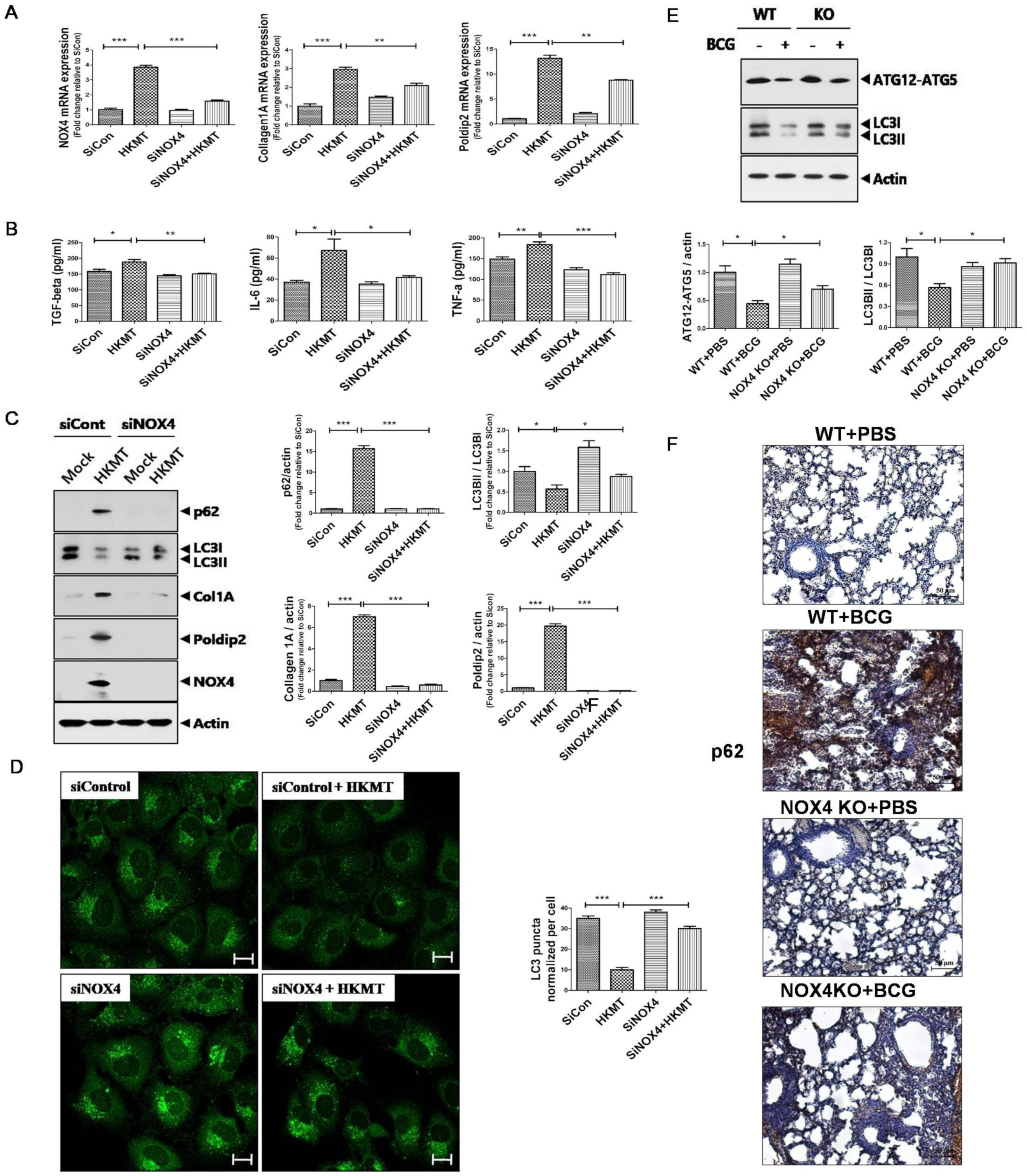
2.1. Downregulation of NOX4 Signaling Reduces HKMT-Induced Collagen and POLDIP2Synthesis by Activating Autophagy Signaling
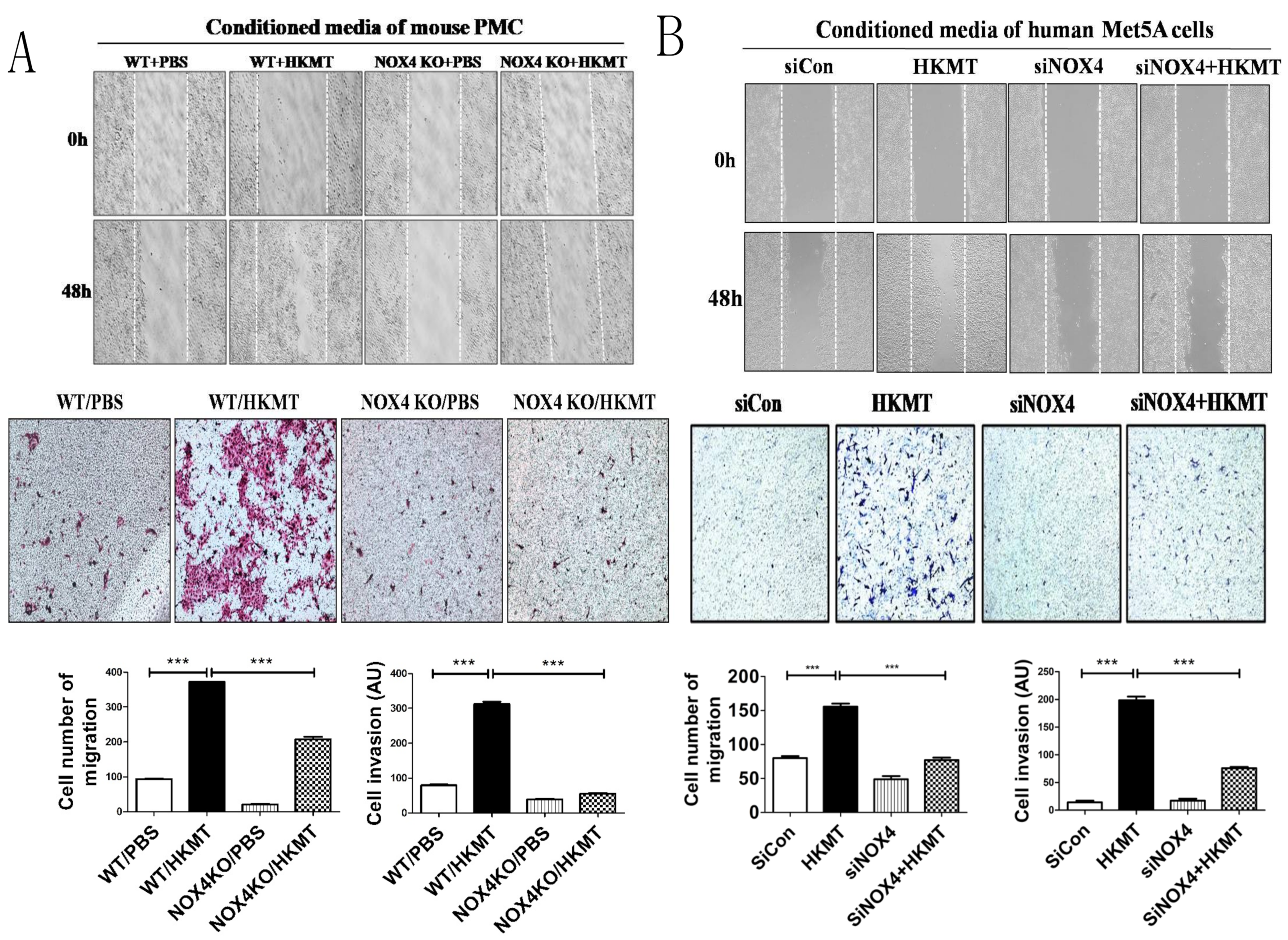
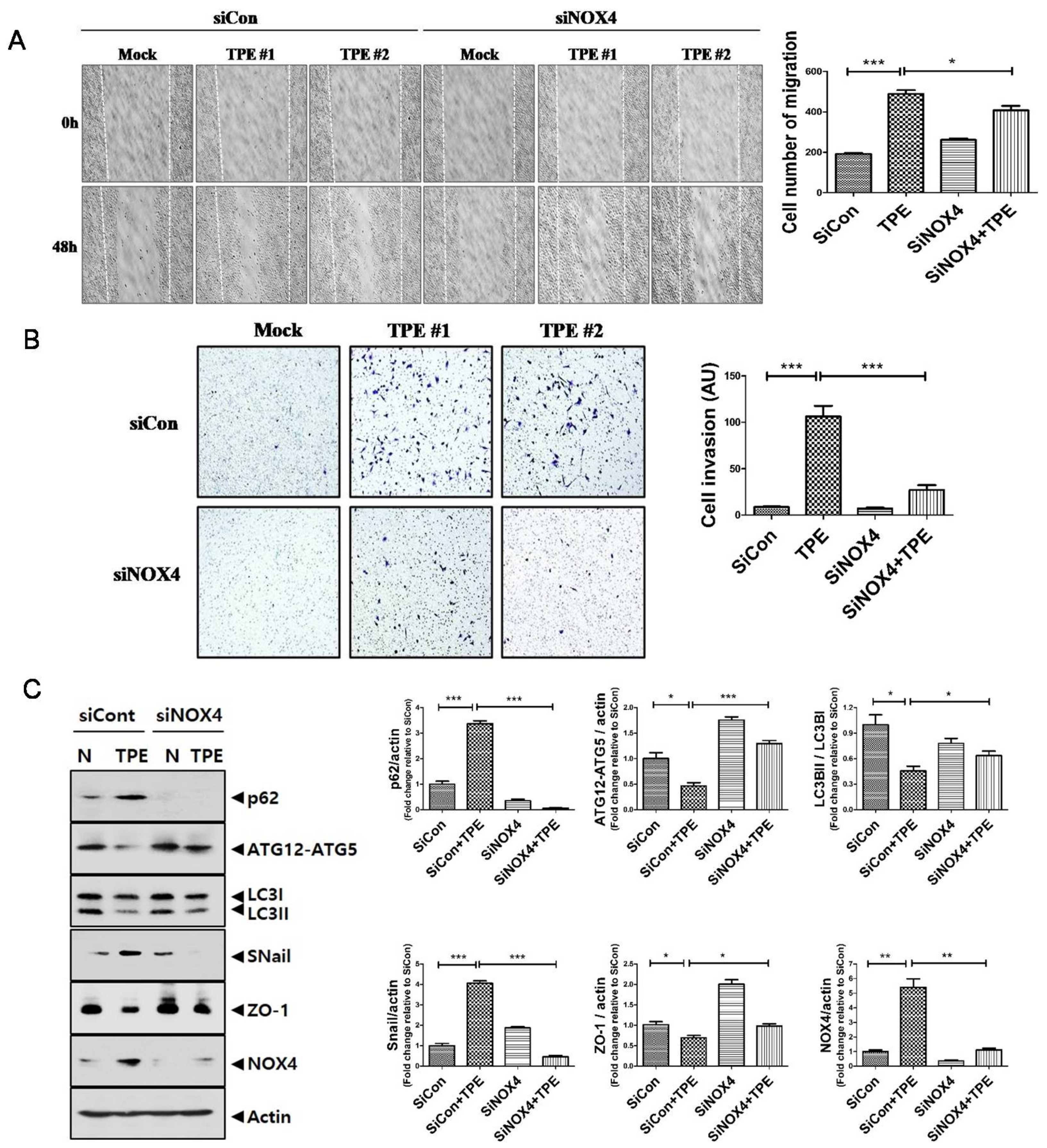
2.2. HKMT-Induced PMCs Enhance Migration and Invasion of Lung Cancer Cells via NOX4 Signaling
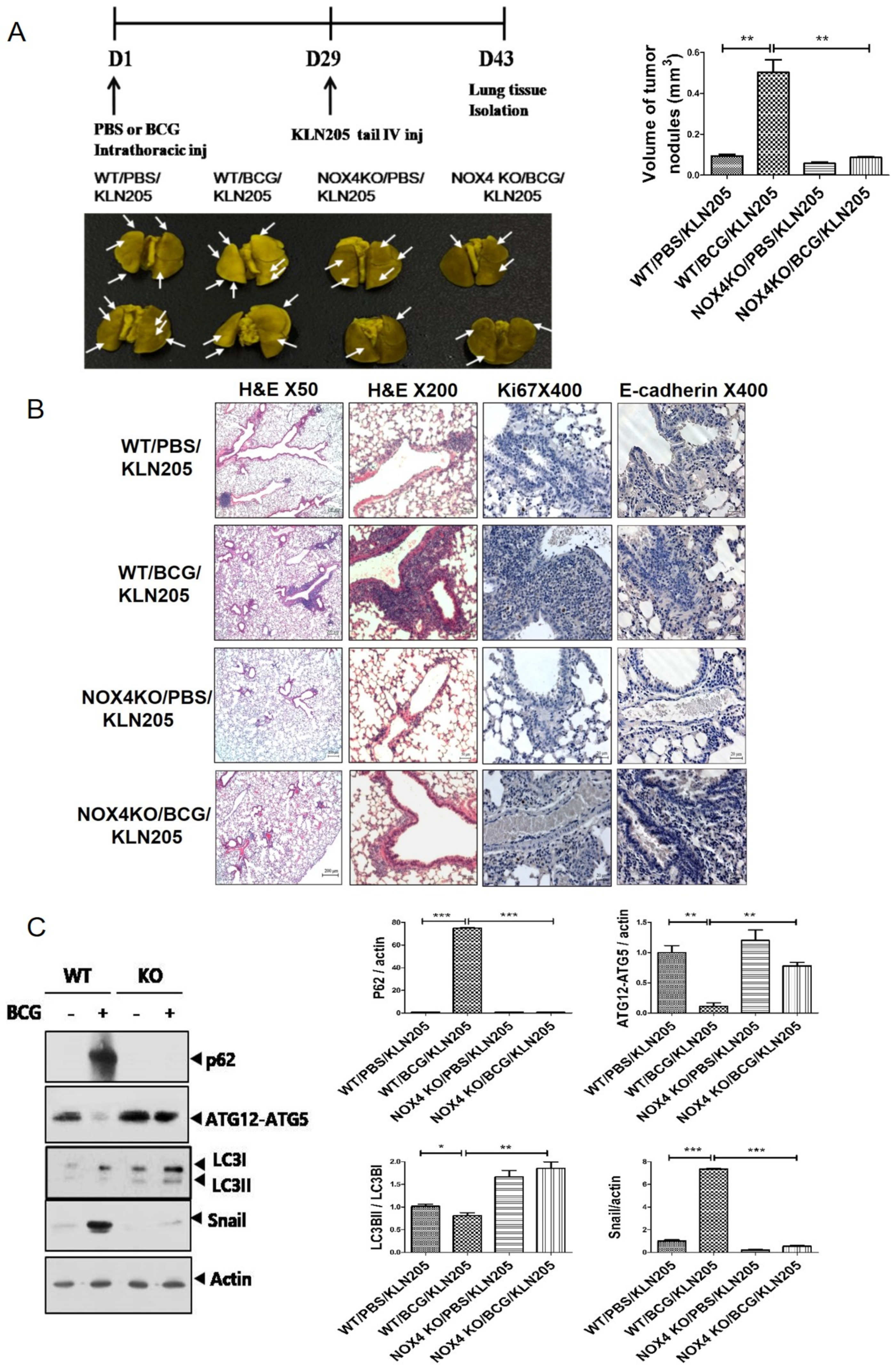
2.3. NOX4 Is Required for the Increased Metastatic Potential of Cancer Cells by Tuberculous Fibrosis
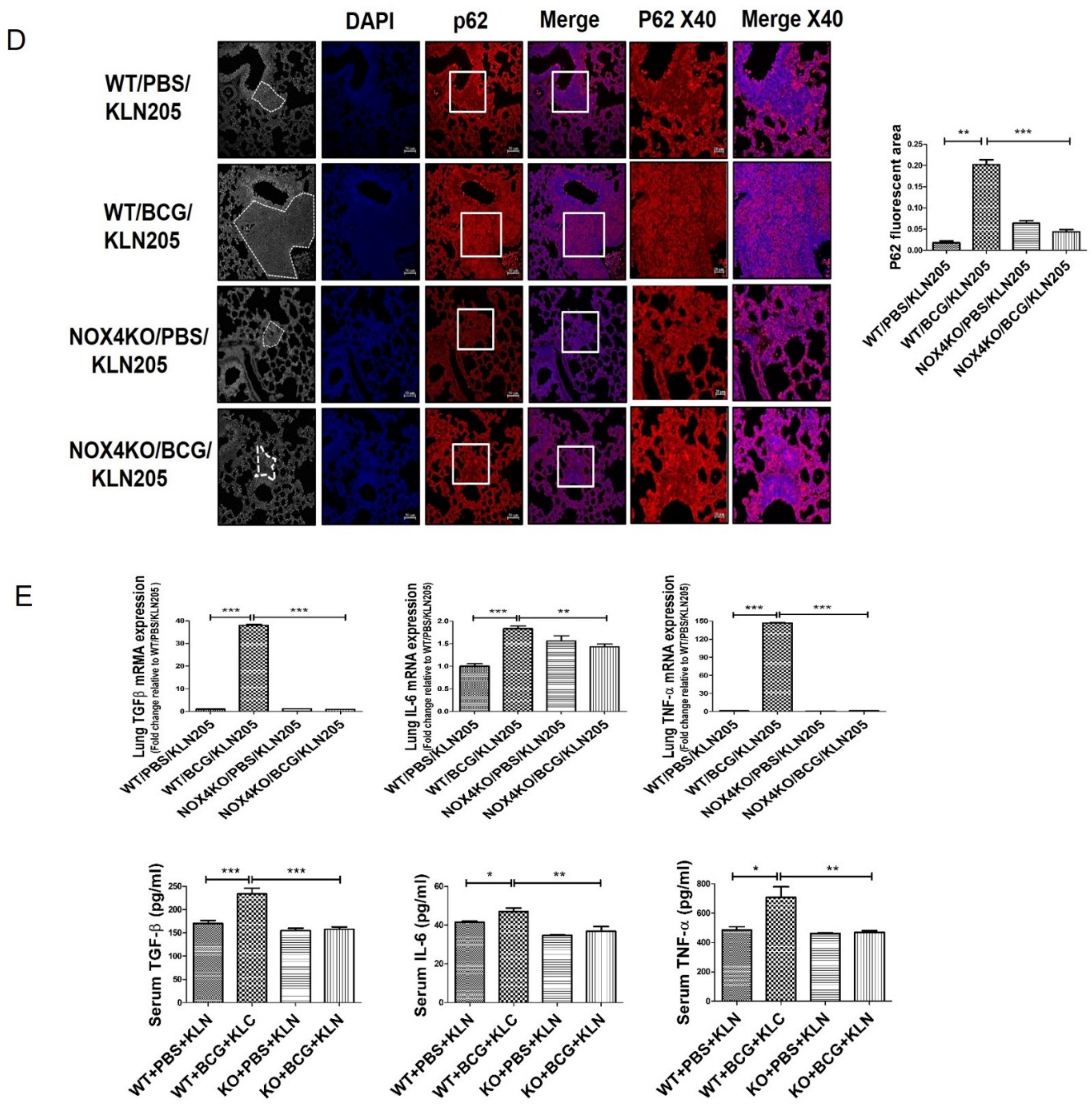
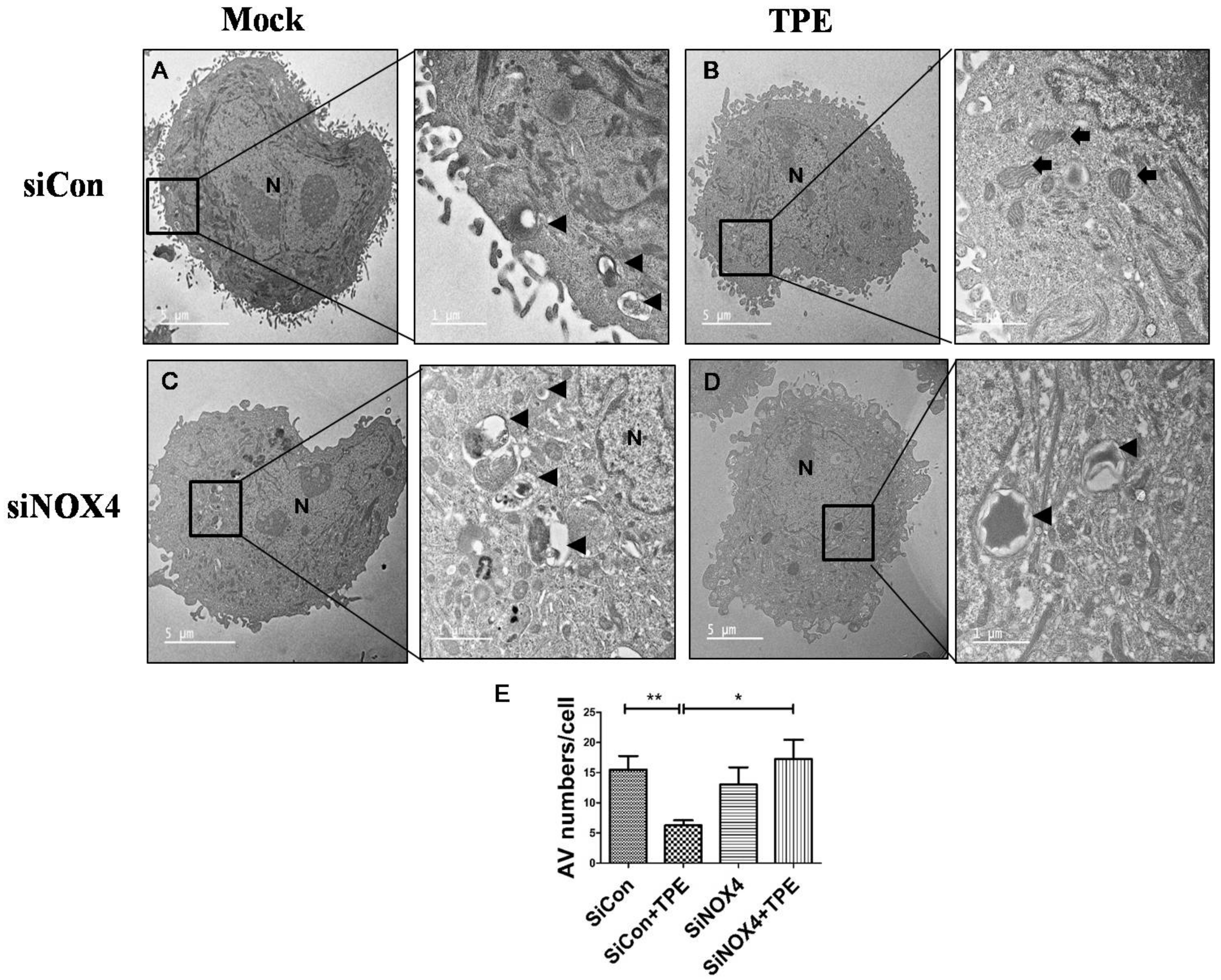
2.4. Autophagy Is Attenuated in Lung Cancer Cells after Tuberculous Effusion Treatment
2.5. TPE Enhances Migration, Invasion, and the EMT in Lung Cancer Cells in a NOX4-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Animals
4.2. Mouse Pleural Mesothelial Cells
4.3. Heat-Killed M. tuberculosis Treatment, Cell Transfection, and Immunofluorescence of LC3
4.4. Human Pleural Effusion Collection
4.5. Effect of BCG-Induced Pleural Fibrosis on Metastatic Potential
4.6. Scratch-Wound Assay and Cell Invasion Assay
4.7. Real-Time Reverse Transcription PCR, Western Blotting, and Enzyme-Linked Immunosorbent Assay
4.8. Lung Tissue Staining
4.9. Transmission Electron Microscopy
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Nalbandian, A.; Yan, B.S.; Pichugin, A.; Bronson, R.T.; Kramnik, I. Lung carcinogenesis induced by chronic tuberculosis infection: The experimental model and genetic control. Oncogene 2009, 28, 1928–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, J. Pleural tuberculosis. Eur. Respir. J. 1997, 10, 942–947. [Google Scholar] [PubMed]
- Yang, J.; Xiang, F.; Cai, P.C.; Lu, Y.Z.; Xu, X.X.; Yu, F.; Li, F.Z.; Greer, P.A.; Shi, H.Z.; Zhou, Q.; et al. Activation of calpain by renin-angiotensin system in pleural mesothelial cells mediates tuberculous pleural fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L145–L153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Park, S.Y.; Jung, H.; Noh, Y.S.; Lee, J.J.; Hong, J.Y. Inhibition of NADPH Oxidase 4 (NOX4) Signaling Attenuates Tuberculous Pleural Fibrosis. J. Clin. Med. 2019, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Lan, T.; Hou, J.; Li, J.; Fang, R.; Yang, Z.; Zhang, M.; Liu, J.; Liu, B. NOX4 promotes non-small cell lung cancer cell proliferation and metastasis through positive feedback regulation of PI3K/Akt signaling. Oncotarget 2014, 5, 4392–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Hong, S.; Li, W.; Wang, P.; You, J.; Zhang, X.; Tang, F.; Wang, P.; Zhang, C. MiR-99a regulates ROS-mediated invasion and migration of lung adenocarcinoma cells by targeting NOX4. Oncol. Rep. 2016, 35, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.; Vouret-Craviari, V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes 2012, 3, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jess, T.; Loftus, E.V., Jr.; Velayos, F.S.; Harmsen, W.S.; Zinsmeister, A.R.; Smyrk, T.C.; Schleck, C.D.; Tremaine, W.J.; Melton, L.J., 3rd; Munkholm, P.; et al. Risk of intestinal cancer in inflammatory bowel disease: A population-based study from olmsted county, Minnesota. Gastroenterology 2006, 130, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kato, J.; Inoue, I.; Yoshimura, N.; Deguchi, H.; Mukoubayashi, C.; Oka, M.; Watanabe, M.; Enomoto, S.; Niwa, T.; et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int. J. Cancer 2014, 134, 1445–1457. [Google Scholar] [CrossRef]
- Dacosta, N.A.; Kinare, S.G. Association of lung carcinoma and tuberculosis. J. Postgrad. Med. 1991, 37, 185–189. [Google Scholar]
- Sakurai, R.; Sasaki, R.; Yamaguchi, M.; Shibata, A.; Aoki, K. Prognosis of female patients with pulmonary tuberculosis. Jpn. J. Med. 1989, 28, 471–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, Y.M.; Church, W.R.; Ravikrishnan, K.P. Lung carcinoma superimposed on pulmonary tuberculosis. Radiology 1976, 119, 307–312. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.; Miller, J.E.; McLaughlin, J.S. Pulmonary tuberculosis and coexisting carcinoma: A 10-year experience and review of the literature. Am. Surg. 1975, 41, 405–408. [Google Scholar]
- Ardies, C.M. Inflammation as cause for scar cancers of the lung. Integr. Cancer Ther. 2003, 2, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassegue, B.; Griendling, K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.T.; Liu, H.; Mao, M.J.; Tan, Y.; Mo, X.Q.; Meng, X.J.; Cao, M.T.; Zhong, C.Y.; Liu, Y.; Shan, H.; et al. Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol. Cancer 2019, 18, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, H.; Wang, F.; Gu, Q.; Xu, X. Snail involves in the transforming growth factor beta1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS ONE 2011, 6, e23322. [Google Scholar] [CrossRef] [Green Version]
- Murata-Kamiya, N.; Kurashima, Y.; Teishikata, Y.; Yamahashi, Y.; Saito, Y.; Higashi, H.; Aburatani, H.; Akiyama, T.; Peek, R.M., Jr.; Azuma, T.; et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 2007, 26, 4617–4626. [Google Scholar] [CrossRef] [Green Version]
- Cane, G.; Ginouves, A.; Marchetti, S.; Busca, R.; Pouyssegur, J.; Berra, E.; Hofman, P.; Vouret-Craviari, V. HIF-1alpha mediates the induction of IL-8 and VEGF expression on infection with Afa/Dr diffusely adhering E. coli and promotes EMT-like behaviour. Cell. Microbiol. 2010, 12, 640–653. [Google Scholar] [CrossRef]
- Gupta, P.K.; Tripathi, D.; Kulkarni, S.; Rajan, M.G. Mycobacterium tuberculosis H37Rv infected THP-1 cells induce epithelial mesenchymal transition (EMT) in lung adenocarcinoma epithelial cell line (A549). Cell. Immunol. 2016, 300, 33–40. [Google Scholar] [CrossRef]
- Holla, S.; Ghorpade, D.S.; Singh, V.; Bansal, K.; Balaji, K.N. Mycobacterium bovis BCG promotes tumor cell survival from tumor necrosis factor-alpha-induced apoptosis. Mol. Cancer 2014, 13, 210. [Google Scholar] [CrossRef] [Green Version]
- Bernard, K.; Hecker, L.; Luckhardt, T.R.; Cheng, G.; Thannickal, V.J. NADPH oxidases in lung health and disease. Antioxid. Redox Signal. 2014, 20, 2838–2853. [Google Scholar] [CrossRef] [Green Version]
- Amara, N.; Goven, D.; Prost, F.; Muloway, R.; Crestani, B.; Boczkowski, J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 2010, 65, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Sutcliffe, A.; Hollins, F.; Gomez, E.; Saunders, R.; Doe, C.; Cooke, M.; Challiss, R.A.; Brightling, C.E. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 267–274. [Google Scholar] [CrossRef]
- Fu, P.; Mohan, V.; Mansoor, S.; Tiruppathi, C.; Sadikot, R.T.; Natarajan, V. Role of nicotinamide adenine dinucleotide phosphate-reduced oxidase proteins in Pseudomonasaeruginosa-induced lung inflammation and permeability. Am. J. Respir. Cell Mol. Biol. 2013, 48, 477–488. [Google Scholar] [CrossRef] [Green Version]
- Manickam, N.; Patel, M.; Griendling, K.K.; Gorin, Y.; Barnes, J.L. RhoA/Rho kinase mediates TGF-beta1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am. J. Physiol. Ren. Physiol. 2014, 307, F159–F171. [Google Scholar] [CrossRef] [Green Version]
- Gasche, J.A.; Hoffmann, J.; Boland, C.R.; Goel, A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int. J. Cancer 2011, 129, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Hilbert, D.M.; Kopf, M.; Mock, B.A.; Kohler, G.; Rudikoff, S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J. Exp. Med. 1995, 182, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Connolly, E.C.; Freimuth, J.; Akhurst, R.J. Complexities of TGF-beta targeted cancer therapy. Int. J. Biol. Sci. 2012, 8, 964–978. [Google Scholar] [CrossRef] [Green Version]
- Li, C.W.; Xia, W.; Huo, L.; Lim, S.O.; Wu, Y.; Hsu, J.L.; Chao, C.H.; Yamaguchi, H.; Yang, N.K.; Ding, Q.; et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012, 72, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.Y.; Tang, S.J.; Chuang, M.J.; Cha, T.L.; Li, J.Y.; Sun, G.H.; Sun, K.H. TNF-alpha induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3beta-dependent mechanism. Mol. Cancer Res. 2012, 10, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Forte, M.; Palmerio, S.; Yee, D.; Frati, G.; Sciarretta, S. Functional Role of Nox4 in Autophagy. Adv. Exp. Med. Biol. 2017, 982, 307–326. [Google Scholar] [CrossRef]
- Sobhakumari, A.; Schickling, B.M.; Love-Homan, L.; Raeburn, A.; Fletcher, E.V.; Case, A.J.; Domann, F.E.; Miller, F.J., Jr.; Simons, A.L. NOX4 mediates cytoprotective autophagy induced by the EGFR inhibitor erlotinib in head and neck cancer cells. Toxicol. Appl. Pharmacol. 2013, 272, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Luo, W.; Sun, W.; Niu, Y.; Ma, D.L.; Leung, C.H.; Wang, Y.; Lu, J.J.; Chen, X. Psoralidin induced reactive oxygen species (ROS)-dependent DNA damage and protective autophagy mediated by NOX4 in breast cancer cells. Phytomedicine 2016, 23, 939–947. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, L.; Li, P.; Zhao, P. MicroRNA-100 Enhances Autophagy and Suppresses Migration and Invasion of Renal Cell Carcinoma Cells via Disruption of NOX4-Dependent mTOR Pathway. Clin. Transl. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Light, R.W.; Macgregor, M.I.; Luchsinger, P.C.; Ball, W.C., Jr. Pleural effusions: The diagnostic separation of transudates and exudates. Ann. Intern. Med. 1972, 77, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genoula, M.; Marin Franco, J.L.; Dupont, M.; Kviatcovsky, D.; Milillo, A.; Schierloh, P.; Morana, E.J.; Poggi, S.; Palmero, D.; Mata-Espinosa, D.; et al. Formation of Foamy Macrophages by Tuberculous Pleural Effusions Is Triggered by the Interleukin-10/Signal Transducer and Activator of Transcription 3 Axis through ACAT Upregulation. Front. Immunol. 2018, 9, 459. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. J. Cell Biol. 1965, 27, A1–A149. [Google Scholar]
- Yuan, K.; Huang, C.; Fox, J.; Laturnus, D.; Carlson, E.; Zhang, B.; Yin, Q.; Gao, H.; Wu, M. Autophagy plays an essential role in the clearance of Pseudomonas aeruginosa by alveolar macrophages. J. Cell Sci. 2012, 125, 507–515. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.J.; Kim, Y.; Jung, H.; Lee, J.J.; Hong, J.Y. Tuberculous Fibrosis Enhances Tumorigenic Potential via the NOX4–Autophagy Axis. Cancers 2021, 13, 687. https://doi.org/10.3390/cancers13040687
Woo SJ, Kim Y, Jung H, Lee JJ, Hong JY. Tuberculous Fibrosis Enhances Tumorigenic Potential via the NOX4–Autophagy Axis. Cancers. 2021; 13(4):687. https://doi.org/10.3390/cancers13040687
Chicago/Turabian StyleWoo, Seong Ji, Youngmi Kim, Harry Jung, Jae Jun Lee, and Ji Young Hong. 2021. "Tuberculous Fibrosis Enhances Tumorigenic Potential via the NOX4–Autophagy Axis" Cancers 13, no. 4: 687. https://doi.org/10.3390/cancers13040687
APA StyleWoo, S. J., Kim, Y., Jung, H., Lee, J. J., & Hong, J. Y. (2021). Tuberculous Fibrosis Enhances Tumorigenic Potential via the NOX4–Autophagy Axis. Cancers, 13(4), 687. https://doi.org/10.3390/cancers13040687





