Integrative Genomic Analyses of Patient-Matched Intracranial and Extracranial Metastases Reveal a Novel Brain-Specific Landscape of Genetic Variants in Driver Genes of Malignant Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
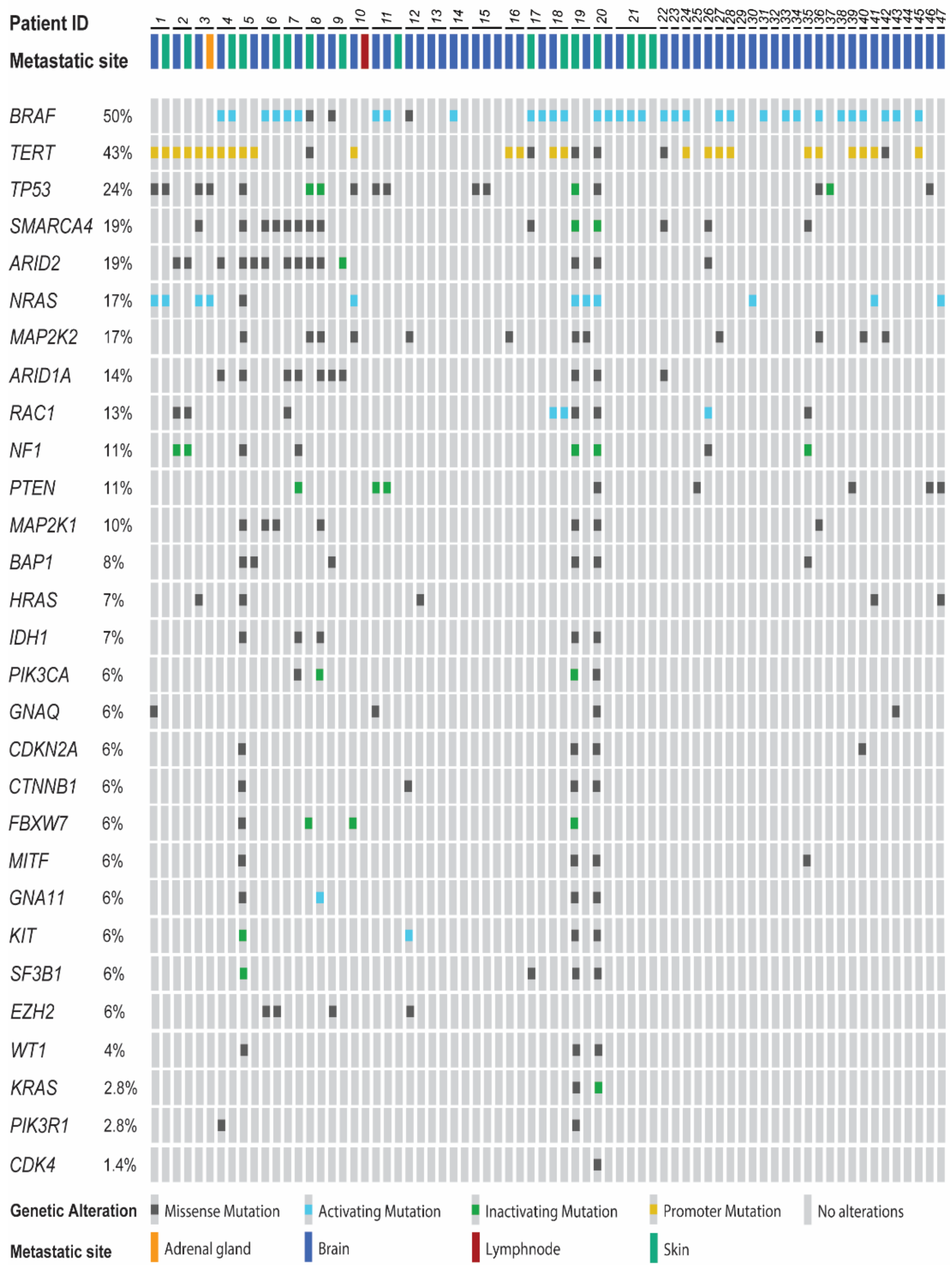
2.1. Patient Characteristics
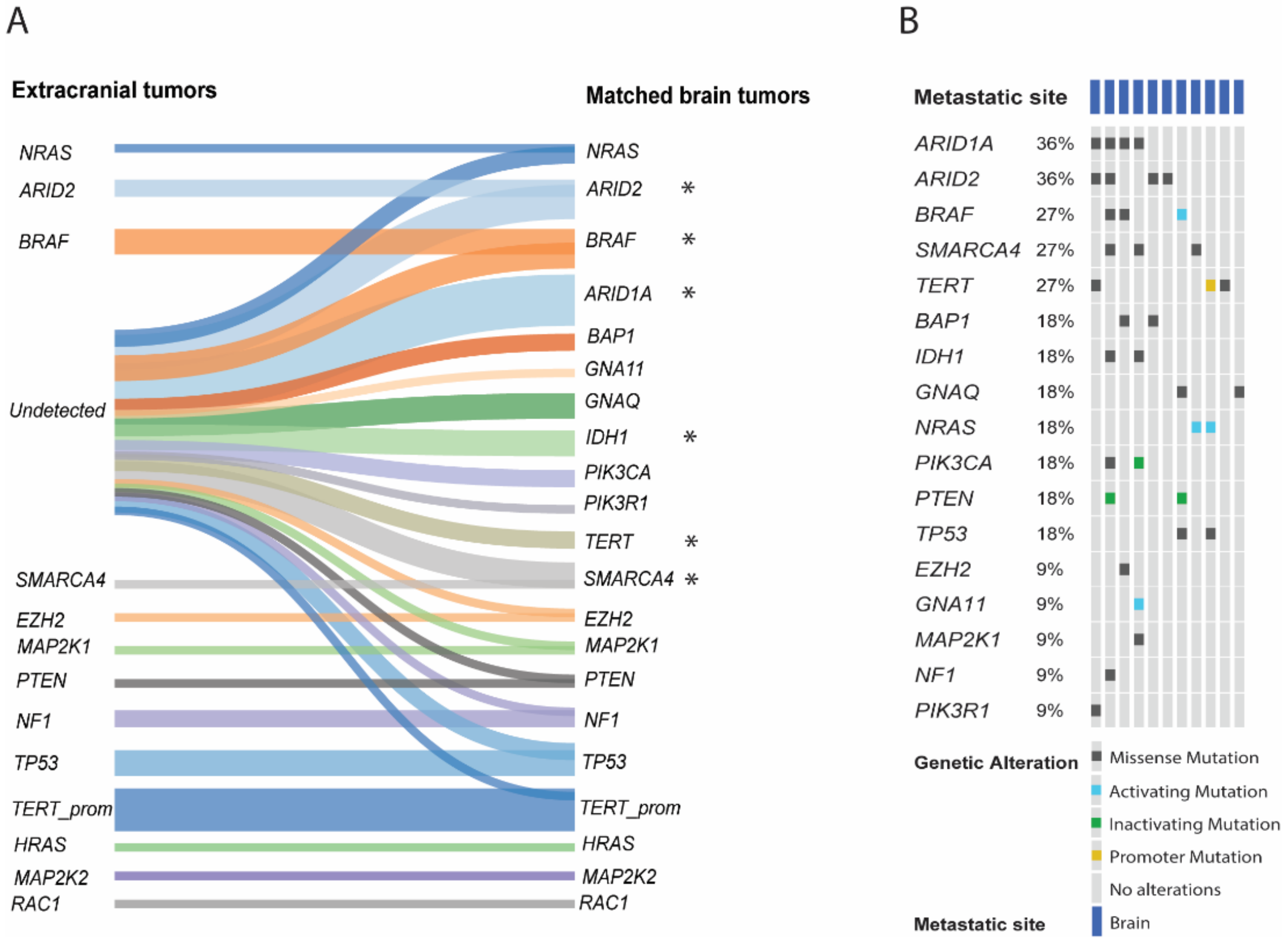
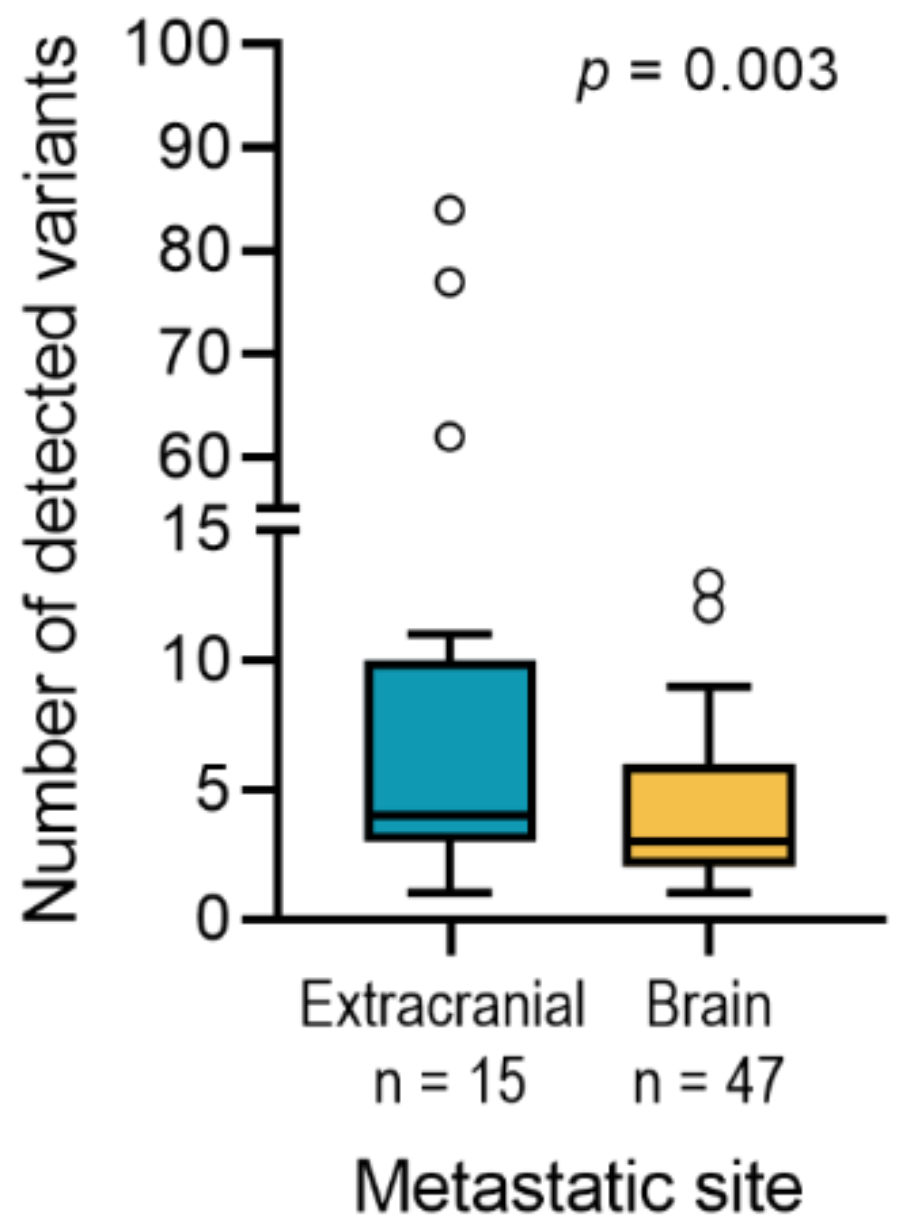
2.2. Comparison of Brain Metastases and Metastases of Other Sites
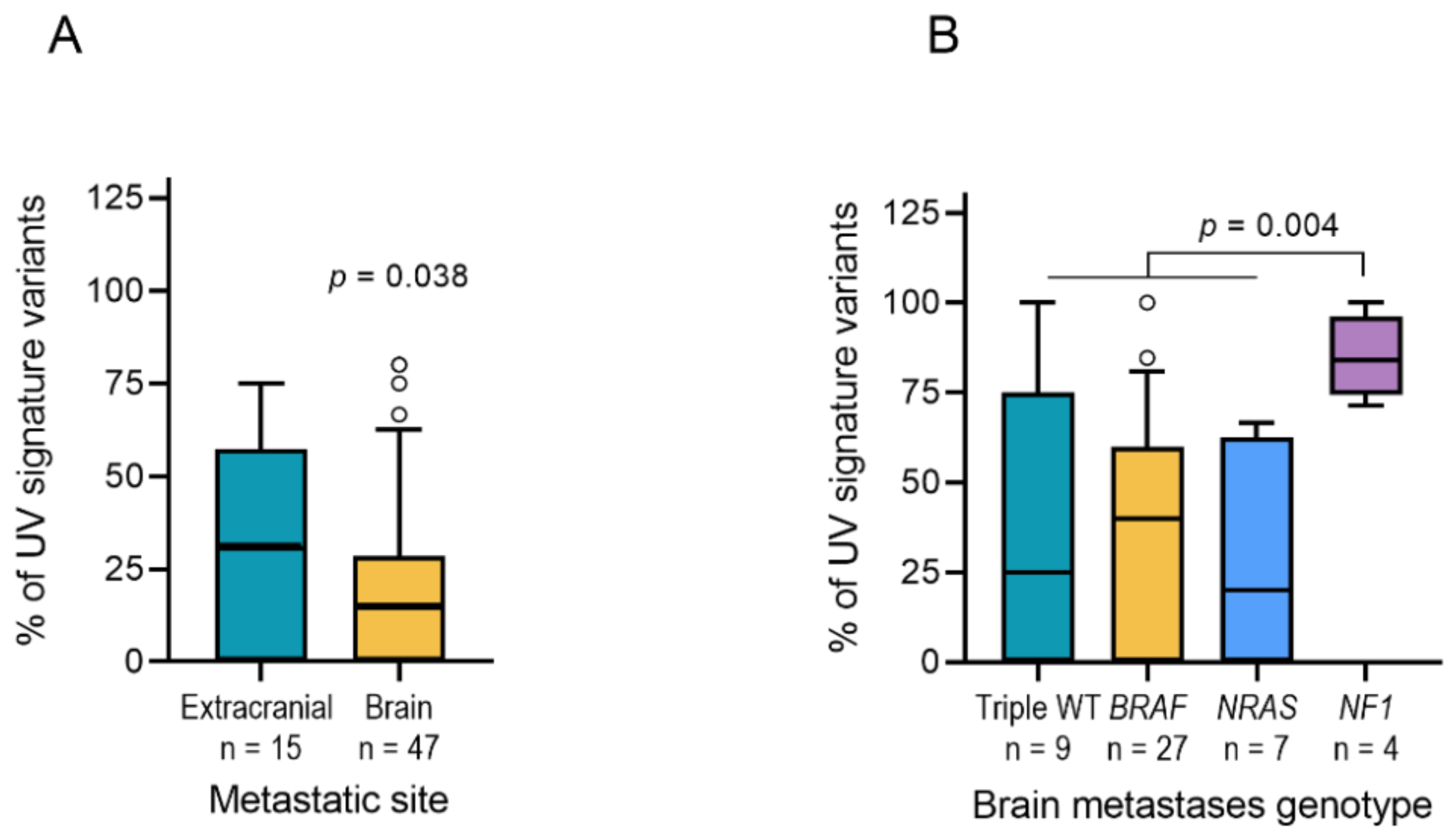
2.3. UV Mutation Signature
3. Discussion
4. Materials and Methods
4.1. Patient Material
4.2. DNA Isolation
4.3. Targeted Sequencing
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, E.S.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro-Oncology 2017, 19, 1511–1521. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Fabi, A.; Felici, A.; Metro, G.; Mirri, A.; Bria, E.; Telera, S.; Moscetti, L.; Russillo, M.; Lanzetta, G.; Mansueto, G.; et al. Brain metastases from solid tumors: Disease outcome according to type of treatment and therapeutic resources of the treating center. J. Exp. Clin. Cancer Res. 2011, 30, 10. [Google Scholar] [CrossRef]
- Balch, C.M.; Balch, G.C.; Sharma, R.R. Identifying Early Melanomas at Higher Risk for Metastases. J. Clin. Oncol. 2012, 30, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Gramsch, C.; Göricke, S.L.; Behrens, F.; Zimmer, L.; Schadendorf, D.; Krasny, A.; Forsting, M.; Schlamann, M.U. Isolated cerebral susceptibility artefacts in patients with malignant melanoma: Metastasis or not? Eur. Radiol. 2013, 23, 2622–2627. [Google Scholar] [CrossRef]
- Miller, D.; Zappala, V.; El Hindy, N.; Livingstone, E.; Schadendorf, D.; Sure, U.; Sandalcioglu, I.E. Intracerebral metastases of malignant melanoma and their recurrences—A clinical analysis. Clin. Neurol. Neurosurg. 2013, 115, 1721–1728. [Google Scholar] [CrossRef]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.-C.; McQuade, J.L.; Haydu, L.E.; Joon, A.Y.; Reuben, A.; De Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov. 2019, 9, 628–645. [Google Scholar] [CrossRef]
- Gorantla, V.; Kirkwood, J.M.; Tawbi, H.A. Melanoma Brain Metastases: An Unmet Challenge in the Era of Active Therapy. Curr. Oncol. Rep. 2013, 15, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Long, G.V.; Kurzrock, R.; Kim, K.B.; Arkenau, T.H.; Brown, M.P.; Hamid, O.; Infante, J.R.; Millward, M.; Pavlick, A.C.; et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet 2012, 379, 1893–1901. [Google Scholar] [CrossRef]
- Long, G.V.; Trefzer, U.; Davies, M.A.; Kefford, R.F.; Ascierto, P.A.; Chapman, P.B.; Puzanov, I.; Hauschild, A.; Robert, C.; Algazi, A.; et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 1087–1095. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nat. Cell Biol. 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.-P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A Landscape of Driver Mutations in Melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef]
- Krauthammer, M.; Kong, Y.; Bacchiocchi, A.; Evans, P.; Pornputtapong, N.; Wu, C.; McCusker, J.P.; Ma, S.; Cheng, E.; Straub, R.; et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat. Genet. 2015, 47, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chakravarti, N.; Aardalen, K.; Lazar, A.J.; Tetzlaff, M.T.; Wubbenhorst, B.; Kim, S.-B.; Kopetz, S.; LeDoux, A.A.; Gopal, Y.V.; et al. Molecular Profiling of Patient-Matched Brain and Extracranial Melanoma Metastases Implicates the PI3K Pathway as a Therapeutic Target. Clin. Cancer Res. 2014, 20, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Laitinen, S.; Khan, S.; Vihinen, M.; Kowalski, J.; Yu, G.; Chen, L.; Ewing, C.M.; Eisenberger, M.A.; Carducci, M.A.; et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat. Med. 2009, 15, 559–565. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Zhang, H.-F.; Liang, R.; Xie, Z.-R.; Luo, H.-Y.; Zeng, Y.-J.; Xu, Y.; Wang, L.-M.; Kong, X.-Y.; Wang, K.-H. Colorectal Cancer Genetic Heterogeneity Delineated by Multi-Region Sequencing. PLoS ONE 2016, 11, e0152673. [Google Scholar] [CrossRef]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Krauthammer, M.; Kong, Y.; Ha, B.H.; Evans, P.; Bacchiocchi, A.; McCusker, J.P.; Cheng, E.; Davis, M.J.; Goh, G.; Choi, M.; et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012, 44, 1006–1014. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Wagle, N.; Sucker, A.; Treacy, D.J.; Johannessen, C.M.; Goetz, E.M.; Place, C.S.; Taylor-Weiner, A.; Whittaker, S.; Kryukov, G.V.; et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014, 4, 94–109. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef]
- Van de Nes, J.; Wrede, K.; Ringelstein, A.; Stiller, M.; Horn, S.; Sucker, A.; Möller, I.; Scholz, S.L.; Murali, R.; Gessi, M.; et al. Diagnosing a Primary Leptomeningeal Melanoma by Gene Mutation Signature. J. Investig. Dermatol. 2016, 136, 1526–1528. [Google Scholar] [CrossRef]
- Berger, M.F.; Hodis, E.; Heffernan, T.P.; Deribe, Y.L.; Lawrence, M.S.; Protopopov, A.; Ivanova, E.; Watson, I.R.; Nickerson, E.; Ghosh, P.; et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nat. Cell Biol. 2012, 485, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.; Varela, I.; Lin, M.-L.; Ordóñez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nat. Cell Biol. 2009, 463, 191–196. [Google Scholar] [CrossRef]
- Shain, A.H.; Garrido, M.C.; Botton, T.; Talevich, E.; Yeh, I.; Sanborn, J.Z.; Chung, J.; Wang, N.J.; Kakavand, H.; Mann, G.J.; et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat. Genet. 2015, 47, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Curry, W.T.; Oh, K.S. Clinical Discussion and Review of the Management of Brain Metastases. J. Natl. Compr. Cancer Netw. 2013, 11, 1153–1164. [Google Scholar] [CrossRef]
- Milsch, L.; Gesierich, A.; Kreft, S.; Livingstone, E.; Zimmer, L.; Goebeler, M.; Schadendorf, D.; Schilling, B. Patterns of disease control and survival in patients with melanoma brain metastases undergoing immune-checkpoint blockade. Eur. J. Cancer 2018, 99, 58–65. [Google Scholar] [CrossRef]
- Sloot, S.; Chen, Y.A.; Zhao, X.; Weber, J.S.; Benedict, J.J.; Mulé, J.J.; Smalley, K.S.; Weber, J.S.; Zager, J.S.; Forsyth, P.A.; et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer 2018, 124, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Pigozzo, J.; Di Maggio, A.; Tosi, A.L.; Sileni, V.C.; Rossi, C.R. Neoadjuvant treatment with dabrafenib of unresectable localizations from occult melanoma. Melanoma Res. 2014, 24, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Peuvrel, L.; Saint-Jean, M.; Quéreux, G.; Brocard, A.; Khammari, A.; Knol, A.C.; Dréno, B. Incidence and characteristics of melanoma brain metastases developing during treatment with vemurafenib. J. Neuro-Oncology 2014, 120, 147–154. [Google Scholar] [CrossRef]
- Puzanov, I.; Amaravadi, R.K.; McArthur, G.A.; Flaherty, K.T.; Chapman, P.B.; Sosman, J.A.; Ribas, A.; Shackleton, M.; Hwu, P.; Chmielowski, B.; et al. Long-term outcome in BRAF(V600E) melanoma patients treated with vemurafenib: Patterns of disease progression and clinical management of limited progression. Eur. J. Cancer 2015, 51, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.A.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef]
- Nishino, M.; Giobbie-Hurder, A.; Manos, M.P.; Bailey, N.D.; Buchbinder, E.I.; Ott, P.A.; Ramaiya, N.H.; Hodi, F.S. Immune-Related Tumor Response Dynamics in Melanoma Patients Treated with Pembrolizumab: Identifying Markers for Clinical Outcome and Treatment Decisions. Clin. Cancer Res. 2017, 23, 4671–4679. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.A.; Scolyer, R.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Chapman, P.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Silk, A.W.; Tian, S.; Mehnert, J.; Danish, S.; Ranjan, S.; Kaufman, H.L. Clinical Management of Multiple Melanoma Brain Metastases: A Systematic Review. JAMA Oncol. 2015, 1, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Boehling, N.S.; Koay, E.J.; Bucheit, A.D.; Jakob, J.A.; Settle, S.H.; Brown, P.D.; Davies, M.A.; Sulman, E.P. Melanoma brain metastases harboring BRAF V600K or NRAS mutations are associated with an increased local failure rate following conventional therapy. J. Neuro-Oncology 2018, 137, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nagl, N.G.; Jr Flowers, S.; Zweitzig, D.; Dallas, P.B.; Moran, E. Expression of p270 (ARID1A), a component of human SWI/SNF complexes, in human tumors. Int. J. Cancer 2004, 112, 636. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.-L.; Shih, I.-M.; Mao, T.-L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A.; Vogelstein, B.; et al. Frequent Mutations of Chromatin Remodeling Gene ARID1A in Ovarian Clear Cell Carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Sy, K.; Kalloger, S.E.; Li-Chang, H.; Woods, R.; Kumar, A.; Streutker, C.J.; Hafezi-Bakhtiari, S.; Zhou, C.; Lim, H.J.; et al. ARID1A/BAF250a as a prognostic marker for gastric carcinoma: A study of 2 cohorts. Hum. Pathol. 2014, 45, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.G.; Roberts, C.W.M. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 2011, 11, 481–492. [Google Scholar] [CrossRef]
- Wu, R.; Wang, T.-L.; Shih, I.-M. The emerging roles of ARID1A in tumor suppression. Cancer Biol. Ther. 2014, 15, 655–664. [Google Scholar] [CrossRef]
- Rahmanto, Y.S.; Jung, J.-G.; Wu, R.-C.; Kobayashi, Y.; Heaphy, C.M.; Meeker, A.K.; Wang, T.-L.; Shih, I.-M. Inactivating ARID1A Tumor Suppressor Enhances TERT Transcription and Maintains Telomere Length in Cancer Cells. J. Biol. Chem. 2016, 291, 9690–9699. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Rimoldi, D.; Iseli, C.; Valsesia, A.; Robyr, D.; Gehrig, C.; Harshman, K.; Guipponi, M.; Bukach, O.; Zoete, V.; et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat. Genet. 2011, 44, 133–139. [Google Scholar] [CrossRef]
- Stark, M.S.; Woods, S.L.; Gartside, M.G.; Bonazzi, V.F.; Dutton-Regester, K.; Aoude, L.G.; Chow, D.; Sereduk, C.; Niemi, N.M.; Tang, N.; et al. Frequent somatic mutations in MAP3K5 and MAP3K9 in metastatic melanoma identified by exome sequencing. Nat. Genet. 2011, 44, 165–169. [Google Scholar] [CrossRef]
- Wei, X.; Program, N.C.S.; Walia, V.; Lin, J.C.; Teer, J.K.; Prickett, T.D.; Gartner, J.; Davis, S.; Stemke-Hale, K.A.; Davies, M.; et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat. Genet. 2011, 43, 442–446. [Google Scholar] [CrossRef]
- Medina, P.P.; Carretero, J.; Fraga, M.F.; Esteller, M.; Sidransky, D.; Sanchez-Cespedes, M. Genetic and Epigenetic screening for gene alterations of the chromatin-remodeling factor, SMARCA4/BRG1, in lung tumors. Genes Chromosom. Cancer 2004, 41, 170–177. [Google Scholar] [CrossRef]
- Sanchez-Tillo, E.; Lazaro, A.; Torrent, R.; Cuatrecasas, M.; Vaquero, E.C.; Castells, A.; Engel, P.; Postigo, A. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 2010, 29, 3490–3500. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Njauw, C.-N.J.; Kim, I.; Piris, A.; Gabree, M.; Taylor, M.; Lane, A.M.; DeAngelis, M.M.; Gragoudas, E.; Duncan, L.M.; Tsao, H. Germline BAP1 Inactivation Is Preferentially Associated with Metastatic Ocular Melanoma and Cutaneous-Ocular Melanoma Families. PLoS ONE 2012, 7, e35295. [Google Scholar] [CrossRef]
- Wiesner, T.; Obenauf, A.C.; Murali, R.; Fried, I.; Griewank, K.G.; Ulz, P.; Windpassinger, C.; Wackernagel, W.; Loy, S.; Wolf, I.; et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat. Genet. 2011, 43, 1018–1021. [Google Scholar] [CrossRef]
- Tesch, M.E.; Pater, J.A.; Vandekerkhove, G.; Wang, G.; Binnington, K.; So, A.I.; Wyatt, A.W.; Eigl, B.J. Concurrent germline and somatic pathogenic BAP1 variants in a patient with metastatic bladder cancer. NPJ Genom. Med. 2020, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Van de Nes, J.; Gessi, M.; Sucker, A.; Moller, I.; Stiller, M.; Horn, S.; Scholz, S.L.; Pischler, C.; Stadtler, N.; Schilling, B.; et al. Targeted next generation sequencing reveals unique mutation profile of primary melanocytic tumors of the central nervous system. J. Neurooncol. 2016, 127, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Chandramohan, R.; Möller, I.; Scholz, S.L.; Berger, M.; Huberman, K.; Viale, A.; Pirun, M.; Socci, N.D.; Bouvier, N.; et al. Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget 2015, 6, 36041–36052. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2018, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- cBioPortal. Available online: http://www.cbioportal.org/ (accessed on 5 October 2020).
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number of Cases | Age at Diagnosis [Years ± Range] | Age at Surgery [Years ± Range] |
|---|---|---|---|
| Total patients | 47 | ||
| Sex | |||
| Male | 27 | 55 ± 14 | 59 ± 14 |
| Female | 20 | 60 ± 14 | 60 ± 11 |
| Metastases | 72 | ||
| Brain | 54 | 56 ± 14 | 57 ± 14 |
| Skin | 16 | 53 ± 20 | 55 ± 1 |
| Lymph node | 1 | 44 | 45 |
| Adrenal gland | 1 | 47 | 47 |
| Localization of BM | |||
| Cerebellar | 5 | - | - |
| Cerebral | 48 | - | - |
| Spinal | 1 | - | - |
| Therapy | |||
| Mono-CT | 2 | ||
| Mono-RT | 4 | ||
| Mono-IMT | 2 | ||
| CT + RT | 1 | ||
| CT + IMT | 3 | ||
| RT + IMT | 5 | ||
| Unknown | 30 | ||
| Status | - | - | |
| Alive | 0 | - | - |
| Dead | 45 | - | - |
| Lost to follow up | 2 | - | - |
| Pair | Gene | Chr | Region | Cov | Freq (%) | Subs | Protein Change | Relevance |
|---|---|---|---|---|---|---|---|---|
| 9 | EZH2 | 7 | 148523587 | 82 | 14.6 | C > T | C289Y | Missense-US |
| 9 | ARID1A | 1 | 27087374 | 813 | 35.6 | C > T | P650S | Missense-US |
| 9 | ARID1A | 1 | 27101420 | 266 | 5.6 | C > T | P1568S | Missense-US |
| 9 | BAP1 | 3 | 52439829 | 90 | 32.2 | C > T | V295M | Missense-US |
| 9 | BRAF | 7 | 140494241 | 2446 | 5.2 | G > A | P336L | Missense-US |
| 8 | SMARCA4 | 19 | 11097605 | 378 | 39.2 | C > T | P262L | Missense-US |
| 8 | MAP2K1 | 15 | 66727441 | 1118 | 48.3 | T > A | F53I | Missense-US |
| 8 | ARID1A | 1 | 27106745 | 10123 | 57.1 | T > C | L2119S | Missense-US |
| 8 | IDH1 | 2 | 209106800..209106801 | 223 | 41.3 | GG > AA | A212V | Missense-US |
| 8 | PIK3CA | 3 | 178951900 | 330 | 43.3 | C > A | Y1157* | inactivating |
| 8 | GNA11 | 19 | 3115011..3115012 | 130 | 40.8 | CC > TT | R183C | activating |
| 2 | TERT | 5 | 1255474 | 148 | 5.4 | G > A | P1029S | Missense-US |
| 7 | IDH1 | 2 | 209108164 | 229 | 5.2 | C > T | E229K | Missense-US |
| 7 | BRAF | 7 | 140481411 | 796 | 12.8 | C > T | G73E | Missense-US |
| 7 | ARID1A | 1 | 27101403 | 513 | 6.0 | C > T | P1562L | Missense-US |
| 7 | ARID1A | 1 | 27089536 | 686 | 6.1 | G > A | G831A | Missense-US |
| 7 | SMARCA4 | 19 | 11132539 | 363 | 10.2 | C > T | P919S | Missense-US |
| 7 | PIK3CA | 3 | 178927423 | 111 | 6.3 | C > T | L396F | Missense-US |
| 7 | NF1 | 17 | 29560040 | 485 | 6.4 | C > T | L1173F | Missense-US |
| 7 | NF1 | 17 | 29663403 | 412 | 5.3 | C > T | T1999I | Missense-US |
| 7 | SMARCA4 | 19 | 11138581 | 95 | 12.6 | G > A | E1113K | Missense-US |
| 7 | ARID2 | 12 | 46230381 | 91 | 8.8 | G > A | D239N | Missense-US |
| 7 | IDH1 | 2 | 209113246 | 216 | 5.1 | C > G | K87N | Missense-US |
| 7 | PTEN | 10 | 89717630 | 115 | 6.1 | C > T | Q219* | inactivating |
| 5 | BAP1 | 3 | 52436829 | 139 | 5.8 | A > T | L650H | Missense-US |
| 5 | ARID2 | 12 | 46246372 | 377 | 39.0 | C > T | S1489L | Missense-US |
| 6 | ARID2 | 12 | 46244970 | 1795 | 25.5 | C > T | P1022S | Missense-US |
| 10 | NRAS | 1 | 115256529 | 378 | 43.4 | T > C | Q61R | activating |
| 10 | TP53 | 17 | 7578389 | 440 | 38.2 | G > A | R142C | Missense-US |
| 10 | TERT promoter | 5 | 1295250 | 324 | 33.0 | G > A | C250T | activating |
| 11 | TP53 | 17 | 7578418 | 444 | 93.5 | T > C | E171G | Missense-US |
| 11 | BRAF | 7 | 140453136 | 124 | 54.0 | A > T | V207E | activating |
| 11 | TP53 | 17 | 7578418 | 2332 | 28.1 | T > C | E171G | Missense-US |
| 11 | GNAQ | 9 | 80537098..80537099 | 171 | 6.4 | TG > GA | P100L | Missense-US |
| 11 | PTEN | 10 | 89720833 | 224 | 29.0 | A > - | A328fs | inactivating |
| 11 | BRAF | 7 | 140453136 | 906 | 23.2 | A > T | V207E | activating |
| 1 | GNAQ | 9 | 80537113 | 420 | 9.3 | G > C | D95E | Missense-US |
| 1 | GNAQ | 9 | 80537098..80537099 | 218 | 17.4 | TG > GA | P100L | Missense-US |
| 3 | NRAS | 1 | 115256530 | 370 | 41.9 | G > T | Q61K | activating |
| 3 | SMARCA4 | 19 | 11132434 | 1563 | 28.9 | C > T | H884Y | Missense-US |
| 4 | TERT | 5 | 1254594 | 294 | 34.4 | C > T | A1062T | Missense-US |
| 4 | ARID1A | 1 | 27056272 | 758 | 35.0 | G > A | G423E | Missense-US |
| 4 | PIK3R1 | 5 | 67522632 | 181 | 7.2 | T > G | S43R | Missense-US |
| 4 | PIK3R1 | 5 | 67522628 | 182 | 7.7 | T > G | F41C | Missense-US |
| 4 | ARID2 | 12 | 46230641 | 749 | 24.0 | C > T | S297F | Missense-US |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Váraljai, R.; Horn, S.; Sucker, A.; Piercianek, D.; Schmitt, V.; Carpinteiro, A.; Becker, K.A.; Reifenberger, J.; Roesch, A.; Felsberg, J.; et al. Integrative Genomic Analyses of Patient-Matched Intracranial and Extracranial Metastases Reveal a Novel Brain-Specific Landscape of Genetic Variants in Driver Genes of Malignant Melanoma. Cancers 2021, 13, 731. https://doi.org/10.3390/cancers13040731
Váraljai R, Horn S, Sucker A, Piercianek D, Schmitt V, Carpinteiro A, Becker KA, Reifenberger J, Roesch A, Felsberg J, et al. Integrative Genomic Analyses of Patient-Matched Intracranial and Extracranial Metastases Reveal a Novel Brain-Specific Landscape of Genetic Variants in Driver Genes of Malignant Melanoma. Cancers. 2021; 13(4):731. https://doi.org/10.3390/cancers13040731
Chicago/Turabian StyleVáraljai, Renáta, Susanne Horn, Antje Sucker, Daniela Piercianek, Verena Schmitt, Alexander Carpinteiro, Katrin Anne Becker, Julia Reifenberger, Alexander Roesch, Jörg Felsberg, and et al. 2021. "Integrative Genomic Analyses of Patient-Matched Intracranial and Extracranial Metastases Reveal a Novel Brain-Specific Landscape of Genetic Variants in Driver Genes of Malignant Melanoma" Cancers 13, no. 4: 731. https://doi.org/10.3390/cancers13040731
APA StyleVáraljai, R., Horn, S., Sucker, A., Piercianek, D., Schmitt, V., Carpinteiro, A., Becker, K. A., Reifenberger, J., Roesch, A., Felsberg, J., Reifenberger, G., Sure, U., Schadendorf, D., & Helfrich, I. (2021). Integrative Genomic Analyses of Patient-Matched Intracranial and Extracranial Metastases Reveal a Novel Brain-Specific Landscape of Genetic Variants in Driver Genes of Malignant Melanoma. Cancers, 13(4), 731. https://doi.org/10.3390/cancers13040731








