The Vault Nanoparticle: A Gigantic Ribonucleoprotein Assembly Involved in Diverse Physiological and Pathological Phenomena and an Ideal Nanovector for Drug Delivery and Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Minor Components of the Vault Nanoparticle
2.1. Poly(ADP-Ribose) Polymerase
2.2. Telomerase-Associated Protein-1 and Untranslated RNAs
3. The Involvement of the Vault Nanoparticle in Normal and Pathological Processes
3.1. How the Vault Nanoparticle Participates in Diverse Cellular Regulatory Mechanisms and Pathways
3.2. The Role of the Vault Nanoparticle in Virus Infection and Inflammation
3.3. Regulatory Mechanisms under Control of vtRNAs
3.4. Control Mechanisms of MVP Gene Expression
4. Vault-Related Multidrug Resistance
5. Investigations on Vault Localization and Trafficking Provide Further Hints on Its Biological Roles
6. The Evolutionary History of the Vault Nanoparticle
7. Vault as a Tool for Drug Delivery
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kedersha, N.L.; Rome, L.H. Isolation and Characterization of a Novel Ribonucleoprotein Particle: Large Structures Contain a Single Species of Small RNA. J. Cell Biol. 1986, 103, 699–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedersha, N.L.; Heuser, J.E.; Chugani, D.C.; Rome, L.H. Vaults. III. Vault ribonucleoprotein particles open into flower-like structures with octagonal symmetry. J. Cell Biol. 1991, 112, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Kickhoefer, V.A.; Searles, R.P.; Kedersha, N.L.; Garber, M.E.; Johnson, D.L.; Rome, L.H. Vault ribonucleoprotein particles from rat and bullfrog contain a related small RNAthat is transcribed by RNA polymerase III. J. Biol. Chem. 1993, 268, 7868–7873. [Google Scholar] [CrossRef]
- Kickhoefer, V.A.; Rajavel, K.S.; Scheffer, G.L.; Dalton, W.S.; Scheper, R.J.; Rome, L.H. Vaults are up-regulated in multidrug-resistant cancer cell lines. J. Biol. Chem. 1998, 273, 8971–8974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zon, A.; Mossink, M.H.; Schoester, M.; Scheffer, G.L.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. Multiple human vault RNAs. Expression and association with the vault complex. J. Biol. Chem. 2001, 276, 37715–37721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kickhoefer, V.A.; Siva, A.C.; Kedersha, N.L.; Inman, E.M.; Ruland, C.; Streuli, M.; Rome, L.H. Vault protein, VPARP, is a novel poly(ADP-ribose) polymerase. J. Cell Biol. 1999, 146, 917–928. [Google Scholar] [CrossRef] [Green Version]
- Kickhoefer, V.A.; Stephen, A.G.; Harrington, L.; Robinson, M.O.; Rome, L.H. Vaults and telomerase share a common subunit, TEP1. J. Biol. Chem. 1999, 274, 32712–32717. [Google Scholar] [CrossRef] [Green Version]
- Kickhoefer, V.A.; Poderycki, M.J.; Chan, E.K.; Rome, L.H. The La RNA-binding protein interacts with the vault RNA and is a vault-associated protein. J. Biol. Chem. 2002, 277, 41282–41286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, W.; Steiner, E.; Grusch, M.; Elbling, L.; Micksche, M. Vaults and the major vault protein: Novel roles in signal pathway regulation and immunity. Cell. Mol. Life Sci. 2009, 66, 43–61. [Google Scholar] [CrossRef]
- Han, M.; Kickhoefer, V.A.; Nemerow, G.R.; Rome, L.H. Targeted vault nanoparticles engineered with an endosomolytic peptide deliver biomolecules to the cytoplasm. ACS Nano 2011, 5, 6128–6137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kickhoefer, V.A.; Han, M.; Raval-Fernandes, S.; Poderycki, M.J.; Moniz, R.J.; Vaccari, D.; Silvestry, M.; Stewart, P.L.; Kelly, K.A.; Rome, L.H. Targeting vault nanoparticles to specific cell surface receptors. ACS Nano 2009, 3, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Kato, K.; Yamashita, E.; Sumizawa, T.; Zhou, Y.; Yao, M.; Iwasaki, K.; Yoshimura, M.; Tsukihara, T. The structure of rat liver vault at 3.5 Angstrom resolution. Science 2009, 323, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Zhang, X.; Mrazek, J.; Kickhoefer, V.A.; Lai, M.; Ng, H.L.; Yang, O.O.; Rome, L.H.; Zhou, Z.H. Solution Structures of Engineered Vault Particles. Structure 2018, 26, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.G.; Raval-Fernandes, S.; Huynh, T.; Torres, M.; Kickhoefer, V.A.; Rome, L.H. Assembly of vault-like particles in insect cells expressing only the major vault protein. J. Biol. Chem. 2001, 276, 23217–23220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrazek, J.; Toso, D.; Ryazantsev, S.; Zhang, X.; Zhou, Z.H.; Fernandez, B.C.; Kickhoefer, V.A.; Rome, L.H. Polyribosomes are molecular 3D nanoprinters that orchestrate the assembly of vault particles. ACS Nano 2014, 8, 11552–11559. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Kickhoefer, V.A.; Rome, L.H.; Foellmer, O.K.; Mahendra, S. Synthesis and assembly of human vault particles in yeast. Biotechnol. Bioeng. 2018, 115, 2941–2950. [Google Scholar] [CrossRef]
- Yang, J.; Kickhoefer, V.A.; Ng, B.C.; Gopal, A.; Bentolila, L.A.; John, S.; Tolbert, S.H.; Rome, L.H. Vaults are dynamically unconstrained cytoplasmic nanoparticles capable of half vault exchange. ACS Nano 2010, 4, 7229–7240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suprenant, K.A. Vault ribonucleoprotein particles: Sarcophagi, gondolas, or safety deposit boxes? Biochemistry 2002, 41, 14447–14454. [Google Scholar] [CrossRef]
- Daly, T.K.; Sutherland-Smith, A.J.; Penny, D. In silico resurrection of the major vault protein suggests it is ancestral in modern eukaryotes. Genome Biol. Evol. 2013, 5, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Steiner, E.; Holzmann, K.; Elbling, L.; Micksche, M.; Berger, W. Cellular functions of vaults and their involvement in multidrug resistance. Curr. Drug Targets 2006, 7, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Ficca, M.L.; Meyer, R.G.; Jacobson, E.L.; Jacobson, M.K. Poly(ADPribose) polymerases: Managing genome stability. Int. J. Biochem. Cell Biol. 2005, 37, 920–926. [Google Scholar] [CrossRef]
- Nguewa, P.A.; Miguel, A.; Fuertes, M.A.; Valladares, B.; Alonso, C.; Pérez, J.M. Poly(ADP-ribose) polymerases: Homology, structural domains and functions: Novel therapeutical applications. Progr. Biophys. Mol. Biol. 2005, 88, 143–172. [Google Scholar] [CrossRef]
- Morales, J.C.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of poly (ADP-ribose) polymerase (PARP). Mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Yau, Y.H.; Sinha, A.; Tan, T.; Kickhoefer, V.A.; Rome, L.H.; Lee, H.; Shochat, S.G.; Lim, S. Modulation of the vault protein-protein interaction for tuning of molecular release. Sci. Rep. 2017, 7, 14816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citarelli, M.; Teotia, S.; Lamb, R.S. Evolutionary history of the poly(ADP-ribose) polymerase gene family in eukaryotes. BMC Evol. Biol. 2010, 10, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bork, P.; Hofmann, K.; Bucher, P.; Neuwald, A.F.; Altschul, S.F.; Koonin, E.V. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997, 11, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bork, P.; Rohde, K. More von Willebrand factor type A domains? Sequence similarities with malaria thrombospondin-related anonymous protein, dihydropyridine-sensitive calcium channel and inter-alpha-trypsin inhibitor. Biochem. J. 1991, 279, 908–910. [Google Scholar] [CrossRef]
- Whittaker, C.A.; Hynes, R.O. Distribution and Evolution of von Willebrand/Integrin a Domains: Widely Dispersed Domains with Roles in Cell. Mol. Biol. Cell. 2002, 13, 3369–3387. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Nagasawa, D.T.; Spasic, M.; Amolis, M.; Choy, W.; Garcia, H.M.; Prins, R.M.; Liau, L.M.; Yang, I. Endogenous vaults and bioengineered vault nanoparticles for treatment of glioblastomas: Implications for future targeted therapies. Neurosurg. Clin. N. Am. 2012, 23, 451–458. [Google Scholar] [CrossRef]
- Poderycki, M.J.; Kickhoefer, V.A.; Kaddis, C.S.; Raval-Fernandes, S.; Johansson, E.; Zink, J.I.; Loo, J.A.; Rome, L.H. The vault exterior shell is a dynamic structure that allows incorporation of vault-associated proteins into its interior. Biochemistry 2006, 45, 12184–12193. [Google Scholar] [CrossRef] [Green Version]
- Van Zon, A.; Mossink, M.H.; Schoester, M.; Houtsmuller, A.B.; Scheffer, G.L.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. The formation of vault-tubes: A dynamic interaction between vaults and vault PARP. J. Cell. Sci. 2003, 116, 4391–4400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, L.; McPhail, T.; Mar, V.; Zhou, W.; Oulton, R.; Bass, M.B.; Arruda, I.; Robinson, M.O. A mammalian telomerase-associated protein. Science 1997, 275, 973–977. [Google Scholar] [CrossRef]
- Collins, K.; Kobayashi, R.; Greider, C.W. Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell 1995, 81, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Lipinska, N.; Romaniuk, A.; Paszel-Jaworska, A.; Toton, E.; Kopczynski, P.; Rubis, B. Telomerase and drug resistance in cancer. Cell Mol. Life Sci. 2017, 74, 4121–4132. [Google Scholar] [CrossRef]
- Harrington, L.; Zhou, W.; McPhail, T.; Oulton, R.; Yeung, D.S.; Mar, V.; Bass, M.B.; Robinson, M.O. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997, 11, 3109–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kickhoefer, V.A.; Liu, Y.; Kong, L.B.; Snow, B.E.; Stewart, P.L.; Harrington, L.; Rome, L.H. The telomerase/vault-associated protein TEP1 is required for vault RNA stability and its association with the vault particle. J. Cell Biol. 2001, 152, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Snow, B.E.; Hande, M.P.; Baerlocher, G.; Kickhoefer, V.A.; Yeung, D.; Wakeham, A.; Itie, A.; Siderovski, D.P.; Lansdorp, P.M.; et al. Telomerase-associated protein TEP1 is not essential for telomerase activity or telomere length maintenance in vivo. Mol. Cell. Biol. 2000, 20, 8178–8184. [Google Scholar] [CrossRef]
- Liu, Y.; Snow, B.E.; Kickhoefer, V.A.; Erdmann, N.; Zhou, W.; Wakeham, A.; Gomez, M.; Rome, L.H.; Harrington, L. Vault poly(ADP-ribose) polymerase is associated with mammalian telomerase and is dispensable for telomerase function and vault structure in vivo. Mol. Cell. Biol. 2004, 24, 5314–5323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poderycki, M.J.; Rome, L.H.; Harrington, L.; Kickhoefer, V.A. The p80 homology region of TEP1 is sufficient for its association with the telomerase and vault RNAs, and the vault particle. Nucleic Acids Res. 2005, 33, 893–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval-Fernandes, S.; Kickhoefer, V.A.; Kitchen, C.; Rome, L.H. Increased susceptibility of vault poly(-ADP-ribose) polymerase-deficient mice to carcinogen-induced tumorigenesis. Cancer Res. 2005, 65, 8846–8852. [Google Scholar] [CrossRef] [Green Version]
- Van Zon, A.; Mossink, M.H.; Schoester, M.; Scheffer, G.L.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. Structural domains of vault proteins: A role for the coiled coil domain in vault assembly. Biochem. Biophys. Res. Commun. 2002, 291, 535–541. [Google Scholar] [CrossRef]
- Mikyas, Y.; Makabi, M.; Raval-Fernandes, S.; Harrington, L.; Kickhoefer, V.A.; Rome, L.H.; Stewart, P.L. Cryoelectron microscopy imaging of recombinant and tissue derived vaults: Localization of the MVP N termini and VPARP. J. Mol. Biol. 2004, 344, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Kickhoefer, V. The TROVE module: A common element in Telomerase, Ro, and Vault ribonucleoproteins. BMC Bioinform. 2003, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Taylor, D.W.; Fowler, C.C.; Galan, J.E.; Wang, H.-W.; Wolin, S.L. An RNA Degradation Machine Sculpted by Ro Autoantigen and Noncoding RNA. Cell 2013, 153, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Kolev, N.G.; Rajan, K.S.; Tycowski, K.T.; Toh, J.Y.; Shi, H.; Lei, Y.; Michaeli, S.; Tschudi, C. The vault RNA of Trypanosoma brucei plays a role in the production of trans-spliced mRNA. J. Biol. Chem. 2019, 294, 15559–15574. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.J.; Fuchs, G.; Fu, C.; Wolin, S.L.; Reinisch, K.M. Structural insights into RNA quality control: The Ro autoantigen binds misfolded RNAs via its central cavity. Cell 2005, 121, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Koonin, E.V.; Aravind, L. The NACHT family—a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem. Sci. 2000, 25, 223–224. [Google Scholar] [CrossRef]
- Li, D.; Roberts, R. WD-repeat proteins: Structure characteristics, biological function, and their involvement in human diseases. Cell. Mol. Life Sci. 2001, 58, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Sabin, L.R.; Delas, M.J.; Hannon, G.J. Dogma derailed: The many influences of RNA on the genome. Mol. Cell 2013, 49, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Stadler, P.F.; Chen, J.J.; Hackermuller, J.; Hoffmann, S.; Horn, F.; Khaitovich, P.; Kretzschmar, A.K.; Mosig, A.; Prohaska, S.J.; Qi, X.; et al. Evolution of vault RNAs. Mol. Biol. Evol. 2009, 26, 1975–1991. [Google Scholar] [CrossRef] [Green Version]
- Büscher, M.; Horos, R.; Hentze, M.W. ‘High vault-age’: Non-coding RNA control of autophagy. Open Biol. 2020, 10, 190307. [Google Scholar] [CrossRef] [Green Version]
- Hahne, J.C.; Lampis, A.; Valeri, N. Vault RNAs: Hidden gems in RNA and protein regulation. Cell Mol. Life Sci. 2020. [Google Scholar] [CrossRef]
- Nandy, C.; Mrázek, J.; Stoiber, H.; Grässer, F.A.; Hüttenhofer, A.; Polacek, N. Epstein–Barr virus-induced expression of a novel human vault RNA. J. Mol. Biol. 2009, 388, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Abbondanza, C.; Rossi, V.; Roscigno, A.; Gallo, L.; Belsito, A.; Piluso, G.; Medici, N.; Nigro, V.; Molinari, A.M.; Moncharmont, B.; et al. Interaction of vault particles with estrogen receptor in the MCF-7 breast cancer cell. J. Cell. Biol. 1998, 141, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Lötsch, D.; Steiner, E.; Holzmann, K.; Spiegl-Kreinecker, S.; Pirker, C.; Hlavaty, J.; Petznek, H.; Hegedus, B.; Garay, T.; Mohr, T.; et al. Major vault protein supports glioblastoma survival and migration by upregulating the EGFR/PI3K signalling axis. Oncotarget 2013, 4, 1904–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Fotouhi-Ardakani, N.; Wu, L.; Maoui, M.; Wang, S.; Banville, D.; Shen, S.H. PTEN associates with the vault particles in HeLa cells. J. Biol. Chem. 2002, 277, 40247–40252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Neo, S.P.; Gunaratne, J.; Poulsen, A.; Boping, L.; Ong, E.H.; Sangthongpitag, K.; Pendharkar, V.; Hill, J.; Cohen, S.M. Feedback regulation on PTEN/AKT pathway by the ER stress kinase PERK mediated by interaction with the Vault complex. Cell. Signal. 2015, 27, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Pasillas, M.P.; Shields, S.; Reilly, R.; Strnadel, J.; Behl, C.; Park, R.; Yates, J.R., 3rd; Klemke, R.; Gonias, S.L.; Coppinger, J.A. Proteomic analysis reveals a role for Bcl2-associated athanogene 3 and major vault protein in resistance to apoptosis in senescent cells by regulating ERK1/2 activation. Mol. Cell. Proteom. 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, P.; Wan, Y.; Yan, Y.; Wang, Y.; Luo, N.; Deng, Y.; Fan, X.; Zhou, J.; Li, Y.; Wang, Z.; et al. MVP interacts with YPEL4 and inhibits YPEL4-mediated-activities of the ERK signal pathway. Biochem. Cell Biol. 2010, 88, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, W.; Phillips, J.B.; Arora, R.; McClellan, S.; Li, J.; Kim, J.H.; Sobol, R.W.; Tan, M. Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene 2019, 38, 88–102. [Google Scholar] [CrossRef]
- Kolli, S.; Zito, C.I.; Mossink, M.H.; Wiemer, E.A.; Bennett, A.M. The major vault protein is a novel substrate for the tyrosine phosphatase SHP-2 and scaffold protein in epidermal growth factor signaling. J. Biol. Chem. 2004, 279, 29374–29385. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Lee, S.; Mian, M.F.; Yun, S.U.; Song, M.; Yi, K.S.; Ryu, S.H.; Suh, P.G. Crosstalk between Src and major vault protein in epidermal growth factor-dependent cell signalling. FEBS J. 2006, 273, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Wang, Y.H.; Hsieh, C.Y.; Suzuki, Y.J. Major vault protein regulates cell growth/survival signaling through oxidative modifications. Cell Signal. 2016, 28, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, C.; Li, S.; Chen, X.; Wiemer, E.A.; Wang, J.; Wei, N.; Deng, X.W. Major vault protein, in concert with constitutively photomorphogenic 1, negatively regulates c-Jun-mediated activator protein 1 transcription in mammalian cells. Cancer Res. 2005, 65, 5835–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashita, K.; Ikeda, R.; Takeda, Y.; Sumizawa, T.; Furukawa, T.; Yamaguchi, T.; Akiyama, S.; Yamada, K. Major vault protein forms complexes with hypoxia-inducible factor (HIF)-1α and reduces HIF-1 α level in ACHN human renal adenocarcinoma cells. Cancer Sci. 2010, 101, 920–926. [Google Scholar] [CrossRef]
- Ben, J.; Zhang, Y.; Zhou, R.; Zhang, H.; Zhu, X.; Li, X.; Zhang, H.; Li, N.; Zhou, X.; Bai, H.; et al. Major vault protein regulates class a scavenger receptor-mediated tumor necrosis factor-alpha synthesis and apoptosis in macrophages. J. Biol. Chem. 2013, 288, 20076–20084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossi, S.; Fenini, G.; Kockmann, T.; Hennig, P.; Di Filippo, M.; Beer, H.D. Inactivation of the Cytoprotective Major Vault Protein by Caspase-1 and -9 in Epithelial Cells during Apoptosis. J. Investig. Dermatol. 2020, 140, 1335–1345. [Google Scholar] [CrossRef]
- Liu, S.; Hao, Q.; Peng, N.; Yue, X.; Wang, Y.; Chen, Y.; Wu, J.; Zhu, Y. Major vault protein: A virus-induced host factor against viral replication through the induction of type-I interferon. Hepatology 2012, 56, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Peng, N.; Xie, J.; Hao, Q.; Zhang, M.; Zhang, Y.; Zhanchuan, X.; Gang, X.; Zhao, F.; Wang, Q.; et al. Human hepatitis B virus surface and e antigens inhibit major vault protein signaling in interferon induction pathways. J. Hepatol. 2015, 62, 1015–1023. [Google Scholar] [CrossRef]
- Yu, H.; Li, M.; He, R.; Fang, P.; Wang, Q.; Yi, Y.; Wang, F.; Zhou, L.; Zhang, Y.; Chen, A.; et al. Major vault protein promotes hepatocellular carcinoma through targeting interferon regulatory factor 2 and decreasing p53 activity. Hepatology 2020, 72, 518–534. [Google Scholar] [CrossRef]
- Peng, N.; Liu, S.; Xia, Z.; Ren, S.; Feng, J.; Jing, M.; Gao, X.; Wiemer, E.A.C.; Zhu, J. Inducible Major Vault Protein Plays a Pivotal Role in Double-Stranded RNA- or Virus-Induced Proinflammatory Response. J. Immunol. 2016, 196, 2753–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben, J.; Jiang, B.; Wang, D.; Liu, Q.; Zhang, Y.; Qi, Y.; Tong, X.; Chen, L.; Liu, X.; Zhang, Y.; et al. Major vault protein suppresses obesity and atherosclerosis through inhibiting IKK-NF-κB signaling mediated inflammation. Nat. Commun. 2019, 10, 1801. [Google Scholar] [CrossRef]
- Horos, R.; Büscher, M.; Kleinendorst, R.; Alleaume, A.M.; Tarafder, A.K.; Schwarzl, T.; Dziuba, D.; Tischer, C.; Zielonka, E.M.; Adak, A.; et al. The small noncoding vault RNA1-1 acts as a riboregulator of autophagy. Cell 2019, 176, 1054–1067. [Google Scholar] [CrossRef] [Green Version]
- Amort, M.; Nachbauer, B.; Tuzlak, S.; Kieser, A.; Schepers, A.; Villunger, A.; Polacek, N. Expression of the vault RNA protects cells from undergoing apoptosis. Nat. Commun. 2015, 6, 7030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracher, L.; Ferro, I.; Pulido-Quetglas, C.; Ruepp, M.D.; Johnson, R.; Polacek, N. Human vtRNA1-1 Levels Modulate Signaling Pathways and Regulate Apoptosis in Human Cancer Cells. Biomolecules 2020, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.; Kvist, A.; Vallon-Christersson, J.; Medstrand, P.; Borg, Å.; Rovira, C. The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat. Cell Biol. 2009, 11, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Sajini, A.A.; Choudhury, N.R.; Wagner, R.E.; Bornelov, S.; Selmi, T.; Spanos, C.; Dietmann, S.; Rappsilber, J.; Michlewski, G.; Frye, M. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat. Commun. 2019, 10, 2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ou Yang, H.; An, X.; Liu, S. Vault RNA partially induces drug resistance of human tumor cells MCF-7 by binding to the RNA/DNA-binding protein PSF and inducing oncogene GAGE6. PLoS ONE 2018, 13, e0191325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, G.L.; Wijngaard, P.L.; Flens, M.J.; Izquierdo, M.A.; Slovak, M.L.; Pinedo, H.M.; Meijer, C.J.; Clevers, H.C.; Scheper, R.J. The drug resistance-related protein LRP is the human major vault protein. Nat. Med. 1995, 6, 578–582. [Google Scholar] [CrossRef]
- Johnson, H.; Del Rosario, A.M.; Bryson, B.D.; Schroeder, M.A.; Sarkaria, J.N.; White, F.M. Molecular characterization of EGFR and EGFRvIII signaling networks in human glioblastoma tumor xenografts. Mol. Cell. Proteom. 2012, 11, 1724–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losert, A.; Lötsch, D.; Lackner, A.; Koppensteiner, H.; Peter-Vörösmarty, B.; Steiner, E.; Holzmann, K.; Grunt, T.; Schmid, K.; Marian, B.; et al. The major vault protein mediates resistance to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Lett. 2012, 2, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Ginn-Pease, M.E.; Eng, C. Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) has nuclear localization signal-like sequences for nuclear import mediated by major vault protein. Cancer Res. 2005, 65, 4108–4116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.H.; Zhang, P.; Chen, W.D.; Li, D.D.; Wu, X.Q.; Deng, R.; Jiao, L.; Li, X.; Ji, J.; Feng, G.K.; et al. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy 2015, 11, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Minaguchi, T.; Waite, K.A.; Eng, C. Nuclear localization of PTEN is regulated by Ca2+ through a tyrosil phosphorylation-independent conformational modification in major vault protein. Cancer Res. 2006, 66, 11677–11682. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.-H.; Eng, C. Nuclear-Cytoplasmic Partitioning of Phosphatase and Tensin Homologue Deleted on Chromosome 10 (PTEN) Differentially Regulates the Cell Cycle and Apoptosis. Cancer Res. 2005, 65, 8096–8100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fels, D.R.; Koumenis, C. The PERK/eIF2α/ATF4 Module of the UPR in Hypoxia Resistance and Tumor Growth. Cancer Biol. Ther. 2006, 5, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Behl, C. Breaking BAG: The co-chaperone BAG3 in health and disease. Trends Pharmacol. Sci. 2016, 37, 672–688. [Google Scholar] [CrossRef]
- Chiappetta, C.; Basile, A.; Barbieri, A.; Falco, A.; Rosati, A.; Festa, M.; Pasquinelli, R.; Califano, D.; Palma, G.; Costanzo, R.; et al. The anti-apoptotic BAG3 protein is expressed in lung carcinomas and regulates small cell lung carcinoma (SCLC) tumor growth. Oncotarget 2014, 5, 6846–6853. [Google Scholar] [CrossRef] [Green Version]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [Green Version]
- Flem-Karlsen, K.; Fodstad, Ø.; Tan, M.; Nunes-Xavier, C.E. B7-H3 in Cancer—Beyond Immune Regulation. Trends Cancer 2018, 4, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhao, G.-D.; Shi, Z.; Qi, L.-L.; Zhou, L.Y.; Fu, Z.-X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016, 12, 3045–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.; Chen, W.; Zhu, J.; Wu, G.; Shen, R.; Xi, M.; Sun, H. Therapeutic potential of targeting SHP2 in human developmental disorders and cancers. Eur. J. Med. Chem. 2020, 190, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Kellner, R.; Volknandt, W. Major vault protein of electric ray is a phosphoprotein. Neurochem. Res. 1998, 23, 39–46. [Google Scholar] [CrossRef]
- Ehrnsperger, C.; Volknandt, W. Major vault protein is a substrate of endogenous protein kinases in CHO and PC12 cells. Biol. Chem. 2001, 382, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Bjorge, J.D.; Jakymiw, A.; Fujita, D.J. Selected glimpses into the activation and function of Src kinase. Oncogene 2000, 19, 5620–5635. [Google Scholar] [CrossRef] [Green Version]
- Tzivion, G.; Gupta, V.S.; Kaplun, L.; Balan, V. 14-3-3 proteins as potential oncogenes. Semin. Cancer Biol. 2006, 16, 203–213. [Google Scholar] [CrossRef]
- Tang, S.; Bai, C.; Yang, P.; Chen, X. 14-3-3ε boosts bleomycin-induced DNA damage response by inhibiting the drug-resistant activity of MVP. J. Proteome Res. 2013, 12, 2511–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avalle, L.; Pensa, S.; Regis, G.; Novelli, F.; Poli, V. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAKSTAT 2012, 1, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.L.; Nakamura, K. The c-jun kinase/stress-activated pathway: Regulation, function and role in human disease. Biochim. Biophys. Acta 2007, 1773, 1341–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, A.; Gelmann, E.P. The ubiquitin-proteasome pathway and its role in cancer. J. Clin. Oncol. 2005, 23, 4776–4789. [Google Scholar] [CrossRef]
- Vizcaíno, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015, 152, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadatzadeh, M.R.; Elmi, A.N.; Pandya, P.H.; Bijangi-Vishehsaraei, K.; Ding, J.; Stamatkin, C.W.; Cohen-Gadol, A.A.; Pollok, K.E. The role of MDM2 in promoting genome stability versus instability. Int. J. Mol. Sci. 2017, 18, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.C.; Lee, J.; Choi, Y. Tumor necrosis factor receptor associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 2015, 266, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 2, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantazopoulou, V.I.; Georgiou, S.; Kakoulidis, P.; Giannakopoulou, S.N.; Tseleni, S.; Stravopodis, D.J.; Anastasiadou, E. From the Argonauts Mythological Sailors to the Argonautes RNA-Silencing Navigators: Their Emerging Roles in Human-Cell Pathologies. Int. J. Mol. Sci. 2020, 21, 4007. [Google Scholar] [CrossRef]
- Patton, J.G.; Porro, E.B.; Galceran, J.; Tempst, P.; Nadal-Ginard, B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 1993, 7, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Feng, T.T.; Lian, Y.; Zhang, G.; Garen, A.; Song, X. Role of human noncoding RNAs in the control of tumorigenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12956–12961. [Google Scholar] [CrossRef] [Green Version]
- Kickhoefer, V.A. A new role for vault RNA-TEP1 complexes in mRNA production in trypanosomes. J. Biol. Chem. 2019, 294, 15575–15576. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, M.A.; Scheffer, G.L.; Flens, M.J.; Giaccone, G.; Broxterman, H.J.; Meijer, C.J.; van der Valk, P.; Scheper, R.J. Broad distribution of the multidrug resistance-related vault lung resistance protein in normal human tissues and tumors. Am. J. Pathol. 1996, 148, 877–887. [Google Scholar] [PubMed]
- Ikeda, R.; Nishizawa, Y.; Tajitsu, Y.; Minami, K.; Mataki, H.; Masuda, S.; Furukawa, T.; Akiyama, S.; Yamada, K.; Takeda, Y. Regulation of major vault protein expression by upstream stimulating factor 1 in SW620 human colon cancer cells. Oncol. Rep. 2014, 31, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Golkaramnay, E.; Inman, E.; Rome, L.; Volknandt, W. Recombinant major vault protein is targeted to neuritic tips of PC12 cells. J. Cell Biol. 1999, 144, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Sisodiya, S.M.; Martinian, L.; Scheffer, G.L.; van der Valk, P.; Cross, J.H.; Scheper, R.J.; Harding, B.N.; Thom, M. Major vault protein, a marker for drug resistance, is upregulated in refractory epilepsy. Epilepsia 2003, 44, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Paspalas, C.D.; Perley, C.C.; Venkitaramani, D.V.; Goebel-Goody, S.M.; Zhang, Y.; Kurup, P.; Mattis, J.H.; Lombroso, P.J. Major vault protein is expressed along the nucleus-neurite axis and associates with mRNAs in cortical neurons. Cereb. Cortex 2009, 19, 1666–1677. [Google Scholar] [CrossRef] [Green Version]
- Ip, J.P.K.; Nagakura, I.; Petravicz, J.; Li, K.; Wiemer, E.A.C.; Sur, M. Major Vault Protein, a Candidate Gene in 16p11.2 Microdeletion Syndrome, Is Required for the Homeostatic Regulation of Visual Cortical Plasticity. J. Neurosci. 2018, 38, 3890–3900. [Google Scholar] [CrossRef] [Green Version]
- Stein, U.; Bergmann, S.; Scheffer, G.L.; Scheper, R.J.; Royer, H.D.; Schlag, P.M.; Walther, W. YB-1 facilitates basal and 5-fluorouracil-inducible expression of the human major vault protein (MVP) gene. Oncogene 2005, 24, 3606–3618. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, T.; Wang, L.; Wang, C.; Zhang, H.; Gao, G.D. Up-regulation of major vault protein in the frontal cortex of patients with intractable frontal lobe epilepsy. J. Neurol. Sci. 2011, 308, 88–93. [Google Scholar] [CrossRef]
- Banerjee Dixit, A.; Sharma, D.; Srivastava, A.; Banerjee, J.; Tripathi, M.; Prakash, D.; Sarat Chandra, P. Upregulation of breast cancer resistance protein and major vault protein in drug resistant epilepsy. Seizure 2017, 47, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, R.; Iwashita, K.; Sumizawa, T.; Beppu, S.; Tabata, S.; Tajitsu, Y.; Shimamoto, Y.; Yoshida, K.; Furukawa, T.; Che, X.F.; et al. Hyperosmotic stress up-regulates the expression of major vault protein in SW620 human colon cancer cells. Exp. Cell. Res. 2008, 314, 3017–3026. [Google Scholar] [CrossRef]
- Ryu, S.J.; Park, S.C. Targeting major vault protein in senescence-associated apoptosis resistance. Expert Opin. Ther. Targets 2009, 13, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S.; Alessio, N.; Acar, M.B.; Mert, E.; Omerli, F.; Peluso, G.; Galderisi, U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 2016, 8, 1316–1329. [Google Scholar] [CrossRef] [Green Version]
- Celestino, A.T.; Levy, D.; Maria Ruiz, J.L.; Bydlowski, S.P. ABCB1, ABCC1, and LRP gene expressions are altered by LDL, HDL, and serum deprivation in a human doxorubicin-resistant uterine sarcoma cell line. Biochem. Biophys. Res. Commun. 2015, 457, 664–668. [Google Scholar] [CrossRef]
- Lehuédé, C.; Li, X.; Dauvillier, S.; Vaysse, C.; Franchet, C.; Clement, E.; Esteve, D.; Longué, M.; Chaltiel, L.; Le Gonidec, S.; et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: Role of the major vault protein (MVP). Breast Cancer Res. 2019, 21, 7. [Google Scholar] [CrossRef] [Green Version]
- Mossink, M.; van Zon, A.; Fränzel-Luiten, E.; Schoester, M.; Scheffer, G.L.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. The genomic sequence of the murine major vault protein and its promoter. Gene 2002, 294, 225–232. [Google Scholar] [CrossRef]
- Lange, C.; Walther, W.; Schwabe, H.; Stein, U. Cloning and initial analysis of the human multidrug resistance-related MVP/LRP gene promoter. Biochem. Biophys. Res. Commun. 2000, 278, 125–133. [Google Scholar] [CrossRef]
- Steiner, E.; Holzmann, K.; Pirker, C.; Elbling, L.; Micksche, M.; Berger, W. SP-transcription factors are involved in basal MVP promoter activity and its stimulation by HDAC inhibitors. Biochem. Biophys. Res. Commun. 2004, 317, 235–243. [Google Scholar] [CrossRef]
- Xiao, Y.S.; Zeng, D.; Liang, Y.K.; Wu, Y.; Li, M.F.; Qi, Y.Z.; Wei, X.L.; Huang, W.H.; Chen, M.; Zhang, G.J. Major vault protein is a direct target of Notch1 signaling and contributes to chemoresistance in triple-negative breast cancer cells. Cancer Lett. 2019, 440–441, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Emre, N.; Raval-Fernandes, S.; Kickhoefer, V.A.; Rome, L.H. Analysis of MVP and VPARP promoters indicates a role for chromatin remodeling in the regulation of MVP. Biochim. Biophys. Acta 2004, 1678, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Tajitsu, Y.; Ikeda, R.; Nishizawa, Y.; Mataki, H.; Che, X.F.; Sumizawa, T.; Nitta, M.; Yamaguchi, T.; Yamamoto, M.; Tabata, S.; et al. Molecular basis for the expression of major vault protein induced by hyperosmotic stress in SW620 human colon cancer cells. Int. J. Mol. Med. 2013, 32, 703–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, E.; Holzmann, K.; Pirker, C.; Elbling, L.; Micksche, M.; Sutterluty, H.; Berger, W. The major vault protein is responsive to and interferes with interferon-gamma-mediated STAT1 signals. J. Cell Sci. 2006, 119, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Liu, J.; Liu, B.; Dong, Y.; Liu, J.; Song, Y.; Sun, Z. p53 suppresses lung resistance-related protein expression through Y-box binding protein 1 in the MCF-7 breast tumor cell line. J. Cell. Physiol. 2011, 226, 3433–3441. [Google Scholar] [CrossRef]
- Hyogotani, A.; Ito, K.; Yoshida, K.; Izumi, H.; Kohno, K.; Amano, J. Association of nuclear YB-1 localization with lung resistance-related protein and epidermal growth factor receptor expression in lung cancer. Clin. Lung Cancer 2012, 13, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, M. An overview of the Notch signalling pathway. Semin. Cell Dev. Biol. 2003, 14, 113–119. [Google Scholar] [CrossRef]
- Fortini, M.E.; Artavanis-Tsakonas, S. The suppressor of hairless protein participates in notch receptor signaling. Cell 1994, 79, 273–282. [Google Scholar] [CrossRef]
- Shimamoto, Y.; Sumizawa, T.; Haraguchi, M.; Gotanda, T.; Jueng, H.C.; Furukawa, T.; Sakata, R.; Akiyama, S. Direct activation of the human major vault protein gene by DNA-damaging agents. Oncol. Rep. 2006, 15, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Fu, Y.; Li, Y.; Han, X. Cisplatin induces expression of drug resistance‑related genes through c‑jun N‑terminal kinase pathway in human lung cancer cells. Cancer Chemother. Pharmacol. 2017, 80, 235–242. [Google Scholar] [CrossRef]
- Shen, W.; Qiu, Y.; Li, J.; Wu, C.; Liu, Z.; Zhang, X.; Hu, X.; Liao, Y.; Wang, H. IL-25 promotes cisplatin resistance of lung cancer cells by activating NF-κB signaling pathway to increase of major vault protein. Cancer Med. 2019, 8, 3491–3501. [Google Scholar] [CrossRef]
- Zheng, C.L.; Sumizawa, T.; Che, X.F.; Tsuyama, S.; Furukawa, T.; Haraguchi, M.; Gao, H.; Gotanda, T.; Jueng, H.C.; Murata, F.; et al. Characterization of MVP and VPARP assembly into vault ribonucleoprotein complexes. Biochem. Biophys. Res. Commun. 2005, 326, 100–107. [Google Scholar] [CrossRef]
- Shults, N.V.; Das, D.; Suzuki, Y.J. Major vault protein in cardiac and smooth muscle. Recept. Clin. Investig. 2016, 3, e1310. [Google Scholar]
- Kitazono, M.; Sumizawa, T.; Takebayashi, Y.; Chen, Z.S.; Furukawa, T.; Nagayama, S.; Tani, A.; Takao, S.; Aikou, T.; Akiyama, S. Multidrug resistance and the lung resistance-related protein in human colon carcinoma SW-620 cells. J. Natl. Cancer Inst. 1999, 91, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Stephen, A.G.; Cao, J.; Tanzer, L.R.; Slapak, C.A.; Harrison, S.D.; Devanarayan, V.; Dantzig, A.H.; Starling, J.J.; Rome, L.H.; et al. A very early induction of major vault protein accompanied by increased drug resistance in U-937 cells. Int. J. Cancer 2002, 97, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo, M.A.; van der Zee, A.G.; Vermorken, J.B.; van der Valk, P.; Beliën, J.A.; Giaccone, G.; Scheffer, G.L.; Flens, M.J.; Pinedo, H.M.; Kenemans, P.; et al. Drug resistance-associated marker Lrp for prediction of response to chemotherapy and prognoses in advanced ovarian carcinoma. J. Natl. Cancer Inst. 1995, 87, 1230–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Arestrom, I.; Virtala, R.; Pisa, P.; Peterson, C.; Gruber, A. High levels of lung resistance related protein mRNA in leukaemic cells from patients with acute myelogenous leukaemia are associated with inferior response to chemotherapy and prior treatment with mitoxantrone. Br. J. Haematol. 1999, 106, 627–633. [Google Scholar] [CrossRef]
- Navarro, L.; Gil-Benso, R.; Megias, J.; Munoz-Hidalgo, L.; San-Miguel, T.; Callaghan, R.C.; Gonzalez-Darder, J.M.; Lopez-Gines, C.; Cerda-Nicolas, M.J. Alteration of major vault protein in human glioblastoma and its relation with EGFR and PTEN status. Neuroscience 2015, 297, 243–251. [Google Scholar] [CrossRef]
- Herlevsen, M.; Oxford, G.; Owens, C.R.; Conaway, M.; Theodorescu, D. Depletion of major vault protein increases doxorubicin sensitivity and nuclear accumulation and disrupts its sequestration in lysosomes. Mol. Cancer Ther. 2007, 6, 1804–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, L.; Wang, J.; Lv, F.; Wang, G.; Li, Y.; Xing, L.; Shen, H.; Zhang, X. Y-box binding protein 1 (YB-1) promotes gefitinib resistance in lung adenocarcinoma cells by activating AKT signaling and epithelial-mesenchymal transition through targeting major vault protein (MVP). Cell. Oncol. 2020. [Google Scholar] [CrossRef]
- Fukushima, H.; Abe, T.; Sakamoto, K.; Tsujimoto, H.; Mizuarai, S.; Oie, S. 3′-ethynylcytidine, an RNA polymerase inhibitor, combined with cisplatin exhibits a potent synergistic growth-inhibitory effect via Vaults dysfunction. BMC Cancer 2014, 14, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, S.C.B.; Matsugami, A.; Katahira, M.; Kumar, P.K.R. Human vault-associated non-coding RNAs bind to mitoxantrone, a chemotherapeutic compound. Nucleic Acids Res. 2005, 33, 4874–4881. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, S.C.B.; Wadhwa, R.; Kumar, P.K.R. Expression of noncoding vault RNA in human malignant cells and its importance in mitoxantrone resistance. Mol. Cancer Res. 2010, 8, 1536–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Scheffer, G.L.; Schroeijers, A.B.; Izquierdo, M.A.; Wiemer, E.A.C.; Scheper, R.J. Lung resistance-related protein/major vault protein and vaults in multidrug-resistant cancer. Curr. Opin. Oncol. 2000, 12, 550–556. [Google Scholar] [CrossRef]
- Ruiz, J.L.; Fernandes, L.R.; Levy, D.; Bydlowski, S.P. Interrelationship between ATP-binding cassette transporters and oxysterols. Biochem. Pharmacol. 2013, 86, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Kimchi-Sarfaty, C.; Sauna, Z.E.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468–7485. [Google Scholar] [CrossRef] [Green Version]
- Bakos, E.; Homolya, L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). Pflug. Arch. 2007, 453, 621–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedlitschky, G.; Hoffmann, U.; Kroemer, H.K. Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin. Drug Metab. Toxicol. 2006, 2, 351–366. [Google Scholar] [CrossRef]
- Zaïr, Z.M.; Singer, D.R. Efflux transporter variants as predictors of drug toxicity in lung cancer patients: Systematic review and meta-analysis. Pharmacogenomics 2016, 17, 1089–1112. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.A.; Scheffer, G.L.; Flens, M.J.; Shoemaker, R.H.; Rome, L.H.; Scheper, R.J. Relationship of LRP-human major vault protein to in vitro and clinical resistance to anticancer drugs. Cytotechnology 1996, 19, 191–197. [Google Scholar] [CrossRef] [PubMed]
- List, A.F.; Spier, C.S.; Grogan, T.M.; Johnson, C.; Roe, D.J.; Greer, J.P.; Wolff, S.N.; Broxterman, H.J.; Scheffer, G.L.; Scheper, R.J.; et al. Overexpression of the major vault transporter protein lung-resistance protein predicts treatment outcome in acute myeloid leukemia. Blood 1996, 87, 2464–2469. [Google Scholar] [CrossRef]
- Izquierdo, M.A.; Shoemaker, R.H.; Flens, M.J.; Scheffer, G.L.; Wu, L.; Prather, T.R.; Scheper, R.J. Overlapping phenotypes of multidrug resistance among panels of human cancer-cell lines. Int. J. Cancer 1996, 65, 230–237. [Google Scholar] [CrossRef]
- Mossink, M.H.; van Zon, A.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. Vaults: A ribonucleoprotein particle involved in drug resistance? Oncogene 2003, 22, 7458–7467. [Google Scholar] [CrossRef] [Green Version]
- Szaflarski, W.; Sujka-Kordowska, P.; Pula, B.; Jaszczyńska-Nowinka, K.; Andrzejewska, M.; Zawierucha, P.; Dziegiel, P.; Nowicki, M.; Ivanov, P.; Zabel, M. Expression profiles of vault components MVP, TEP1 and vPARP and their correlation to other multidrug resistance proteins in ovarian cancer. Int. J. Oncol. 2013, 43, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henríquez-Hernández, L.A.; Moreno, M.; Rey, A.; Lloret, M.; Lara, P.C. MVP expression in the prediction of clinical outcome of locally advanced oral squamous cell carcinoma patients treated with radiotherapy. Radiat. Oncol. 2012, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Siva, A.C.; Raval-Fernandes, S.; Stephen, A.G.; LaFemina, M.J.; Scheper, R.J.; Kickhoefer, V.A.; Rome, L.H. Up-regulation of vaults may be necessary but not sufficient for multidrug resistance. Int. J. Cancer 2001, 92, 195–202. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Januchowski, R.; Nowicki, M.; Zabel, M. vPARP Adjusts MVP Expression in Drug-resistant Cell Lines in Conjunction with MDR Proteins. Anticancer Res. 2017, 37, 3015–3023. [Google Scholar] [PubMed]
- Oguri, T.; Fujiwara, Y.; Ochiai, M.; Fujitaka, K.; Miyazaki, M.; Takahashi, T.; Yokozaki, M.; Isobe, T.; Ohune, T.; Tsuya, T.; et al. Expression of lung-resistance protein gene is not associated with platinum drug exposure in lung cancer. Anticancer Res. 1998, 18, 4159–4162. [Google Scholar] [PubMed]
- Mossink, M.H.; van Zon, A.; Fränzel-Luiten, E.; Schoester, M.; Kickhoefer, V.A.; Scheffer, G.L.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. Disruption of the murine major vault protein (MVP/LRP) gene does not induce hypersensitivity to cytostatics. Cancer Res. 2002, 62, 7298–7304. [Google Scholar]
- Huffman, K.E.; Corey, D.R. Major vault protein does not play a role in chemoresistance or drug localization in a non-small cell lung cancer cell line. Biochemistry 2005, 44, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A. Lung resistance-related protein (LRP) predicts favorable therapeutic outcome in Acute Myeloid Leukemia. Sci. Rep. 2019, 9, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megger, D.A.; Bracht, T.; Kohl, M.; Ahrens, M.; Naboulsi, W.; Weber, F.; Hoffmann, A.C.; Stephan, C.; Kuhlmann, K.; Eisenacher, M.; et al. Proteomic differences between hepatocellular carcinoma and nontumorous liver tissue investigated by a combined gel-based and label-free quantitative proteomics study. Mol. Cell. Proteom. 2013, 12, 2006–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, P.; West, C.M.; Slevin, N.; Valentine, H.; Ryder, W.D.; Hampson, L.; Bibi, R.; Sloan, P.; Thakker, N.; Homer, J.; et al. Tumor expression of major vault protein is an adverse prognostic factor for radiotherapy outcome in oropharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Hernández, L.A.; Lloret, M.; Pinar, B.; Bordón, E.; Rey, A.; Lubrano, A.; Lara, P.C. BCL-2, in combination with MVP and IGF-1R expression, improves prediction of clinical outcome in complete response cervical carcinoma patients treated by radiochemotherapy. Gynecol. Oncol. 2011, 122, 585–589. [Google Scholar] [CrossRef]
- Filipits, M.; Drach, J.; Pohl, G.; Schuster, J.; Stranzl, T.; Ackermann, J.; Königsberg, R.; Kaufmann, H.; Gisslinger, H.; Huber, H.; et al. Expression of the lung resistance protein predicts poor outcome in patients with multiple myeloma. Clin. Cancer Res. 1999, 5, 2426–2430. [Google Scholar]
- Ohno, N.; Tani, A.; Uozumi, K.; Hanada, S.; Furukawa, T.; Akiba, S.; Sumizawa, T.; Utsunomiya, A.; Arima, T.; Akiyama, S. Expression of functional lung resistance-related protein predicts poor outcome in adult T–cell leukemia. Blood 2001, 98, 1160–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minones-Moyano, E.; Friedlander, M.R.; Pallares, J.; Kagerbauer, B.; Porta, S.; Escaramis, G.; Ferrer, I.; Estivill, X.; Marti, E. Upregulation of a small vault RNA (svtRNA2-1a) is an early event in Parkinson disease and induces neuronal dysfunction. RNA Biol. 2013, 10, 1093–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinou, D.; Katsifis, G.; Barouta, G.; Liaskos, C.; Sakkas, L.I.; Tsakris, A.; Routsias, J.G. Major vault protein/lung resistance related protein: A novel biomarker for rheumatoid arthritis. Clin. Exp. Rheumatol. 2020. online ahead of print. [Google Scholar]
- Chugani, D.C.; Rome, L.H.; Kedersha, N.L. Evidence that vault ribonucleoprotein particles localize to the nuclear pore complex. J. Cell Sci. 1993, 106, 23–29. [Google Scholar]
- Berger, W.; Elbling, L.; Micksche, M. Expression of the major vault protein LRP in human non-small-cell lung cancer cells: Activation by short-term exposure to antineoplastic drugs. Int. J. Cancer 2000, 88, 293–300. [Google Scholar] [CrossRef]
- Van Zon, A.; Mossink, M.H.; Houtsmuller, A.B.; Schoester, M.; Scheffer, G.L.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. Vault mobility depends in part on microtubules and vaults can be recruited to the nuclear envelope. Exp. Cell. Res. 2006, 312, 245–255. [Google Scholar] [PubMed]
- Slesina, M.; Inman, E.M.; Rome, L.H.; Volknandt, W. Nuclear localization of the major vault protein in U373 cells. Cell Tissue Res. 2005, 321, 97–104. [Google Scholar] [CrossRef]
- Hamill, D.R.; Suprenant, K.A. Characterization of the sea urchin major vault protein: A possible role for vault ribonucleoprotein particles in nucleocytoplasmic transport. Dev. Biol. 1997, 190, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.L.; Makabi, M.; Lang, J.; Dickey-Sims, C.; Robertson, A.J.; Coffman, J.A.; Suprenant, K.A. Sea urchin vault structure, composition, and differential localization during development. BMC Dev. Biol. 2005, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Vuorinen, E.M.; Rajala, N.K.; Rauhala, H.E.; Nurminen, A.T.; Hytönen, V.P.; Kallioniemi, A. Search for KPNA7 cargo proteins in human cells reveals MVP and ZNF414 as novel regulators of cancer cell growth. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Slesina, M.; Inman, E.M.; Moore, A.E.; Goldhaber, J.I.; Rome, L.H.; Volknandt, W. Movement of vault particles visualized by GFP-tagged major vault protein. Cell Tissue Res. 2006, 324, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.P.; Dubouix-Bourandy, A.; Bajmoczi, M.; Golan, D.E.; Zaidi, T.; Coutinho-Sledge, Y.S.; Gygi, M.P.; Gygi, S.P.; Wiemer, E.A.; Pier, G.B. Host resistance to lung infection mediated by major vault protein in epithelial cells. Science 2007, 317, 130–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.M.; Joh, J.W.; Seo, S.R.; Kim, W.T.; Kim, M.K.; Choi, H.S.; Kim, S.Y.; Jang, Y.J.; Sinn, D.H.; Choi, G.S.; et al. Cell-surface major vault protein promotes cancer progression through harboring mesenchymal and intermediate circulating tumor cells in hepatocellular carcinomas. Sci. Rep. 2017, 7, 13201. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Hu, X.; Mu, J.; Samykutty, A.; Zhuang, X.; Deng, Z.; Kumar, A.; Zhang, L.; Merchant, M.L.; et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat. Commun. 2017, 8, 14448. [Google Scholar] [CrossRef]
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rome, L.H.; Kickhoefer, V.A. Development of the Vault Particle as a Platform Technology. ACS Nano 2013, 7, 889–902. [Google Scholar] [CrossRef]
- Galbiati, E.; Avvakumova, S.; La Rocca, A.; Pozzi, M.; Messali, S.; Magnaghi, P.; Colombo, M.; Prosperi, D.; Tortora, P. A fast and straightforward procedure for vault nanoparticle purification and the characterization of its endocytic uptake. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2254–2260. [Google Scholar] [CrossRef]
- Mossink, M.H.; de Groot, J.; van Zon, A.; Fränzel-Luiten, E.; Schoester, M.; Scheffer, G.L.; Sonneveld, P.; Scheper, R.J.; Wiemer, E.A. Unimpaired dendritic cell functions in MVP/LRP knockout mice. Immunology 2003, 110, 58–65. [Google Scholar] [CrossRef]
- Daly, T.K.; Sutherland-Smith, A.J.; Penny, D. Beyond BLASTing: Tertiary and quaternary structure analysis helps identify major vault proteins. Genome Biol. Evol. 2013, 5, 217–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moult, J.; Pedersen, J.T.; Judson, R.; Fidelis, K. A large-scale experiment to assess protein structure prediction methods. Proteins 1995, 23, ii–iv. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.J.; Moughon, S.; Wang, C.; Schueler-Furman, O.; Kuhlman, B.; Rohl, C.A.; Baker, D. Protein-protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J. Mol. Biol. 2003, 331, 281–299. [Google Scholar] [CrossRef]
- Fritz-Laylin, L.K.; Prochnik, S.E.; Ginger, M.L.; Dacks, J.B.; Carpenter, M.L.; Field, M.C.; Kuo, A.; Paredez, A.; Chapman, J.; Pham, J.; et al. The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell 2010, 140, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, L.E.; Pupols, M.; Kickhoefer, V.A.; Rome, L.H.; Monbouquette, H.G. Utilization of a protein “shuttle” to load vault nanocapsules with gold probes and proteins. ACS Nano 2009, 3, 3175–3183. [Google Scholar] [CrossRef]
- Ng, B.C.; Yu, M.; Gopal, A.; Rome, L.H.; Monbouquette, H.G.; Tolbert, S.H. Encapsulation of semiconducting polymers in vault protein cages. Nano Lett. 2008, 8, 3503–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suprenant, K.A.; Bloom, N.; Fang, J.W.; Lushington, G. The major vault protein is related to the toxic anion resistance protein (TelA) family. J. Exp. Biol. 2007, 210, 946–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef]
- Muñoz-Juan, A.; Carreño, A.; Mendoza, R.; Corchero, J.L. Latest Advances in the Development of Eukaryotic Vaults as Targeted Drug Delivery Systems. Pharmaceutics 2019, 11, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kickhoefer, V.A.; Garcia, Y.; Mikyas, Y.; Johansson, E.; Zhou, J.C.; Raval-Fernandes, S.; Minoofar, P.; Zink, J.I.; Dunn, B.; Stewart, P.L.; et al. Engineering of vault nanocapsules with enzymatic and fluorescent properties. Proc. Natl. Acad. Sci. USA 2005, 102, 4348–4352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braisted, A.C.; Wells, J.A. Minimizing a binding domain from protein A. Proc. Natl. Acad. Sci. USA 1996, 93, 5688–5692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlov, G.; Vavelyuk, O.; Minailiuc, O.; Banville, D.; Gehring, K.; Ekiel, I. solution structure of a two-repeat fragment of major vault protein. J. Mol. Biol. 2006, 356, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005, 79, 1992–2000. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.Y.; Wiethoff, C.M.; Kickhoefer, V.A.; Rome, L.H.; Nemerow, G.R. Vault nanoparticles containing an adenovirus-derived membrane lytic protein facilitate toxin and gene transfer. ACS Nano 2009, 3, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benner, N.L.; Zang, X.; Buehler, D.C.; Kickhoefer, V.A.; Rome, M.E.; Rome, L.H.; Wender, P.A. Vault nanoparticles: Chemical modifications for imaging and enhanced delivery. ACS Nano 2017, 11, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Buehler, D.C.; Toso, D.B.; Kickhoefer, V.A.; Zhou, Z.H.; Rome, L.H. Vaults engineered for hydrophobic drug delivery. Small 2011, 7, 1432–1439. [Google Scholar] [CrossRef] [Green Version]
- Patatanian, E.; Thompson, D.F. Retinoic acid syndrome: A review. J. Clin. Pharm. Ther. 2008, 33, 331–338. [Google Scholar] [CrossRef]
- Buehler, D.C.; Marsden, M.D.; Shen, S.; Toso, D.B.; Wu, X.; Loo, J.A.; Zhou, Z.H.; Kickhoefer, V.A.; Wender, P.A.; Zack, J.A.; et al. Bioengineered vaults: Self-assembling protein shell−lipophilic core nanoparticles for drug delivery. ACS Nano 2014, 8, 7723–7732. [Google Scholar] [CrossRef] [Green Version]
- Champion, C.I.; Kickhoefer, V.A.; Liu, G.; Moniz, R.J.; Freed, A.S.; Bergmann, L.L.; Vaccari, D.; Raval-Fernandes, S.; Chan, A.M.; Rome, L.H.; et al. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS ONE 2009, 4, e5409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Liu, G.; Kickhoefer, V.A.; Rome, L.H.; Li, L.X.; McSorley, S.J.; Kelly, K.A. A protective vaccine against Chlamydia genital infection using vault nanoparticles without an added adjuvant. Vaccines 2017, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Kar, U.K.; Srivastava, M.K.; Andersson, A.; Baratelli, F.; Huang, M.; Kickhoefer, V.A.; Dubinett, S.M.; Rome, L.H.; Sharma, S. Novel CCL21-vault nanocapsule intratumoral delivery inhibits lung cancer growth. PLoS ONE 2011, 6, e18758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voth, B.L.; Pelargos, P.E.; Barnette, N.E.; Bhatt, N.S.; Chen, C.H.J.; Lagman, C.; Chung, L.K.; Nguyen, T.; Sheppard, J.P.; Romiyo, P.; et al. Intratumor injection of CCL21-coupled vault nanoparticles is associated with reduction in tumor volume in an in vivo model of glioma. J. Neurooncol. 2020, 147, 599–605. [Google Scholar] [CrossRef]
- Nagasawa, D.T.; Yang, J.; Romiyo, P.; Lagman, C.; Chung, L.K.; Voth, B.L.; Duong, C.; Kickhoefer, V.A.; Rome, L.H.; Yang, I. Bioengineered recombinant vault nanoparticles coupled with NY-ESO-1 glioma-associated antigens induce maturation of native dendritic cells. J. Neurooncol. 2020, 148, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Querol-Audí, J.; Casañas, A.; Usón, I.; Luque, D.; Castón, J.R.; Fita, I.; Verdaguer, N. The mechanism of vault opening from the high resolution structure of the N-terminal repeats of MVP. EMBO J. 2009, 28, 3450–3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llauró, A.; Guerra, P.; Kant, R.; Bothner, B.; Verdaguer, N.; De Pablo, P.J. Decrease in pH destabilizes individual vault nanocages by weakening the inter-protein lateral interaction. Sci. Rep. 2016, 6, 34143. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Srinivasan, A.; Sun, Y.; Mrazek, J.; Shu, Z.; Kickhoefer, V.A.; Rome, L.H. Vault nanoparticles engineered with the protein transduction domain, tat48, enhances cellular uptake. Integr. Biol. 2013, 5, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, N.M.; Prabhakaran, P.; Rome, L.H.; Maynard, H.D. Smart vaults: Thermally-responsive protein nanocapsules. ACS Nano 2013, 7, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, N.M.; Buchman, G.W.; Rome, L.H.; Maynard, H.D. Dual pH- and temperature-responsive protein nanoparticles. Eur. Polym. J. 2015, 69, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Vasu, S.K.; Rome, L.H. Dictyostelium vaults: Disruption of the major proteins reveals growth and morphological defects and uncovers a new associated protein. J. Biol. Chem. 1995, 270, 16588–16594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lintel, N.J.; Luebker, S.A.; Lele, S.M.; Koepsell, S.A. MVP immunohistochemistry is a useful adjunct in distinguishing leiomyosarcoma from leiomyoma and leiomyoma with bizarre nuclei. Hum. Pathol. 2018, 73, 122–127. [Google Scholar] [CrossRef] [PubMed]
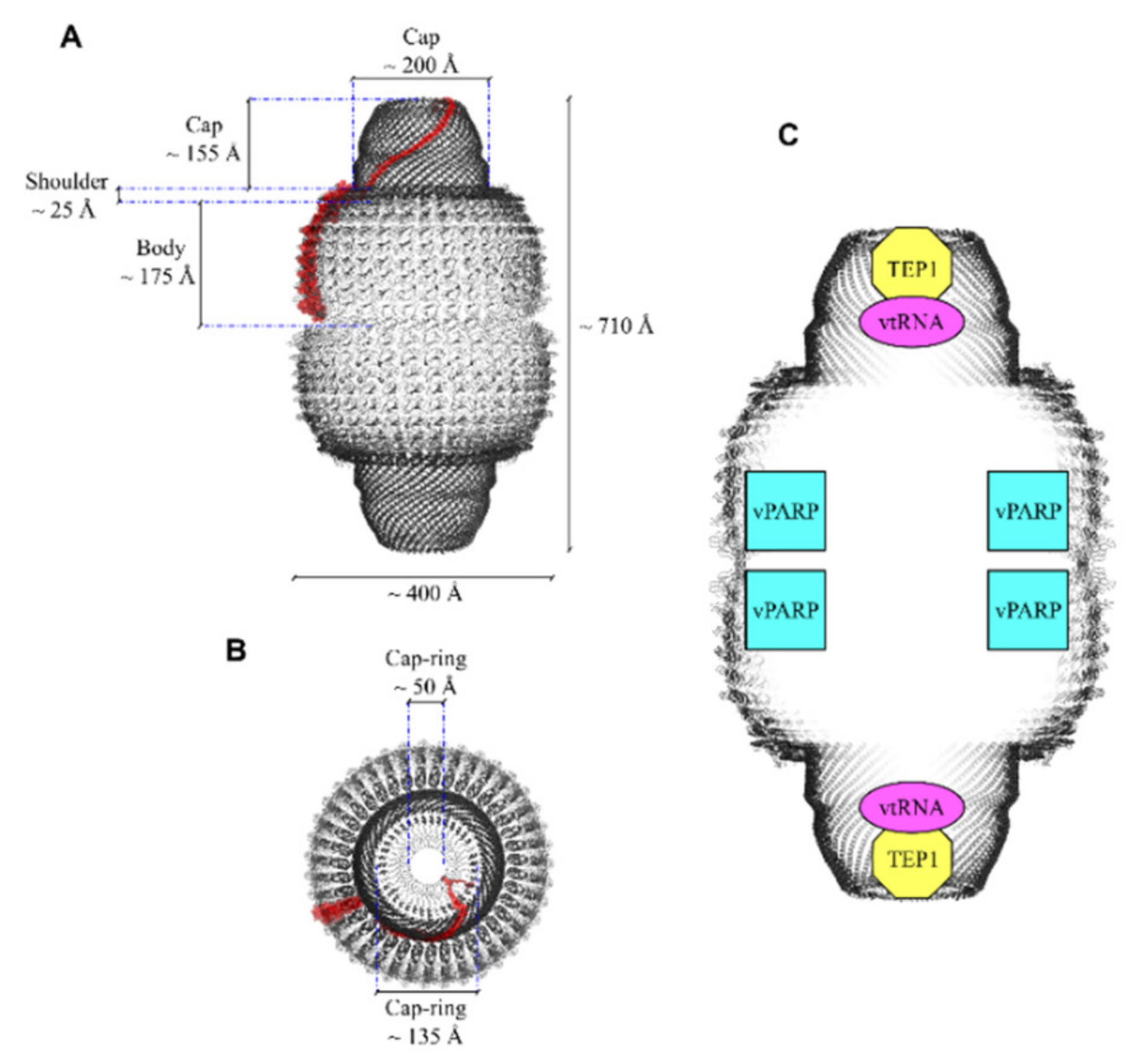
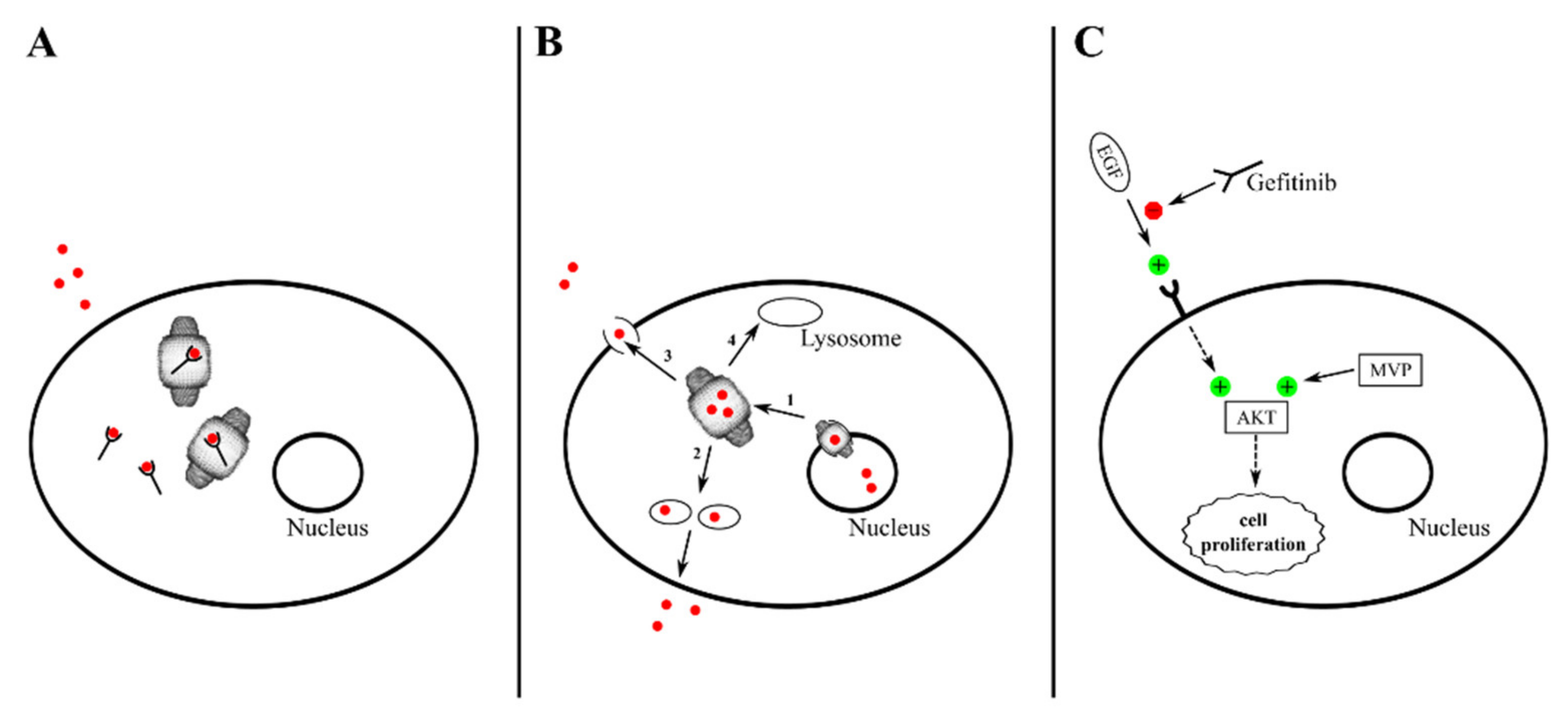
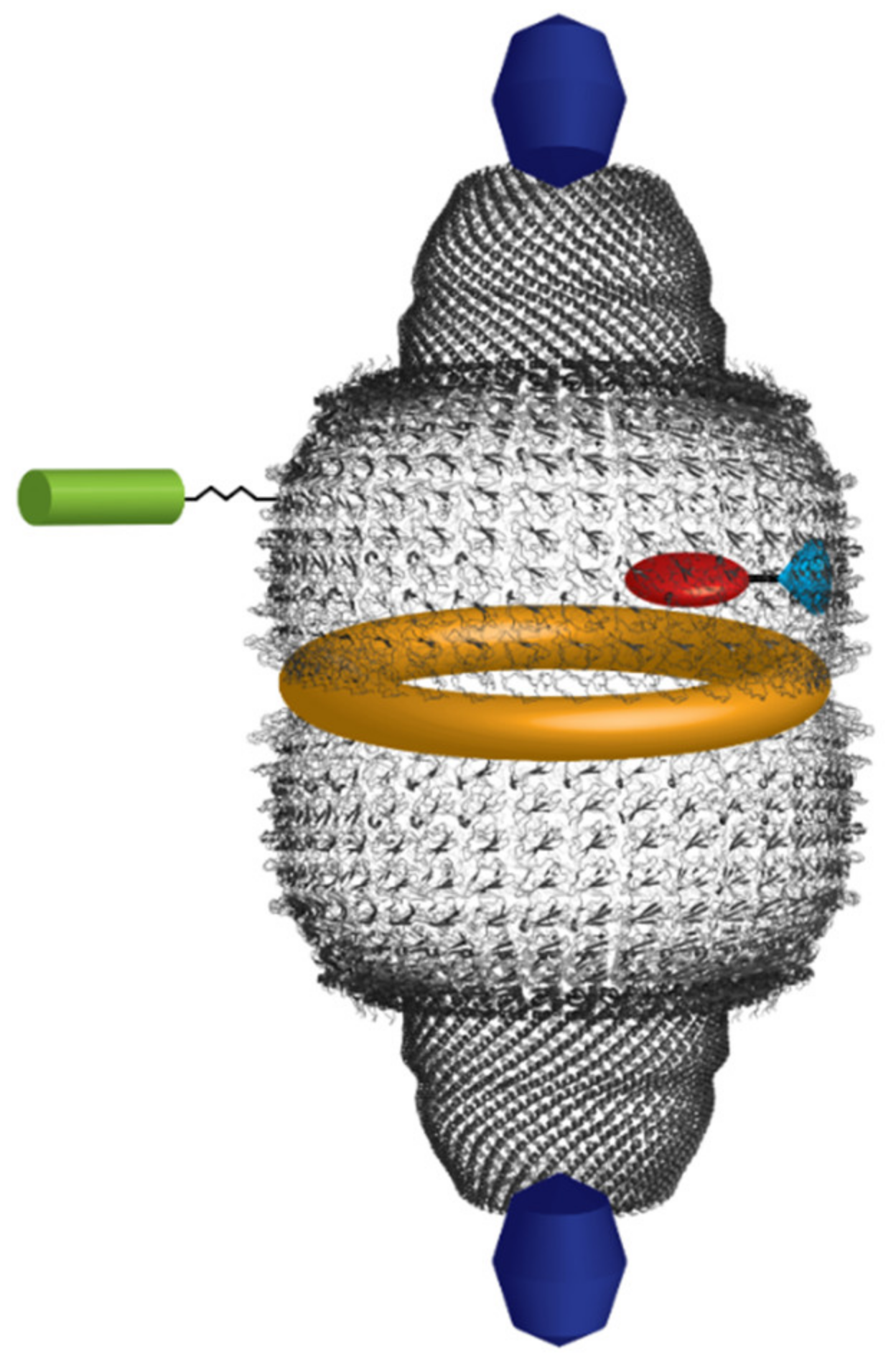
| Cellular Pathways and Mechanisms under Control | Vault Components | Interactor(s) | Tissue/Cells | Cellular Effects | Reference |
|---|---|---|---|---|---|
| Estrogen signaling | MVP | Estrogen receptor | MCF-7 breast cancer cells | Vault translocation to the nucleus | [54] |
| EGF/PI3K/AKT | MVP | PTEN | Glioblasotoma | PTEN nuclear import | [55,56] |
| ER stress | Probably MVP | PERK | Nonsmall cell lung cancer (H1299) | Vault retention in cytosol | [57] |
| BAG3 | MVP | BAG3 | Several breast cancer cell lines | Vault nuclear import | [58] |
| YPEL4 | MVP | YPEL4 | COS-7 fibroblast-like cells | Reduced Elk-1 activation | [59] |
| ERK | MVP | B7-H3 | Human mammary epithelial cells | MEK activation | [60] |
| ERK | MVP | SHP-2 (*) | Human embryonic kidney 293 cells; mouse embryo fibroblasts | Elk-1 activation; prosurvival | [61] |
| ERK | MVP | Src (**) | stomach | Possibly prosurvival | [62] |
| 14-3-3ε | MVP | 14-3-3ε | Hepatocellular carcinoma cells | MVP-induced DR inhibition | [63] |
| IL-22/PDGF/STAT3/AKT | MVP | Myosin-9 | Smooth muscle cells | STAT3 and AKT activation; apoptosis inhibition | [64] |
| c-Jun | MVP; probably also vPARP | COP1 (E3) | Human embryonic kidney 293 cells; HeLa cells | c-Jun-mediated response to UV stress | [65] |
| Hypoxia signaling | MVP | HIF-1α | Human renal adenocarcinoma ACHN cells (ACHN) | Favoring hypoxia adaptation | [66] |
| SR-A receptor | MVP | SR-A | Mouse peritoneal macrophages | TNF-α production; apoptosis | [67] |
| Apoptotic pathway | MVP | Caspase-1; caspase-9 (***) | human primary keratinocytes; human primary fibroblasts | Antiapoptotic effect | [68] |
| Innate immune response to HCV | MVP | unknown | Peripheral blood mononuclear cells; Huh7 hepatoma cells | MVP upregulation; type-I IFN activation | [69] |
| Innate immune response to HBV | MVP | MyD88 | Peripheral blood mononuclear cells; HepG2 and HuH7 hepatoma cells | NF-κB and IFN-β activation | [70] |
| p53 | MVP | IRF2 | Mouse liver | p53 degradation | [71] |
| Proinflammatory response to influenza A virus | MVP | c-Fos; C/ERBβ-LAP; p50/p65 | epithelial A549 cells; peripheral blood mononuclear cells | Virus-evokedIL-6 and IL-8 production | [72] |
| Modulation of inflammation; prevention of metabolic disorders and atherosclerosis | MVP | TRAF6 | Macrophages | Regulation of NF-κB-dependent transcriptional effects | [73] |
| Unknown | vtRNA | La autoantigen | HeLa cells | Unknown | [8] |
| Autophagy | vtRNA1-1 | sequestome-1/p62 | Several human and murine cell lines | Antiautophagic effect | [74] |
| EBV infection | vtRNA1-1 | unknown | Burkitt lymphoma BL2 cells | Antiapoptotic effect | [75] |
| PI3K/AKT and ERK pathways | vtRNA1-1 | unknown | Hela cells | Antiapoptotic effect | [76] |
| Gene expression | vtRNA1-1(via svRNAb) | Dicer and Argonaute proteins | MCF-7 breast cancer cells | CYP3A4 (P450-expressing) gene silencing | [77] |
| Gene expression | vtRNA1-1(via svRNA4) | NSUN2 methylase; SRSF2 | Human dermal fibroblasts | Inhibition of keratinocyte differentiation | [78] |
| Gene expression | vtRNA1-1 | PSF | MCF-7 breast cancer cells | GAGE6-MDR gene activation | [79] |
| Gene expression | vtRNA | Unknown | Trypanosoma brucei | Trans-splicing | [45] |
| Binding Site | Transcription Factor | Transcriptional Effect | Reference |
|---|---|---|---|
| STAT1 | STAT1 | Activation | [132] |
| p53 | - | - | [127] |
| GC | Sp1 | Activation | [128] |
| E-box | USF1 | Activation | [113] |
| GATA-box | - | - | [126] |
| MyoD | - | - | [126] |
| CCAT-box (Y-box) | p53; Y-box-binding protein | Repression | [133] |
| Upstream CBF1-binding site | N1ICD Notch1 fragment; CBF1 | Activation | [129] |
| Tissue/Cell Lines | Drug(s) | Associated MDR Proteins | Vault Component | Mechanism (When Known) | Reference |
|---|---|---|---|---|---|
| Several lung cancer cell lines | DOX | - | MVP, vtRNA | - | [4] |
| Human colon carcinoma SW620 cells | DOX; vincristine; etoposide; gramicidin D; paclitaxel | - | MVP | - | [142] |
| U-937 human leukaemia cells | DOX; etoposide; mitoxantrone; 5-fluorouracil | - | MVP * | - | [143] |
| SW620 human colon cancer cells | Doxorubicin; etoposide; cisplatin; SN-38s | - | MVP * | - | [137] |
| Ovarian carcinoma | Platinum based; Alkylating | - | MVP | - | [144] |
| Human lung adenocarcinoma | Cisplatin | Bcl-2; survivin | MVP | - | [138] |
| Human lung adenocarcinoma A549 | Cisplatin | - | MVP | - | [139] |
| Myeloma cells | Mitoxantrone | - | MVP; vtRNA | - | [4] |
| Leukaemic cells from AML patients | Mitoxantrone | - | MVP * | - | [145] |
| GBM | Several, including temozolomide or bis-chloroethylnitrosourea | - | MVP | - | [146] |
| Breast cancer cell lines cocultured with adipocytes | DOX | - | MVP | Drug efflux from the nucleus | [125] |
| UMUC-3 human urothelial bladder cancer cell line | DOX | - | MVP | Drug export from the nucleus to lysosomes | [147] |
| HCC cell lines | Gefitinib (EGFR inhibitor) | - | MVP | Possible uncoupling of AKT activation from EGFR | [82] |
| Human lung adenocarcinoma | Gefitinib (EGFR inhibitor) | - | MVP | Uncoupling of AKT activation from EGFR | [148] |
| HCC cell lines | Bleomycin | 14-3-3ε | MVP | Drug encapsulation and possible extrusion | [98] |
| KB nasopharingeal carcinoma cell lines | Cisplatin | - | MVP; vtRNA(s) | Drug efflux from nucleus | [149] |
| MG63 and U2OS osteosarcoma; U118MG glioblastoma; U-937 lymphoma; | Mitoxantrone | - | vtRNA1-1 | Mitoxantrone sequestration by vtRNA1-1 | [150,151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frascotti, G.; Galbiati, E.; Mazzucchelli, M.; Pozzi, M.; Salvioni, L.; Vertemara, J.; Tortora, P. The Vault Nanoparticle: A Gigantic Ribonucleoprotein Assembly Involved in Diverse Physiological and Pathological Phenomena and an Ideal Nanovector for Drug Delivery and Therapy. Cancers 2021, 13, 707. https://doi.org/10.3390/cancers13040707
Frascotti G, Galbiati E, Mazzucchelli M, Pozzi M, Salvioni L, Vertemara J, Tortora P. The Vault Nanoparticle: A Gigantic Ribonucleoprotein Assembly Involved in Diverse Physiological and Pathological Phenomena and an Ideal Nanovector for Drug Delivery and Therapy. Cancers. 2021; 13(4):707. https://doi.org/10.3390/cancers13040707
Chicago/Turabian StyleFrascotti, Gianni, Elisabetta Galbiati, Matteo Mazzucchelli, Maria Pozzi, Lucia Salvioni, Jacopo Vertemara, and Paolo Tortora. 2021. "The Vault Nanoparticle: A Gigantic Ribonucleoprotein Assembly Involved in Diverse Physiological and Pathological Phenomena and an Ideal Nanovector for Drug Delivery and Therapy" Cancers 13, no. 4: 707. https://doi.org/10.3390/cancers13040707
APA StyleFrascotti, G., Galbiati, E., Mazzucchelli, M., Pozzi, M., Salvioni, L., Vertemara, J., & Tortora, P. (2021). The Vault Nanoparticle: A Gigantic Ribonucleoprotein Assembly Involved in Diverse Physiological and Pathological Phenomena and an Ideal Nanovector for Drug Delivery and Therapy. Cancers, 13(4), 707. https://doi.org/10.3390/cancers13040707






