Identification of Germline Genetic Variants that Increase Prostate Cancer Risk and Influence Development of Aggressive Disease
Simple Summary
Abstract
1. Introduction
2. Evidence of a Genetic Basis for Prostate Cancer
3. Initial Approaches for the Identification of PrCa Susceptibility Genes
4. Genome-Wide Association Studies for Common, Low Penetrance PrCa Susceptibility Loci
5. Sequencing Studies for Rare, Moderate Penetrance PrCa Susceptibility Genes
6. Translational Potential of Germline PrCa Susceptibility Variation and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/online-analysis-table (accessed on 14 December 2020).
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. Seer Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 14 December 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Koo, K.M.; Mainwaring, P.N.; Tomlins, S.A.; Trau, M. Merging new-age biomarkers and nanodiagnostics for precision prostate cancer management. Nat. Rev. Urol. 2019, 16, 302–317. [Google Scholar] [CrossRef]
- Tikkinen, K.A.O.; Dahm, P.; Lytvyn, L.; Heen, A.F.; Vernooij, R.W.M.; Siemieniuk, R.A.C.; Wheeler, R.; Vaughan, B.; Fobuzi, A.C.; Blanker, M.H.; et al. Prostate cancer screening with prostate-specific antigen (psa) test: A clinical practice guideline. BMJ 2018, 362, k3581. [Google Scholar] [CrossRef]
- Brookman-May, S.D.; Campi, R.; Henriquez, J.D.S.; Klatte, T.; Langenhuijsen, J.F.; Brausi, M.; Linares-Espinos, E.; Volpe, A.; Marszalek, M.; Akdogan, B.; et al. Latest evidence on the impact of smoking, sports, and sexual activity as modifiable lifestyle risk factors for prostate cancer incidence, recurrence, and progression: A systematic review of the literature by the european association of urology section of oncological urology (esou). Eur. Urol. Focus 2019, 5, 756–787. [Google Scholar] [PubMed]
- Harrison, S.; Tilling, K.; Turner, E.L.; Martin, R.M.; Lennon, R.; Lane, J.A.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; Bosch, J.; et al. Systematic review and meta-analysis of the associations between body mass index, prostate cancer, advanced prostate cancer, and prostate-specific antigen. Cancer Causes Control 2020, 31, 431–449. [Google Scholar] [CrossRef]
- Discacciati, A.; Orsini, N.; Wolk, A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1665–1671. [Google Scholar] [CrossRef]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Moul, J.W. The evolving definition of advanced prostate cancer. Rev. Urol. 2004, 6 (Suppl. 8), S10–S17. [Google Scholar]
- Hurwitz, L.M.; Agalliu, I.; Albanes, D.; Barry, K.H.; Berndt, S.I.; Cai, Q.; Chen, C.; Cheng, I.; Genkinger, J.M.; Giles, G.G.; et al. Recommended definitions of aggressive prostate cancer for etiologic epidemiologic research. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Kicinski, M.; Vangronsveld, J.; Nawrot, T.S. An epidemiological reappraisal of the familial aggregation of prostate cancer: A meta-analysis. PLoS ONE 2011, 6, e27130. [Google Scholar] [CrossRef]
- Johns, L.E.; Houlston, R.S. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003, 91, 789–794. [Google Scholar] [CrossRef]
- Stanford, J.L.; Ostrander, E.A. Familial prostate cancer. Epidemiol. Rev. 2001, 23, 19–23. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from sweden, denmark, and finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Hjelmborg, J.B.; Scheike, T.; Holst, K.; Skytthe, A.; Penney, K.L.; Graff, R.E.; Pukkala, E.; Christensen, K.; Adami, H.O.; Holm, N.V.; et al. The heritability of prostate cancer in the nordic twin study of cancer. Cancer Epidemiol. Biom. Prev. 2014, 23, 2303–2310. [Google Scholar] [CrossRef]
- Jansson, K.F.; Akre, O.; Garmo, H.; Bill-Axelson, A.; Adolfsson, J.; Stattin, P.; Bratt, O. Concordance of tumor differentiation among brothers with prostate cancer. Eur. Urol. 2012, 62, 656–661. [Google Scholar] [CrossRef]
- Brandt, A.; Sundquist, J.; Hemminki, K. Risk for incident and fatal prostate cancer in men with a family history of any incident and fatal cancer. Ann. Oncol. 2012, 23, 251–256. [Google Scholar] [CrossRef]
- Hemminki, K.; Ji, J.; Forsti, A.; Sundquist, J.; Lenner, P. Concordance of survival in family members with prostate cancer. J. Clin. Oncol. 2008, 26, 1705–1709. [Google Scholar] [CrossRef]
- Lindstrom, L.S.; Hall, P.; Hartman, M.; Wiklund, F.; Gronberg, H.; Czene, K. Familial concordance in cancer survival: A swedish population-based study. Lancet Oncol. 2007, 8, 1001–1006. [Google Scholar] [CrossRef]
- Albright, F.S.; Stephenson, R.A.; Agarwal, N.; Cannon-Albright, L.A. Relative risks for lethal prostate cancer based on complete family history of prostate cancer death. Prostate 2017, 77, 41–48. [Google Scholar] [CrossRef]
- Bratt, O.; Drevin, L.; Akre, O.; Garmo, H.; Stattin, P. Family history and probability of prostate cancer, differentiated by risk category: A nationwide population-based study. J. Natl. Cancer Inst. 2016. [Google Scholar] [CrossRef]
- Pritchard, C.C. New name for breast-cancer syndrome could help to save lives. Nature 2019, 571, 27–29. [Google Scholar] [CrossRef]
- Barber, L.; Gerke, T.; Markt, S.C.; Peisch, S.F.; Wilson, K.M.; Ahearn, T.; Giovannucci, E.; Parmigiani, G.; Mucci, L.A. Family history of breast or prostate cancer and prostate cancer risk. Clin. Cancer Res. 2018, 24, 5910–5917. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Parker, A.S.; Putnam, S.D.; Chiu, B.C.; Lynch, C.F.; Cohen, M.B.; Torner, J.C.; Cantor, K.P. Family history and prostate cancer risk in a population-based cohort of iowa men. Cancer Epidemiol. Biomed. Prev. 1999, 8, 53–60. [Google Scholar]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppala, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the prospective lynch syndrome database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef]
- Haraldsdottir, S.; Hampel, H.; Wei, L.; Wu, C.; Frankel, W.; Bekaii-Saab, T.; de la Chapelle, A.; Goldberg, R.M. Prostate cancer incidence in males with lynch syndrome. Genet. Med. 2014, 16, 553–557. [Google Scholar] [CrossRef]
- Raymond, V.M.; Mukherjee, B.; Wang, F.; Huang, S.C.; Stoffel, E.M.; Kastrinos, F.; Syngal, S.; Cooney, K.A.; Gruber, S.B. Elevated risk of prostate cancer among men with lynch syndrome. J. Clin. Oncol. 2013, 31, 1713–1718. [Google Scholar] [CrossRef]
- Bauer, C.M.; Ray, A.M.; Halstead-Nussloch, B.A.; Dekker, R.G.; Raymond, V.M.; Gruber, S.B.; Cooney, K.A. Hereditary prostate cancer as a feature of lynch syndrome. Fam. Cancer 2011, 10, 37–42. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Kapron, A.L.; Fraser, A.M.; Smith, K.R.; Cooney, K.A. Risk of prostate cancer associated with familial and hereditary cancer syndromes. J. Clin. Oncol. 2020, 38, 1807–1813. [Google Scholar] [CrossRef]
- Taitt, H.E. Global trends and prostate cancer: A review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am. J. Mens Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for african americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef]
- McGinley, K.F.; Tay, K.J.; Moul, J.W. Prostate cancer in men of african origin. Nat. Rev. Urol. 2016, 13, 99–107. [Google Scholar] [CrossRef]
- Cancer Research, UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence (accessed on 14 December 2020).
- Salinas, C.A.; Tsodikov, A.; Ishak-Howard, M.; Cooney, K.A. Prostate cancer in young men: An important clinical entity. Nat. Rev. Urol. 2014, 11, 317–323. [Google Scholar] [CrossRef]
- Lindstrom, S.; Schumacher, F.R.; Cox, D.; Travis, R.C.; Albanes, D.; Allen, N.E.; Andriole, G.; Berndt, S.I.; Boeing, H.; Bueno-de-Mesquita, H.B.; et al. Common genetic variants in prostate cancer risk prediction--results from the nci breast and prostate cancer cohort consortium (bpc3). Cancer Epidemiol. Biomed. Prev. 2012, 21, 437–444. [Google Scholar] [CrossRef]
- Hirschhorn, J.N.; Lohmueller, K.; Byrne, E.; Hirschhorn, K. A comprehensive review of genetic association studies. Genet. Med. 2002, 4, 45–61. [Google Scholar] [CrossRef]
- Weng, H.; Li, S.; Huang, J.Y.; He, Z.Q.; Meng, X.Y.; Cao, Y.; Fang, C.; Zeng, X.T. Androgen receptor gene polymorphisms and risk of prostate cancer: A meta-analysis. Sci. Rep. 2017, 7, 40554. [Google Scholar] [CrossRef]
- Sissung, T.M.; Price, D.K.; Del Re, M.; Ley, A.M.; Giovannetti, E.; Figg, W.D.; Danesi, R. Genetic variation: Effect on prostate cancer. Biochim. Biophys. Acta 2014, 1846, 446–456. [Google Scholar] [CrossRef]
- Schleutker, J. Polymorphisms in androgen signaling pathway predisposing to prostate cancer. Mol. Cell Endocrinol. 2012, 360, 25–37. [Google Scholar] [CrossRef]
- Price, D.K.; Chau, C.H.; Till, C.; Goodman, P.J.; Baum, C.E.; Ockers, S.B.; English, B.C.; Minasian, L.; Parnes, H.L.; Hsing, A.W.; et al. Androgen receptor cag repeat length and association with prostate cancer risk: Results from the prostate cancer prevention trial. J. Urol. 2010, 184, 2297–2302. [Google Scholar] [CrossRef]
- Zeegers, M.P.; Kiemeney, L.A.; Nieder, A.M.; Ostrer, H. How strong is the association between cag and ggn repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol. Biomed. Prev. 2004, 13, 1765–1771. [Google Scholar]
- Freedman, M.L.; Pearce, C.L.; Penney, K.L.; Hirschhorn, J.N.; Kolonel, L.N.; Henderson, B.E.; Altshuler, D. Systematic evaluation of genetic variation at the androgen receptor locus and risk of prostate cancer in a multiethnic cohort study. Am. J. Hum. Genet. 2005, 76, 82–90. [Google Scholar] [CrossRef]
- Mononen, N.; Schleutker, J. Polymorphisms in genes involved in androgen pathways as risk factors for prostate cancer. J. Urol. 2009, 181, 1541–1549. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Lin, H.; Xiong, L.; Ma, Y.; Lao, H. Esr1 pvuii (rs2234693 t>c) polymorphism and cancer susceptibility: Evidence from 80 studies. J. Cancer 2018, 9, 2963–2972. [Google Scholar] [CrossRef]
- Han, P.Z.; Cao, D.H.; Zhang, X.L.; Ren, Z.J.; Wei, Q. Association between tp53 gene codon72 polymorphism and prostate cancer risk: A systematic review and meta-analysis. Medicine (Baltim.) 2019, 98, e16135. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Castro, E.; Mahmud, N.; Guy, M.; Edwards, S.; O’Brien, L.; Sawyer, E.; et al. Brca2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br. J. Cancer 2011, 105, 1230–1234. [Google Scholar] [CrossRef]
- Edwards, S.M.; Kote-Jarai, Z.; Meitz, J.; Hamoudi, R.; Hope, Q.; Osin, P.; Jackson, R.; Southgate, C.; Singh, R.; Falconer, A.; et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the brca2 gene. Am. J. Hum. Genet. 2003, 72, 1–12. [Google Scholar] [CrossRef]
- Thompson, D.; Easton, D.; Breast Cancer Linkage, C. Variation in cancer risks, by mutation position, in brca2 mutation carriers. Am. J. Hum. Genet. 2001, 68, 410–419. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Thorlacius, S.; Tomasson, J.; Tryggvadottir, L.; Benediktsdottir, K.; Eyfjord, J.E.; Jonsson, E. Brca2 mutation in icelandic prostate cancer patients. J. Mol. Med. (Berl.) 1997, 75, 758–761. [Google Scholar] [CrossRef]
- Agalliu, I.; Karlins, E.; Kwon, E.M.; Iwasaki, L.M.; Diamond, A.; Ostrander, E.A.; Stanford, J.L. Rare germline mutations in the brca2 gene are associated with early-onset prostate cancer. Br. J. Cancer 2007, 97, 826–831. [Google Scholar] [CrossRef]
- Leongamornlert, D.; Mahmud, N.; Tymrakiewicz, M.; Saunders, E.; Dadaev, T.; Castro, E.; Goh, C.; Govindasami, K.; Guy, M.; O’Brien, L.; et al. Germline brca1 mutations increase prostate cancer risk. Br. J. Cancer 2012, 106, 1697–1701. [Google Scholar] [CrossRef]
- Thompson, D.; Easton, D.F.; Breast Cancer Linkage, C. Cancer incidence in brca1 mutation carriers. J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef]
- Ford, D.; Easton, D.F.; Bishop, D.T.; Narod, S.A.; Goldgar, D.E. Risks of cancer in brca1-mutation carriers. Breast cancer linkage consortium. Lancet 1994, 343, 692–695. [Google Scholar] [CrossRef]
- Hale, V.; Weischer, M.; Park, J.Y. Chek2 ( *) 1100delc mutation and risk of prostate cancer. Prost. Cancer 2014, 2014, 294575. [Google Scholar] [CrossRef]
- Cybulski, C.; Wokolorczyk, D.; Kluzniak, W.; Jakubowska, A.; Gorski, B.; Gronwald, J.; Huzarski, T.; Kashyap, A.; Byrski, T.; Debniak, T.; et al. An inherited nbn mutation is associated with poor prognosis prostate cancer. Br. J. Cancer 2013, 108, 461–468. [Google Scholar] [CrossRef]
- Cybulski, C.; Gorski, B.; Debniak, T.; Gliniewicz, B.; Mierzejewski, M.; Masojc, B.; Jakubowska, A.; Matyjasik, J.; Zlowocka, E.; Sikorski, A.; et al. Nbs1 is a prostate cancer susceptibility gene. Cancer Res. 2004, 64, 1215–1219. [Google Scholar] [CrossRef]
- Narod, S.A.; Feunteun, J.; Lynch, H.T.; Watson, P.; Conway, T.; Lynch, J.; Lenoir, G.M. Familial breast-ovarian cancer locus on chromosome 17q12–q23. Lancet 1991, 338, 82–83. [Google Scholar] [CrossRef]
- Hall, J.M.; Lee, M.K.; Newman, B.; Morrow, J.E.; Anderson, L.A.; Huey, B.; King, M.C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 1990, 250, 1684–1689. [Google Scholar] [CrossRef]
- Friedman, L.S.; Ostermeyer, E.A.; Szabo, C.I.; Dowd, P.; Lynch, E.D.; Rowell, S.E.; King, M.C. Confirmation of brca1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat. Genet. 1994, 8, 399–404. [Google Scholar] [CrossRef]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene brca1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef]
- Altshuler, D.; Daly, M.J.; Lander, E.S. Genetic mapping in human disease. Science 2008, 322, 881–888. [Google Scholar] [CrossRef]
- Lander, E.S.; Schork, N.J. Genetic dissection of complex traits. Science 1994, 265, 2037–2048. [Google Scholar] [CrossRef]
- Potter, S.R.; Partin, A.W. Hereditary and familial prostate cancer: Biologic aggressiveness and recurrence. Rev. Urol. 2000, 2, 35–36. [Google Scholar] [PubMed]
- Shriner, D. Overview of admixture mapping. Curr. Protoc. Hum. Genet. 2013. Chapter 1, Unit 1 23. [Google Scholar] [CrossRef]
- Smith, J.R.; Freije, D.; Carpten, J.D.; Gronberg, H.; Xu, J.; Isaacs, S.D.; Brownstein, M.J.; Bova, G.S.; Guo, H.; Bujnovszky, P.; et al. Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 1996, 274, 1371–1374. [Google Scholar] [CrossRef]
- Gronberg, H.; Smith, J.; Emanuelsson, M.; Jonsson, B.A.; Bergh, A.; Carpten, J.; Isaacs, W.; Xu, J.; Meyers, D.; Trent, J.; et al. In swedish families with hereditary prostate cancer, linkage to the hpc1 locus on chromosome 1q24-25 is restricted to families with early-onset prostate cancer. Am. J. Hum. Genet. 1999, 65, 134–140. [Google Scholar] [CrossRef]
- Carpten, J.; Nupponen, N.; Isaacs, S.; Sood, R.; Robbins, C.; Xu, J.; Faruque, M.; Moses, T.; Ewing, C.; Gillanders, E.; et al. Germline mutations in the ribonuclease l gene in families showing linkage with hpc1. Nat. Genet. 2002, 30, 181–184. [Google Scholar] [CrossRef]
- Eeles, R.A.; Durocher, F.; Edwards, S.; Teare, D.; Badzioch, M.; Hamoudi, R.; Gill, S.; Biggs, P.; Dearnaley, D.; Ardern-Jones, A.; et al. Linkage analysis of chromosome 1q markers in 136 prostate cancer families. The cancer research campaign/british prostate group u.K. Familial prostate cancer study collaborators. Am. J. Hum. Genet. 1998, 62, 653–658. [Google Scholar] [CrossRef]
- McIndoe, R.A.; Stanford, J.L.; Gibbs, M.; Jarvik, G.P.; Brandzel, S.; Neal, C.L.; Li, S.; Gammack, J.T.; Gay, A.A.; Goode, E.L.; et al. Linkage analysis of 49 high-risk families does not support a common familial prostate cancer-susceptibility gene at 1q24-25. Am. J. Hum. Genet. 1997, 61, 347–353. [Google Scholar] [CrossRef]
- Cooney, K.A.; McCarthy, J.D.; Lange, E.; Huang, L.; Miesfeldt, S.; Montie, J.E.; Oesterling, J.E.; Sandler, H.M.; Lange, K. Prostate cancer susceptibility locus on chromosome 1q: A confirmatory study. J. Natl. Cancer Inst. 1997, 89, 955–959. [Google Scholar] [CrossRef]
- Cancel-Tassin, G.; Latil, A.; Valeri, A.; Mangin, P.; Fournier, G.; Berthon, P.; Cussenot, O. Pcap is the major known prostate cancer predisposing locus in families from south and west europe. Eur. J. Hum. Genet. 2001, 9, 135–142. [Google Scholar] [CrossRef]
- Xu, J. Combined analysis of hereditary prostate cancer linkage to 1q24-25: Results from 772 hereditary prostate cancer families from the international consortium for prostate cancer genetics. Am. J. Hum. Genet. 2000, 66, 945–957. [Google Scholar] [CrossRef]
- Neuhausen, S.L.; Farnham, J.M.; Kort, E.; Tavtigian, S.V.; Skolnick, M.H.; Cannon-Albright, L.A. Prostate cancer susceptibility locus hpc1 in utah high-risk pedigrees. Hum. Mol. Genet. 1999, 8, 2437–2442. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Dimitrov, L.; Chang, B.L.; Adams, T.S.; Turner, A.R.; Meyers, D.A.; Eeles, R.A.; Easton, D.F.; Foulkes, W.D.; Simard, J.; et al. A combined genomewide linkage scan of 1,233 families for prostate cancer-susceptibility genes conducted by the international consortium for prostate cancer genetics. Am. J. Hum. Genet. 2005, 77, 219–229. [Google Scholar] [CrossRef]
- Berry, R.; Schaid, D.J.; Smith, J.R.; French, A.J.; Schroeder, J.J.; McDonnell, S.K.; Peterson, B.J.; Wang, Z.Y.; Carpten, J.D.; Roberts, S.G.; et al. Linkage analyses at the chromosome 1 loci 1q24-25 (hpc1), 1q42.2-43 (pcap), and 1p36 (capb) in families with hereditary prostate cancer. Am. J. Hum. Genet. 2000, 66, 539–546. [Google Scholar] [CrossRef][Green Version]
- Gibbs, M.; Stanford, J.L.; McIndoe, R.A.; Jarvik, G.P.; Kolb, S.; Goode, E.L.; Chakrabarti, L.; Schuster, E.F.; Buckley, V.A.; Miller, E.L.; et al. Evidence for a rare prostate cancer-susceptibility locus at chromosome 1p36. Am. J. Hum. Genet. 1999, 64, 776–787. [Google Scholar] [CrossRef]
- Matsui, H.; Suzuki, K.; Ohtake, N.; Nakata, S.; Takeuchi, T.; Yamanaka, H.; Inoue, I. Genomewide linkage analysis of familial prostate cancer in the japanese population. J. Hum. Genet. 2004, 49, 9–15. [Google Scholar] [CrossRef]
- Badzioch, M.; Eeles, R.; Leblanc, G.; Foulkes, W.D.; Giles, G.; Edwards, S.; Goldgar, D.; Hopper, J.L.; Bishop, D.T.; Moller, P.; et al. Suggestive evidence for a site specific prostate cancer gene on chromosome 1p36. The crc/bpg uk familial prostate cancer study coordinators and collaborators. The eu biomed collaborators. J. Med. Genet. 2000, 37, 947–949. [Google Scholar] [CrossRef][Green Version]
- Gibbs, M.; Chakrabarti, L.; Stanford, J.L.; Goode, E.L.; Kolb, S.; Schuster, E.F.; Buckley, V.A.; Shook, M.; Hood, L.; Jarvik, G.P.; et al. Analysis of chromosome 1q42.2-43 in 152 families with high risk of prostate cancer. Am. J. Hum. Genet. 1999, 64, 1087–1095. [Google Scholar] [CrossRef]
- Berthon, P.; Valeri, A.; Cohen-Akenine, A.; Drelon, E.; Paiss, T.; Wohr, G.; Latil, A.; Millasseau, P.; Mellah, I.; Cohen, N.; et al. Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am. J. Hum. Genet. 1998, 62, 1416–1424. [Google Scholar] [CrossRef]
- Cropp, C.D.; Simpson, C.L.; Wahlfors, T.; Ha, N.; George, A.; Jones, M.S.; Harper, U.; Ponciano-Jackson, D.; Green, T.A.; Tammela, T.L.; et al. Genome-wide linkage scan for prostate cancer susceptibility in finland: Evidence for a novel locus on 2q37.3 and confirmation of signal on 17q21-q22. Int. J. Cancer 2011, 129, 2400–2407. [Google Scholar] [CrossRef]
- Suarez, B.K.; Lin, J.; Burmester, J.K.; Broman, K.W.; Weber, J.L.; Banerjee, T.K.; Goddard, K.A.; Witte, J.S.; Elston, R.C.; Catalona, W.J. A genome screen of multiplex sibships with prostate cancer. Am. J. Hum. Genet. 2000, 66, 933–944. [Google Scholar] [CrossRef][Green Version]
- Pierce, B.L.; Friedrichsen-Karyadi, D.M.; McIntosh, L.; Deutsch, K.; Hood, L.; Ostrander, E.A.; Austin, M.A.; Stanford, J.L. Genomic scan of 12 hereditary prostate cancer families having an occurrence of pancreas cancer. Prostate 2007, 67, 410–415. [Google Scholar] [CrossRef]
- Larson, G.P.; Ding, Y.; Cheng, L.S.; Lundberg, C.; Gagalang, V.; Rivas, G.; Geller, L.; Weitzel, J.; MacDonald, D.; Archambeau, J.; et al. Genetic linkage of prostate cancer risk to the chromosome 3 region bearing fhit. Cancer Res. 2005, 65, 805–814. [Google Scholar] [PubMed]
- Rokman, A.; Baffoe-Bonnie, A.B.; Gillanders, E.; Fredriksson, H.; Autio, V.; Ikonen, T.; Gibbs, K.D., Jr.; Jones, M.; Gildea, D.; Freas-Lutz, D.; et al. Hereditary prostate cancer in finland: Fine-mapping validates 3p26 as a major predisposition locus. Hum. Genet. 2005, 116, 43–50. [Google Scholar] [CrossRef]
- Schleutker, J.; Baffoe-Bonnie, A.B.; Gillanders, E.; Kainu, T.; Jones, M.P.; Freas-Lutz, D.; Markey, C.; Gildea, D.; Riedesel, E.; Albertus, J.; et al. Genome-wide scan for linkage in finnish hereditary prostate cancer (hpc) families identifies novel susceptibility loci at 11q14 and 3p25-26. Prostate 2003, 57, 280–289. [Google Scholar] [CrossRef]
- Christensen, G.B.; Baffoe-Bonnie, A.B.; George, A.; Powell, I.; Bailey-Wilson, J.E.; Carpten, J.D.; Giles, G.G.; Hopper, J.L.; Severi, G.; English, D.R.; et al. Genome-wide linkage analysis of 1,233 prostate cancer pedigrees from the international consortium for prostate cancer genetics using novel sumlink and sumlod analyses. Prostate 2010, 70, 735–744. [Google Scholar] [CrossRef]
- Bock, C.H.; Schwartz, A.G.; Ruterbusch, J.J.; Levin, A.M.; Neslund-Dudas, C.; Land, S.J.; Wenzlaff, A.S.; Reich, D.; McKeigue, P.; Chen, W.; et al. Results from a prostate cancer admixture mapping study in african-american men. Hum. Genet. 2009, 126, 637–642. [Google Scholar] [CrossRef]
- Schaid, D.J.; McDonnell, S.K.; Zarfas, K.E.; Cunningham, J.M.; Hebbring, S.; Thibodeau, S.N.; Eeles, R.A.; Easton, D.F.; Foulkes, W.D.; Simard, J.; et al. Pooled genome linkage scan of aggressive prostate cancer: Results from the international consortium for prostate cancer genetics. Hum. Genet. 2006, 120, 471–485. [Google Scholar] [CrossRef]
- Friedrichsen, D.M.; Stanford, J.L.; Isaacs, S.D.; Janer, M.; Chang, B.L.; Deutsch, K.; Gillanders, E.; Kolb, S.; Wiley, K.E.; Badzioch, M.D.; et al. Identification of a prostate cancer susceptibility locus on chromosome 7q11-21 in jewish families. Proc. Natl. Acad. Sci. USA 2004, 101, 1939–1944. [Google Scholar] [CrossRef]
- Witte, J.S.; Goddard, K.A.; Conti, D.V.; Elston, R.C.; Lin, J.; Suarez, B.K.; Broman, K.W.; Burmester, J.K.; Weber, J.L.; Catalona, W.J. Genomewide scan for prostate cancer-aggressiveness loci. Am. J. Hum. Genet. 2000, 67, 92–99. [Google Scholar] [CrossRef]
- Neville, P.J.; Conti, D.V.; Paris, P.L.; Levin, H.; Catalona, W.J.; Suarez, B.K.; Witte, J.S.; Casey, G. Prostate cancer aggressiveness locus on chromosome 7q32-q33 identified by linkage and allelic imbalance studies. Neoplasia 2002, 4, 424–431. [Google Scholar] [CrossRef][Green Version]
- Paiss, T.; Worner, S.; Kurtz, F.; Haeussler, J.; Hautmann, R.E.; Gschwend, J.E.; Herkommer, K.; Vogel, W. Linkage of aggressive prostate cancer to chromosome 7q31-33 in german prostate cancer families. Eur. J. Hum. Genet. 2003, 11, 17–22. [Google Scholar] [CrossRef]
- Wiklund, F.; Jonsson, B.A.; Goransson, I.; Bergh, A.; Gronberg, H. Linkage analysis of prostate cancer susceptibility: Confirmation of linkage at 8p22-23. Hum. Genet. 2003, 112, 414–418. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, S.L.; Hawkins, G.A.; Faith, D.A.; Kelly, B.; Isaacs, S.D.; Wiley, K.E.; Chang, B.; Ewing, C.M.; Bujnovszky, P.; et al. Linkage and association studies of prostate cancer susceptibility: Evidence for linkage at 8p22-23. Am. J. Hum. Genet. 2001, 69, 341–350. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, S.L.; Komiya, A.; Mychaleckyj, J.C.; Isaacs, S.D.; Hu, J.J.; Sterling, D.; Lange, E.M.; Hawkins, G.A.; Turner, A.; et al. Germline mutations and sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Nat. Genet. 2002, 32, 321–325. [Google Scholar] [CrossRef]
- Amundadottir, L.T.; Sulem, P.; Gudmundsson, J.; Helgason, A.; Baker, A.; Agnarsson, B.A.; Sigurdsson, A.; Benediktsdottir, K.R.; Cazier, J.B.; Sainz, J.; et al. A common variant associated with prostate cancer in european and african populations. Nat. Genet. 2006, 38, 652–658. [Google Scholar] [CrossRef]
- Freedman, M.L.; Haiman, C.A.; Patterson, N.; McDonald, G.J.; Tandon, A.; Waliszewska, A.; Penney, K.; Steen, R.G.; Ardlie, K.; John, E.M.; et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in african-american men. Proc. Natl. Acad. Sci. USA 2006, 103, 14068–14073. [Google Scholar] [CrossRef]
- Witte, J.S.; Suarez, B.K.; Thiel, B.; Lin, J.; Yu, A.; Banerjee, T.K.; Burmester, J.K.; Casey, G.; Catalona, W.J. Genome-wide scan of brothers: Replication and fine mapping of prostate cancer susceptibility and aggressiveness loci. Prostate 2003, 57, 298–308. [Google Scholar] [CrossRef]
- Xu, J.; Gillanders, E.M.; Isaacs, S.D.; Chang, B.L.; Wiley, K.E.; Zheng, S.L.; Jones, M.; Gildea, D.; Riedesel, E.; Albertus, J.; et al. Genome-wide scan for prostate cancer susceptibility genes in the johns hopkins hereditary prostate cancer families. Prostate 2003, 57, 320–325. [Google Scholar] [CrossRef]
- Fitzgerald, L.M.; McDonnell, S.K.; Carlson, E.E.; Langeberg, W.; McIntosh, L.M.; Deutsch, K.; Ostrander, E.A.; Schaid, D.J.; Stanford, J.L. Genome-wide linkage analyses of hereditary prostate cancer families with colon cancer provide further evidence for a susceptibility locus on 15q11-q14. Eur. J. Hum. Genet. 2010, 18, 1141–1147. [Google Scholar] [CrossRef]
- Lange, E.M.; Ho, L.A.; Beebe-Dimmer, J.L.; Wang, Y.; Gillanders, E.M.; Trent, J.M.; Lange, L.A.; Wood, D.P.; Cooney, K.A. Genome-wide linkage scan for prostate cancer susceptibility genes in men with aggressive disease: Significant evidence for linkage at chromosome 15q12. Hum. Genet. 2006, 119, 400–407. [Google Scholar] [CrossRef][Green Version]
- Gillanders, E.M.; Xu, J.; Chang, B.L.; Lange, E.M.; Wiklund, F.; Bailey-Wilson, J.E.; Baffoe-Bonnie, A.; Jones, M.; Gildea, D.; Riedesel, E.; et al. Combined genome-wide scan for prostate cancer susceptibility genes. J. Natl. Cancer Inst. 2004, 96, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Emi, M.; Nagai, H.; Nishimura, T.; Konishi, N.; Kubota, Y.; Ichikawa, T.; Takahashi, S.; Shuin, T.; Habuchi, T.; et al. Association of common missense changes in elac2 ( hpc2) with prostate cancer in a japanese case-control series. J. Hum. Genet. 2002, 47, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Camp, N.J.; Tavtigian, S.V. Meta-analysis of associations of the ser217leu and ala541thr variants in elac2 (hpc2) and prostate cancer. Am. J. Hum. Genet. 2002, 71, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Tavtigian, S.V.; Simard, J.; Teng, D.H.; Abtin, V.; Baumgard, M.; Beck, A.; Camp, N.J.; Carillo, A.R.; Chen, Y.; Dayananth, P.; et al. A candidate prostate cancer susceptibility gene at chromosome 17p. Nat. Genet. 2001, 27, 172–180. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Walker, A.H.; Zeigler-Johnson, C.; Weisburg, S.; Martin, A.M.; Nathanson, K.L.; Wein, A.J.; Malkowicz, S.B. Association of hpc2/elac2 genotypes and prostate cancer. Am. J. Hum. Genet. 2000, 67, 1014–1019. [Google Scholar] [CrossRef]
- Lange, E.M.; Gillanders, E.M.; Davis, C.C.; Brown, W.M.; Campbell, J.K.; Jones, M.; Gildea, D.; Riedesel, E.; Albertus, J.; Freas-Lutz, D.; et al. Genome-wide scan for prostate cancer susceptibility genes using families from the university of michigan prostate cancer genetics project finds evidence for linkage on chromosome 17 near brca1. Prostate 2003, 57, 326–334. [Google Scholar] [CrossRef]
- Schaid, D.J.; Stanford, J.L.; McDonnell, S.K.; Suuriniemi, M.; McIntosh, L.; Karyadi, D.M.; Carlson, E.E.; Deutsch, K.; Janer, M.; Hood, L.; et al. Genome-wide linkage scan of prostate cancer gleason score and confirmation of chromosome 19q. Hum. Genet. 2007, 121, 729–735. [Google Scholar] [CrossRef]
- Slager, S.L.; Schaid, D.J.; Cunningham, J.M.; McDonnell, S.K.; Marks, A.F.; Peterson, B.J.; Hebbring, S.J.; Anderson, S.; French, A.J.; Thibodeau, S.N. Confirmation of linkage of prostate cancer aggressiveness with chromosome 19q. Am. J. Hum. Genet. 2003, 72, 759–762. [Google Scholar] [CrossRef]
- Neville, P.J.; Conti, D.V.; Krumroy, L.M.; Catalona, W.J.; Suarez, B.K.; Witte, J.S.; Casey, G. Prostate cancer aggressiveness locus on chromosome segment 19q12-q13.1 identified by linkage and allelic imbalance studies. Genes Chrom. Cancer 2003, 36, 332–339. [Google Scholar] [CrossRef]
- Zheng, S.L.; Xu, J.; Isaacs, S.D.; Wiley, K.; Chang, B.; Bleecker, E.R.; Walsh, P.C.; Trent, J.M.; Meyers, D.A.; Isaacs, W.B. Evidence for a prostate cancer linkage to chromosome 20 in 159 hereditary prostate cancer families. Hum. Genet. 2001, 108, 430–435. [Google Scholar] [CrossRef]
- Bock, C.H.; Cunningham, J.M.; McDonnell, S.K.; Schaid, D.J.; Peterson, B.J.; Pavlic, R.J.; Schroeder, J.J.; Klein, J.; French, A.J.; Marks, A.; et al. Analysis of the prostate cancer-susceptibility locus hpc20 in 172 families affected by prostate cancer. Am. J. Hum. Genet. 2001, 68, 795–801. [Google Scholar] [CrossRef]
- Berry, R.; Schroeder, J.J.; French, A.J.; McDonnell, S.K.; Peterson, B.J.; Cunningham, J.M.; Thibodeau, S.N.; Schaid, D.J. Evidence for a prostate cancer-susceptibility locus on chromosome 20. Am. J. Hum. Genet. 2000, 67, 82–91. [Google Scholar] [CrossRef]
- Johanneson, B.; McDonnell, S.K.; Karyadi, D.M.; Quignon, P.; McIntosh, L.; Riska, S.M.; FitzGerald, L.M.; Johnson, G.; Deutsch, K.; Williams, G.; et al. Family-based association analysis of 42 hereditary prostate cancer families identifies the apolipoprotein l3 region on chromosome 22q12 as a risk locus. Hum. Mol. Genet. 2010, 19, 3852–3862. [Google Scholar] [CrossRef]
- Johanneson, B.; McDonnell, S.K.; Karyadi, D.M.; Hebbring, S.J.; Wang, L.; Deutsch, K.; McIntosh, L.; Kwon, E.M.; Suuriniemi, M.; Stanford, J.L.; et al. Fine mapping of familial prostate cancer families narrows the interval for a susceptibility locus on chromosome 22q12.3 to 1.36 mb. Hum. Genet. 2008, 123, 65–75. [Google Scholar] [CrossRef]
- Camp, N.J.; Cannon-Albright, L.A.; Farnham, J.M.; Baffoe-Bonnie, A.B.; George, A.; Powell, I.; Bailey-Wilson, J.E.; Carpten, J.D.; Giles, G.G.; Hopper, J.L.; et al. Compelling evidence for a prostate cancer gene at 22q12.3 by the international consortium for prostate cancer genetics. Hum. Mol. Genet. 2007, 16, 1271–1278. [Google Scholar] [CrossRef][Green Version]
- Camp, N.J.; Farnham, J.M.; Cannon-Albright, L.A. Localization of a prostate cancer predisposition gene to an 880-kb region on chromosome 22q12.3 in utah high-risk pedigrees. Cancer. Res. 2006, 66, 10205–10212. [Google Scholar] [CrossRef][Green Version]
- Baffoe-Bonnie, A.B.; Smith, J.R.; Stephan, D.A.; Schleutker, J.; Carpten, J.D.; Kainu, T.; Gillanders, E.M.; Matikainen, M.; Teslovich, T.M.; Tammela, T.; et al. A major locus for hereditary prostate cancer in finland: Localization by linkage disequilibrium of a haplotype in the hpcx region. Hum. Genet. 2005, 117, 307–316. [Google Scholar]
- Farnham, J.M.; Camp, N.J.; Swensen, J.; Tavtigian, S.V.; Albright, L.A. Confirmation of the hpcx prostate cancer predisposition locus in large utah prostate cancer pedigrees. Hum. Genet. 2005, 116, 179–185. [Google Scholar] [CrossRef]
- Peters, M.A.; Jarvik, G.P.; Janer, M.; Chakrabarti, L.; Kolb, S.; Goode, E.L.; Gibbs, M.; DuBois, C.C.; Schuster, E.F.; Hood, L.; et al. Genetic linkage analysis of prostate cancer families to xq27-28. Hum. Hered. 2001, 51, 107–113. [Google Scholar] [CrossRef]
- Xu, J.; Meyers, D.; Freije, D.; Isaacs, S.; Wiley, K.; Nusskern, D.; Ewing, C.; Wilkens, E.; Bujnovszky, P.; Bova, G.S.; et al. Evidence for a prostate cancer susceptibility locus on the x chromosome. Nat. Genet. 1998, 20, 175–179. [Google Scholar] [CrossRef]
- Schaid, D.J. The complex genetic epidemiology of prostate cancer. Hum. Mol. Genet. 2004, 13, R103–R121. [Google Scholar] [CrossRef]
- Easton, D.F.; Schaid, D.J.; Whittemore, A.S.; Isaacs, W.J.; International Consortium for Prostate Cancer, G. Where are the prostate cancer genes?--a summary of eight genome wide searches. Prostate 2003, 57, 261–269. [Google Scholar] [CrossRef]
- Conti, D.V.; Darst, B.F.; Moss, L.C.; Saunders, E.J.; Sheng, X.; Chou, A.; Schumacher, F.R.; Olama, A.A.A.; Benlloch, S.; Dadaev, T.; et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat. Genet. 2021, 53, 65–75. [Google Scholar] [CrossRef]
- Hao, Z. Rideogram: Drawing svg graphics to visualize and map genome-wide data on the idiograms. PeerJ. Comput. Sci. 2020. [Google Scholar] [CrossRef]
- Lange, E.M.; Robbins, C.M.; Gillanders, E.M.; Zheng, S.L.; Xu, J.; Wang, Y.; White, K.A.; Chang, B.L.; Ho, L.A.; Trent, J.M.; et al. Fine-mapping the putative chromosome 17q21-22 prostate cancer susceptibility gene to a 10 cm region based on linkage analysis. Hum. Genet. 2007, 121, 49–55. [Google Scholar] [CrossRef]
- Ewing, C.M.; Ray, A.M.; Lange, E.M.; Zuhlke, K.A.; Robbins, C.M.; Tembe, W.D.; Wiley, K.E.; Isaacs, S.D.; Johng, D.; Wang, Y.; et al. Germline mutations in hoxb13 and prostate-cancer risk. N. Engl. J. Med. 2012, 366, 141–149. [Google Scholar] [CrossRef]
- Chen, Z.; Greenwood, C.; Isaacs, W.B.; Foulkes, W.D.; Sun, J.; Zheng, S.L.; Condreay, L.D.; Xu, J. The g84e mutation of hoxb13 is associated with increased risk for prostate cancer: Results from the reduce trial. Carcinogenesis 2013, 34, 1260–1264. [Google Scholar] [CrossRef]
- Shang, Z.; Zhu, S.; Zhang, H.; Li, L.; Niu, Y. Germline homeobox b13 (hoxb13) g84e mutation and prostate cancer risk in european descendants: A meta-analysis of 24,213 cases and 73, 631 controls. Eur. Urol. 2013, 64, 173–176. [Google Scholar] [CrossRef]
- Witte, J.S.; Mefford, J.; Plummer, S.J.; Liu, J.; Cheng, I.; Klein, E.A.; Rybicki, B.A.; Casey, G. Hoxb13 mutation and prostate cancer: Studies of siblings and aggressive disease. Cancer Epidemiol. Biom. Prev. 2013, 22, 675–680. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Hathcock, M.; Yee, C.; Okoth, L.A.; Ewing, C.M.; Isaacs, W.B.; Cooney, K.A.; Thibodeau, S.N. The hoxb13 g84e mutation is associated with an increased risk for prostate cancer and other malignancies. Cancer Epidemiol. Biomed. Prev. 2015, 24, 1366–1372. [Google Scholar] [CrossRef]
- Huang, H.; Cai, B. G84e mutation in hoxb13 is firmly associated with prostate cancer risk: A meta-analysis. Tumour Biol. 2014, 35, 1177–1182. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Masson, G.; Agnarsson, B.A.; Benediktsdottir, K.R.; Sigurdsson, A.; Magnusson, O.T.; Gudjonsson, S.A.; Magnusdottir, D.N.; et al. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nat. Genet. 2012, 44, 1326–1329. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Mikropoulos, C.; Leongamornlert, D.A.; Dadaev, T.; Tymrakiewicz, M.; Saunders, E.J.; Jones, M.; Jugurnauth-Little, S.; Govindasami, K.; Guy, M.; et al. Prevalence of the hoxb13 g84e germline mutation in british men and correlation with prostate cancer risk, tumour characteristics and clinical outcomes. Ann. Oncol. 2015, 26, 756–761. [Google Scholar] [CrossRef]
- Laitinen, V.H.; Wahlfors, T.; Saaristo, L.; Rantapero, T.; Pelttari, L.M.; Kilpivaara, O.; Laasanen, S.L.; Kallioniemi, A.; Nevanlinna, H.; Aaltonen, L.; et al. Hoxb13 g84e mutation in finland: Population-based analysis of prostate, breast, and colorectal cancer risk. Cancer Epidemiol. Biomed. Prev. 2013, 22, 452–460. [Google Scholar] [CrossRef]
- Stott-Miller, M.; Karyadi, D.M.; Smith, T.; Kwon, E.M.; Kolb, S.; Stanford, J.L.; Ostrander, E.A. Hoxb13 mutations in a population-based, case-control study of prostate cancer. Prostate 2013, 73, 634–641. [Google Scholar] [CrossRef]
- Xu, J.; Lange, E.M.; Lu, L.; Zheng, S.L.; Wang, Z.; Thibodeau, S.N.; Cannon-Albright, L.A.; Teerlink, C.C.; Camp, N.J.; Johnson, A.M.; et al. Hoxb13 is a susceptibility gene for prostate cancer: Results from the international consortium for prostate cancer genetics (icpcg). Hum. Genet. 2013, 132, 5–14. [Google Scholar] [CrossRef]
- Nyberg, T.; Govindasami, K.; Leslie, G.; Dadaev, T.; Bancroft, E.; Ni Raghallaigh, H.; Brook, M.N.; Hussain, N.; Keating, D.; Lee, A.; et al. Homeobox b13 g84e mutation and prostate cancer risk. Eur. Urol. 2019, 75, 834–845. [Google Scholar] [CrossRef]
- Handorf, E.; Crumpler, N.; Gross, L.; Giri, V.N. Prevalence of the hoxb13 g84e mutation among unaffected men with a family history of prostate cancer. J. Genet. Couns 2014, 23, 371–376. [Google Scholar] [CrossRef]
- Lin, X.; Qu, L.; Chen, Z.; Xu, C.; Ye, D.; Shao, Q.; Wang, X.; Qi, J.; Chen, Z.; Zhou, F.; et al. A novel germline mutation in hoxb13 is associated with prostate cancer risk in chinese men. Prostate 2013, 73, 169–175. [Google Scholar] [CrossRef]
- Momozawa, Y.; Iwasaki, Y.; Hirata, M.; Liu, X.; Kamatani, Y.; Takahashi, A.; Sugano, K.; Yoshida, T.; Murakami, Y.; Matsuda, K.; et al. Germline pathogenic variants in 7636 japanese patients with prostate cancer and 12 366 controls. J. Natl. Cancer Inst. 2020, 112, 369–376. [Google Scholar] [CrossRef]
- Maia, S.; Cardoso, M.; Pinto, P.; Pinheiro, M.; Santos, C.; Peixoto, A.; Bento, M.J.; Oliveira, J.; Henrique, R.; Jeronimo, C.; et al. Identification of two novel hoxb13 germline mutations in portuguese prostate cancer patients. PLoS ONE 2015, 10, e0132728. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef]
- Genomes Project, C.; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- International HapMap, C. A haplotype map of the human genome. Nature 2005, 437, 1299–1320. [Google Scholar] [CrossRef]
- Hirschhorn, J.N.; Daly, M.J. Genome-wide association studies for common diseases and complex traits. Nat. Rev. Genet. 2005, 6, 95–108. [Google Scholar] [CrossRef]
- LaFramboise, T. Single nucleotide polymorphism arrays: A decade of biological, computational and technological advances. Nucl. Acids Res. 2009, 37, 4181–4193. [Google Scholar] [CrossRef]
- Marchini, J.; Howie, B. Genotype imputation for genome-wide association studies. Nat. Rev. Genet. 2010, 11, 499–511. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Fadista, J.; Manning, A.K.; Florez, J.C.; Groop, L. The (in)famous gwas p-value threshold revisited and updated for low-frequency variants. Eur. J. Hum. Genet. 2016, 24, 1202–1205. [Google Scholar] [CrossRef]
- Pe’er, I.; Yelensky, R.; Altshuler, D.; Daly, M.J. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet. Epidemiol. 2008, 32, 381–385. [Google Scholar] [CrossRef]
- Evangelou, E.; Ioannidis, J.P. Meta-analysis methods for genome-wide association studies and beyond. Nat. Rev. Genet. 2013, 14, 379–389. [Google Scholar] [CrossRef]
- Wang, W.Y.; Barratt, B.J.; Clayton, D.G.; Todd, J.A. Genome-wide association studies: Theoretical and practical concerns. Nat. Rev. Genet. 2005, 6, 109–118. [Google Scholar] [CrossRef]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 years of gwas discovery: Biology, function, and translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five years of gwas discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Manolescu, A.; Amundadottir, L.T.; Gudbjartsson, D.; Helgason, A.; Rafnar, T.; Bergthorsson, J.T.; Agnarsson, B.A.; Baker, A.; et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat. Genet. 2007, 39, 631–637. [Google Scholar] [CrossRef]
- Duggan, D.; Zheng, S.L.; Knowlton, M.; Benitez, D.; Dimitrov, L.; Wiklund, F.; Robbins, C.; Isaacs, S.D.; Cheng, Y.; Li, G.; et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene dab2ip. J. Natl. Cancer Inst. 2007, 99, 1836–1844. [Google Scholar] [CrossRef]
- Yeager, M.; Orr, N.; Hayes, R.B.; Jacobs, K.B.; Kraft, P.; Wacholder, S.; Minichiello, M.J.; Fearnhead, P.; Yu, K.; Chatterjee, N.; et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat. Genet. 2007, 39, 645–649. [Google Scholar] [CrossRef]
- Haiman, C.A.; Patterson, N.; Freedman, M.L.; Myers, S.R.; Pike, M.C.; Waliszewska, A.; Neubauer, J.; Tandon, A.; Schirmer, C.; McDonald, G.J.; et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat. Genet. 2007, 39, 638–644. [Google Scholar] [CrossRef]
- Witte, J.S. Multiple prostate cancer risk variants on 8q24. Nat. Genet. 2007, 39, 579–580. [Google Scholar] [CrossRef]
- Matejcic, M.; Saunders, E.J.; Dadaev, T.; Brook, M.N.; Wang, K.; Sheng, X.; Olama, A.A.A.; Schumacher, F.R.; Ingles, S.A.; Govindasami, K.; et al. Germline variation at 8q24 and prostate cancer risk in men of european ancestry. Nat. Commun. 2018, 9, 4616. [Google Scholar] [CrossRef] [PubMed]
- Al Olama, A.A.; Kote-Jarai, Z.; Giles, G.G.; Guy, M.; Morrison, J.; Severi, G.; Leongamornlert, D.A.; Tymrakiewicz, M.; Jhavar, S.; Saunders, E.; et al. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat. Genet. 2009, 41, 1058–1060. [Google Scholar] [CrossRef]
- Darst, B.F.; Wan, P.; Sheng, X.; Bensen, J.T.; Ingles, S.A.; Rybicki, B.A.; Nemesure, B.; John, E.M.; Fowke, J.H.; Stevens, V.L.; et al. A germline variant at 8q24 contributes to familial clustering of prostate cancer in men of african ancestry. Eur. Urol. 2020. [Google Scholar] [CrossRef]
- Han, Y.; Rand, K.A.; Hazelett, D.J.; Ingles, S.A.; Kittles, R.A.; Strom, S.S.; Rybicki, B.A.; Nemesure, B.; Isaacs, W.B.; Stanford, J.L.; et al. Prostate cancer susceptibility in men of african ancestry at 8q24. J. Natl. Cancer Inst. 2016. [Google Scholar] [CrossRef]
- Hazelett, D.J.; Coetzee, S.G.; Coetzee, G.A. A rare variant, which destroys a foxa1 site at 8q24, is associated with prostate cancer risk. Cell Cycl. 2013, 12, 379–380. [Google Scholar] [CrossRef][Green Version]
- Dupont, W.D.; Breyer, J.P.; Plummer, W.D.; Chang, S.S.; Cookson, M.S.; Smith, J.A.; Blue, E.E.; Bamshad, M.J.; Smith, J.R. 8q24 genetic variation and comprehensive haplotypes altering familial risk of prostate cancer. Nat. Commun. 2020, 11, 1523. [Google Scholar] [CrossRef]
- Teerlink, C.C.; Leongamornlert, D.; Dadaev, T.; Thomas, A.; Farnham, J.; Stephenson, R.A.; Riska, S.; McDonnell, S.K.; Schaid, D.J.; Catalona, W.J.; et al. Genome-wide association of familial prostate cancer cases identifies evidence for a rare segregating haplotype at 8q24.21. Hum. Genet. 2016, 135, 923–938. [Google Scholar] [CrossRef]
- Huppi, K.; Pitt, J.J.; Wahlberg, B.M.; Caplen, N.J. The 8q24 gene desert: An oasis of non-coding transcriptional activity. Front. Genet. 2012, 3, 69. [Google Scholar] [CrossRef]
- Hazelett, D.J.; Rhie, S.K.; Gaddis, M.; Yan, C.; Lakeland, D.L.; Coetzee, S.G.; Ellipse, G.-O.N.; Practical, C.; Henderson, B.E.; Noushmehr, H.; et al. Comprehensive functional annotation of 77 prostate cancer risk loci. PLoS Genet. 2014, 10, e1004102. [Google Scholar] [CrossRef]
- Jia, L.; Landan, G.; Pomerantz, M.; Jaschek, R.; Herman, P.; Reich, D.; Yan, C.; Khalid, O.; Kantoff, P.; Oh, W.; et al. Functional enhancers at the gene-poor 8q24 cancer-linked locus. PLoS Genet. 2009, 5, e1000597. [Google Scholar] [CrossRef]
- Wasserman, N.F.; Aneas, I.; Nobrega, M.A. An 8q24 gene desert variant associated with prostate cancer risk confers differential in vivo activity to a myc enhancer. Genom. Res. 2010, 20, 1191–1197. [Google Scholar] [CrossRef]
- Sotelo, J.; Esposito, D.; Duhagon, M.A.; Banfield, K.; Mehalko, J.; Liao, H.; Stephens, R.M.; Harris, T.J.; Munroe, D.J.; Wu, X. Long-range enhancers on 8q24 regulate c-myc. Proc. Natl. Acad. Sci. USA 2010, 107, 3001–3005. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Ahmadiyeh, N.; Jia, L.; Herman, P.; Verzi, M.P.; Doddapaneni, H.; Beckwith, C.A.; Chan, J.A.; Hills, A.; Davis, M.; et al. The 8q24 cancer risk variant rs6983267 shows long-range interaction with myc in colorectal cancer. Nat. Genet. 2009, 41, 882–884. [Google Scholar] [CrossRef]
- Walavalkar, K.; Saravanan, B.; Singh, A.K.; Jayani, R.S.; Nair, A.; Farooq, U.; Islam, Z.; Soota, D.; Mann, R.; Shivaprasad, P.V.; et al. A rare variant of african ancestry activates 8q24 lncrna hub by modulating cancer associated enhancer. Nat. Commun. 2020, 11, 3598. [Google Scholar] [CrossRef]
- Breyer, J.P.; Dorset, D.C.; Clark, T.A.; Bradley, K.M.; Wahlfors, T.A.; McReynolds, K.M.; Maynard, W.H.; Chang, S.S.; Cookson, M.S.; Smith, J.A.; et al. An expressed retrogene of the master embryonic stem cell gene pou5f1 is associated with prostate cancer susceptibility. Am. J. Hum. Genet. 2014, 94, 395–404. [Google Scholar] [CrossRef]
- Takata, R.; Takahashi, A.; Fujita, M.; Momozawa, Y.; Saunders, E.J.; Yamada, H.; Maejima, K.; Nakano, K.; Nishida, Y.; Hishida, A.; et al. 12 new susceptibility loci for prostate cancer identified by genome-wide association study in japanese population. Nat. Commun. 2019, 10, 4422. [Google Scholar] [CrossRef]
- Xiang, J.F.; Yang, L.; Chen, L.L. The long noncoding rna regulation at the myc locus. Curr. Opin. Genet. Dev. 2015, 33, 41–48. [Google Scholar] [CrossRef]
- Meyer, K.B.; Maia, A.T.; O’Reilly, M.; Ghoussaini, M.; Prathalingam, R.; Porter-Gill, P.; Ambs, S.; Prokunina-Olsson, L.; Carroll, J.; Ponder, B.A. A functional variant at a prostate cancer predisposition locus at 8q24 is associated with pvt1 expression. PLoS Genet. 2011, 7, e1002165. [Google Scholar] [CrossRef]
- Guo, H.; Ahmed, M.; Zhang, F.; Yao, C.Q.; Li, S.; Liang, Y.; Hua, J.; Soares, F.; Sun, Y.; Langstein, J.; et al. Modulation of long noncoding rnas by risk snps underlying genetic predispositions to prostate cancer. Nat. Genet. 2016, 48, 1142–1150. [Google Scholar] [CrossRef]
- Chung, S.; Nakagawa, H.; Uemura, M.; Piao, L.; Ashikawa, K.; Hosono, N.; Takata, R.; Akamatsu, S.; Kawaguchi, T.; Morizono, T.; et al. Association of a novel long non-coding rna in 8q24 with prostate cancer susceptibility. Cancer Sci. 2011, 102, 245–252. [Google Scholar] [CrossRef]
- Eeles, R.A.; Kote-Jarai, Z.; Al Olama, A.A.; Giles, G.G.; Guy, M.; Severi, G.; Muir, K.; Hopper, J.L.; Henderson, B.E.; Haiman, C.A.; et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat. Genet. 2009, 41, 1116–1121. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Rafnar, T.; Bergthorsson, J.T.; Manolescu, A.; Gudbjartsson, D.; Agnarsson, B.A.; Sigurdsson, A.; Benediktsdottir, K.R.; Blondal, T.; et al. Common sequence variants on 2p15 and xp11.22 confer susceptibility to prostate cancer. Nat. Genet. 2008, 40, 281–283. [Google Scholar] [CrossRef]
- Eeles, R.A.; Kote-Jarai, Z.; Giles, G.G.; Olama, A.A.; Guy, M.; Jugurnauth, S.K.; Mulholland, S.; Leongamornlert, D.A.; Edwards, S.M.; Morrison, J.; et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat. Genet. 2008, 40, 316–321. [Google Scholar] [CrossRef]
- Thomas, G.; Jacobs, K.B.; Yeager, M.; Kraft, P.; Wacholder, S.; Orr, N.; Yu, K.; Chatterjee, N.; Welch, R.; Hutchinson, A.; et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat. Genet. 2008, 40, 310–315. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Olama, A.A.; Giles, G.G.; Severi, G.; Schleutker, J.; Weischer, M.; Campa, D.; Riboli, E.; Key, T.; Gronberg, H.; et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat. Genet. 2011, 43, 785–791. [Google Scholar] [CrossRef]
- Schumacher, F.R.; Berndt, S.I.; Siddiq, A.; Jacobs, K.B.; Wang, Z.; Lindstrom, S.; Stevens, V.L.; Chen, C.; Mondul, A.M.; Travis, R.C.; et al. Genome-wide association study identifies new prostate cancer susceptibility loci. Hum. Mol. Genet. 2011, 20, 3867–3875. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Blondal, T.; Gylfason, A.; Agnarsson, B.A.; Benediktsdottir, K.R.; Magnusdottir, D.N.; Orlygsdottir, G.; Jakobsdottir, M.; et al. Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat. Genet. 2009, 41, 1122–1126. [Google Scholar] [CrossRef]
- Schumacher, F.R.; Al Olama, A.A.; Berndt, S.I.; Benlloch, S.; Ahmed, M.; Saunders, E.J.; Dadaev, T.; Leongamornlert, D.; Anokian, E.; Cieza-Borrella, C.; et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 2018, 50, 928–936. [Google Scholar] [CrossRef]
- Eeles, R.A.; Olama, A.A.; Benlloch, S.; Saunders, E.J.; Leongamornlert, D.A.; Tymrakiewicz, M.; Ghoussaini, M.; Luccarini, C.; Dennis, J.; Jugurnauth-Little, S.; et al. Identification of 23 new prostate cancer susceptibility loci using the icogs custom genotyping array. Nat. Genet. 2013, 45, 385–391. [Google Scholar] [CrossRef]
- Al Olama, A.A.; Kote-Jarai, Z.; Berndt, S.I.; Conti, D.V.; Schumacher, F.; Han, Y.; Benlloch, S.; Hazelett, D.J.; Wang, Z.; Saunders, E.; et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat. Genet. 2014, 46, 1103–1109. [Google Scholar] [CrossRef]
- O’Hurley, G.; Busch, C.; Fagerberg, L.; Hallstrom, B.M.; Stadler, C.; Tolf, A.; Lundberg, E.; Schwenk, J.M.; Jirstrom, K.; Bjartell, A.; et al. Analysis of the human prostate-specific proteome defined by transcriptomics and antibody-based profiling identifies tmem79 and acoxl as two putative, diagnostic markers in prostate cancer. PLoS ONE 2015, 10, e0133449. [Google Scholar]
- Spisak, S.; Lawrenson, K.; Fu, Y.; Csabai, I.; Cottman, R.T.; Seo, J.H.; Haiman, C.; Han, Y.; Lenci, R.; Li, Q.; et al. Causel: An epigenome- and genome-editing pipeline for establishing function of noncoding gwas variants. Nat. Med. 2015, 21, 1357–1363. [Google Scholar] [CrossRef]
- Huang, Q.; Whitington, T.; Gao, P.; Lindberg, J.F.; Yang, Y.; Sun, J.; Vaisanen, M.R.; Szulkin, R.; Annala, M.; Yan, J.; et al. A prostate cancer susceptibility allele at 6q22 increases rfx6 expression by modulating hoxb13 chromatin binding. Nat. Genet. 2014, 46, 126–135. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, L.; Cai, M.; Li, H.; Xu, H.; Yang, H.; Zhao, Z.; Rhie, S.K.; Farnham, P.J.; Shi, J.; et al. The prostate cancer risk variant rs55958994 regulates multiple gene expression through extreme long-range chromatin interaction to control tumor progression. Sci. Adv. 2019, 5, eaaw6710. [Google Scholar] [CrossRef]
- Hua, J.T.; Ahmed, M.; Guo, H.; Zhang, Y.; Chen, S.; Soares, F.; Lu, J.; Zhou, S.; Wang, M.; Li, H.; et al. Risk snp-mediated promoter-enhancer switching drives prostate cancer through lncrna pcat19. Cell 2018, 174, 564–575. [Google Scholar] [CrossRef]
- Gao, P.; Xia, J.H.; Sipeky, C.; Dong, X.M.; Zhang, Q.; Yang, Y.; Zhang, P.; Cruz, S.P.; Zhang, K.; Zhu, J.; et al. Biology and clinical implications of the 19q13 aggressive prostate cancer susceptibility locus. Cell 2018, 174, 576–589. [Google Scholar] [CrossRef]
- Luo, Z.; Rhie, S.K.; Lay, F.D.; Farnham, P.J. A prostate cancer risk element functions as a repressive loop that regulates hoxa13. Cell Rep. 2017, 21, 1411–1417. [Google Scholar] [CrossRef]
- Lou, H.; Yeager, M.; Li, H.; Bosquet, J.G.; Hayes, R.B.; Orr, N.; Yu, K.; Hutchinson, A.; Jacobs, K.B.; Kraft, P.; et al. Fine mapping and functional analysis of a common variant in msmb on chromosome 10q11.2 associated with prostate cancer susceptibility. Proc. Natl. Acad. Sci. USA 2009, 106, 7933–7938. [Google Scholar] [CrossRef]
- Chang, B.L.; Cramer, S.D.; Wiklund, F.; Isaacs, S.D.; Stevens, V.L.; Sun, J.; Smith, S.; Pruett, K.; Romero, L.M.; Wiley, K.E.; et al. Fine mapping association study and functional analysis implicate a snp in msmb at 10q11 as a causal variant for prostate cancer risk. Hum. Mol. Genet. 2009, 18, 1368–1375. [Google Scholar] [CrossRef]
- Zhang, X.; Cowper-Sal lari, R.; Bailey, S.D.; Moore, J.H.; Lupien, M. Integrative functional genomics identifies an enhancer looping to the sox9 gene disrupted by the 17q24.3 prostate cancer risk locus. Genom. Res. 2012, 22, 1437–1446. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Amin Al Olama, A.; Leongamornlert, D.; Tymrakiewicz, M.; Saunders, E.; Guy, M.; Giles, G.G.; Severi, G.; Southey, M.; Hopper, J.L.; et al. Identification of a novel prostate cancer susceptibility variant in the klk3 gene transcript. Hum. Genet. 2011, 129, 687–694. [Google Scholar] [CrossRef]
- Dadaev, T.; Saunders, E.J.; Newcombe, P.J.; Anokian, E.; Leongamornlert, D.A.; Brook, M.N.; Cieza-Borrella, C.; Mijuskovic, M.; Wakerell, S.; Olama, A.A.A.; et al. Fine-mapping of prostate cancer susceptibility loci in a large meta-analysis identifies candidate causal variants. Nat. Commun. 2018, 9, 2256. [Google Scholar] [CrossRef]
- Whitington, T.; Gao, P.; Song, W.; Ross-Adams, H.; Lamb, A.D.; Yang, Y.; Svezia, I.; Klevebring, D.; Mills, I.G.; Karlsson, R.; et al. Gene regulatory mechanisms underpinning prostate cancer susceptibility. Nat. Genet. 2016, 48, 387–397. [Google Scholar] [CrossRef]
- Han, Y.; Hazelett, D.J.; Wiklund, F.; Schumacher, F.R.; Stram, D.O.; Berndt, S.I.; Wang, Z.; Rand, K.A.; Hoover, R.N.; Machiela, M.J.; et al. Integration of multiethnic fine-mapping and genomic annotation to prioritize candidate functional snps at prostate cancer susceptibility regions. Hum. Mol. Genet. 2015, 24, 5603–5618. [Google Scholar] [CrossRef]
- Amin Al Olama, A.; Dadaev, T.; Hazelett, D.J.; Li, Q.; Leongamornlert, D.; Saunders, E.J.; Stephens, S.; Cieza-Borrella, C.; Whitmore, I.; Benlloch Garcia, S.; et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among europeans. Hum. Mol. Genet. 2015, 24, 5589–5602. [Google Scholar] [CrossRef]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.; Jansen, R.; de Geus, E.J.; Boomsma, D.I.; Wright, F.A.; et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Cai, Q.; Cavazos, T.B.; Emami, N.C.; Long, J.; Shu, X.O.; Lu, Y.; Guo, X.; Bauer, J.A.; et al. Identification of novel susceptibility loci and genes for prostate cancer risk: A transcriptome-wide association study in over 140,000 european descendants. Cancer Res. 2019, 79, 3192–3204. [Google Scholar] [CrossRef]
- Mancuso, N.; Gayther, S.; Gusev, A.; Zheng, W.; Penney, K.L.; Kote-Jarai, Z.; Eeles, R.; Freedman, M.; Haiman, C.; Pasaniuc, B.; et al. Large-scale transcriptome-wide association study identifies new prostate cancer risk regions. Nat. Commun. 2018, 9, 4079. [Google Scholar] [CrossRef]
- Emami, N.C.; Kachuri, L.; Meyers, T.J.; Das, R.; Hoffman, J.D.; Hoffmann, T.J.; Hu, D.; Shan, J.; Feng, F.Y.; Ziv, E.; et al. Association of imputed prostate cancer transcriptome with disease risk reveals novel mechanisms. Nat. Commun. 2019, 10, 3107. [Google Scholar] [CrossRef]
- Du, Z.; Lubmawa, A.; Gundell, S.; Wan, P.; Nalukenge, C.; Muwanga, P.; Lutalo, M.; Nansereko, D.; Ndaruhutse, O.; Katuku, M.; et al. Genetic risk of prostate cancer in ugandan men. Prostate 2018, 78, 370–376. [Google Scholar] [CrossRef]
- Conti, D.V.; Wang, K.; Sheng, X.; Bensen, J.T.; Hazelett, D.J.; Cook, M.B.; Ingles, S.A.; Kittles, R.A.; Strom, S.S.; Rybicki, B.A.; et al. Two novel susceptibility loci for prostate cancer in men of african ancestry. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Cook, M.B.; Wang, Z.; Yeboah, E.D.; Tettey, Y.; Biritwum, R.B.; Adjei, A.A.; Tay, E.; Truelove, A.; Niwa, S.; Chung, C.C.; et al. A genome-wide association study of prostate cancer in west african men. Hum. Genet. 2014, 133, 509–521. [Google Scholar] [CrossRef]
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Genome-wide association study of prostate cancer in men of african ancestry identifies a susceptibility locus at 17q21. Nat. Genet. 2011, 43, 570–573. [Google Scholar] [CrossRef]
- Akamatsu, S.; Takata, R.; Haiman, C.A.; Takahashi, A.; Inoue, T.; Kubo, M.; Furihata, M.; Kamatani, N.; Inazawa, J.; Chen, G.K.; et al. Common variants at 11q12, 10q26 and 3p11.2 are associated with prostate cancer susceptibility in japanese. Nat. Genet. 2012, 44, 426–429. [Google Scholar] [CrossRef]
- Takata, R.; Akamatsu, S.; Kubo, M.; Takahashi, A.; Hosono, N.; Kawaguchi, T.; Tsunoda, T.; Inazawa, J.; Kamatani, N.; Ogawa, O.; et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the japanese population. Nat. Genet. 2010, 42, 751–754. [Google Scholar] [CrossRef]
- Wang, M.; Takahashi, A.; Liu, F.; Ye, D.; Ding, Q.; Qin, C.; Yin, C.; Zhang, Z.; Matsuda, K.; Kubo, M.; et al. Large-scale association analysis in asians identifies new susceptibility loci for prostate cancer. Nat. Commun. 2015, 6, 8469. [Google Scholar] [CrossRef]
- Xu, J.; Mo, Z.; Ye, D.; Wang, M.; Liu, F.; Jin, G.; Xu, C.; Wang, X.; Shao, Q.; Chen, Z.; et al. Genome-wide association study in chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat. Genet. 2012, 44, 1231–1235. [Google Scholar] [CrossRef]
- Du, Z.; Hopp, H.; Ingles, S.A.; Huff, C.; Sheng, X.; Weaver, B.; Stern, M.; Hoffmann, T.J.; John, E.M.; Van Den Eeden, S.K.; et al. A genome-wide association study of prostate cancer in latinos. Int. J. Cancer 2020, 146, 1819–1826. [Google Scholar] [CrossRef]
- Hoffmann, T.J.; Van Den Eeden, S.K.; Sakoda, L.C.; Jorgenson, E.; Habel, L.A.; Graff, R.E.; Passarelli, M.N.; Cario, C.L.; Emami, N.C.; Chao, C.R.; et al. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov. 2015, 5, 878–891. [Google Scholar] [CrossRef]
- Han, Y.; Signorello, L.B.; Strom, S.S.; Kittles, R.A.; Rybicki, B.A.; Stanford, J.L.; Goodman, P.J.; Berndt, S.I.; Carpten, J.; Casey, G.; et al. Generalizability of established prostate cancer risk variants in men of african ancestry. Int. J. Cancer 2015, 136, 1210–1217. [Google Scholar] [CrossRef]
- Liu, F.; Hsing, A.W.; Wang, X.; Shao, Q.; Qi, J.; Ye, Y.; Wang, Z.; Chen, H.; Gao, X.; Wang, G.; et al. Systematic confirmation study of reported prostate cancer risk-associated single nucleotide polymorphisms in chinese men. Cancer Sci. 2011, 102, 1916–1920. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Hurson, A.N.; Zhang, H.; Choudhury, P.P.; Easton, D.F.; Milne, R.L.; Simard, J.; Hall, P.; Michailidou, K.; Dennis, J.; et al. Assessment of polygenic architecture and risk prediction based on common variants across fourteen cancers. Nat. Commun. 2020, 11, 3353. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef]
- Pal, P.; Xi, H.; Guha, S.; Sun, G.; Helfand, B.T.; Meeks, J.J.; Suarez, B.K.; Catalona, W.J.; Deka, R. Common variants in 8q24 are associated with risk for prostate cancer and tumor aggressiveness in men of european ancestry. Prostate 2009, 69, 1548–1556. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.; Li, G.; Zhu, Y.; Zhang, S.; Kim, S.T.; Sun, J.; Wiklund, F.; Wiley, K.; Isaacs, S.D.; et al. Association of a germ-line copy number variation at 2p24.3 and risk for aggressive prostate cancer. Cancer Res. 2009, 69, 2176–2179. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, S.L.; Wiklund, F.; Isaacs, S.D.; Li, G.; Wiley, K.E.; Kim, S.T.; Zhu, Y.; Zhang, Z.; Hsu, F.C.; et al. Sequence variants at 22q13 are associated with prostate cancer risk. Cancer Res. 2009, 69, 10–15. [Google Scholar] [CrossRef]
- Amin Al Olama, A.; Kote-Jarai, Z.; Schumacher, F.R.; Wiklund, F.; Berndt, S.I.; Benlloch, S.; Giles, G.G.; Severi, G.; Neal, D.E.; Hamdy, F.C.; et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum. Mol. Genet. 2013, 22, 408–415. [Google Scholar] [CrossRef]
- Nam, R.K.; Zhang, W.; Siminovitch, K.; Shlien, A.; Kattan, M.W.; Klotz, L.H.; Trachtenberg, J.; Lu, Y.; Zhang, J.; Yu, C.; et al. New variants at 10q26 and 15q21 are associated with aggressive prostate cancer in a genome-wide association study from a prostate biopsy screening cohort. Cancer Biol. Ther. 2011, 12, 997–1004. [Google Scholar] [CrossRef]
- FitzGerald, L.M.; Kwon, E.M.; Conomos, M.P.; Kolb, S.; Holt, S.K.; Levine, D.; Feng, Z.; Ostrander, E.A.; Stanford, J.L. Genome-wide association study identifies a genetic variant associated with risk for more aggressive prostate cancer. Cancer Epidemiol. Biomed. Prev. 2011, 20, 1196–1203. [Google Scholar] [CrossRef]
- Ahn, J.; Kibel, A.S.; Park, J.Y.; Rebbeck, T.R.; Rennert, H.; Stanford, J.L.; Ostrander, E.A.; Chanock, S.; Wang, M.H.; Mittal, R.D.; et al. Prostate cancer predisposition loci and risk of metastatic disease and prostate cancer recurrence. Clin. Cancer Res. 2011, 17, 1075–1081. [Google Scholar] [CrossRef]
- Szulkin, R.; Karlsson, R.; Whitington, T.; Aly, M.; Gronberg, H.; Eeles, R.A.; Easton, D.F.; Kote-Jarai, Z.; Al Olama, A.A.; Benlloch, S.; et al. Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol. Biomed. Prev. 2015, 24, 1796–1800. [Google Scholar] [CrossRef]
- Penney, K.L.; Pyne, S.; Schumacher, F.R.; Sinnott, J.A.; Mucci, L.A.; Kraft, P.L.; Ma, J.; Oh, W.K.; Kurth, T.; Kantoff, P.W.; et al. Genome-wide association study of prostate cancer mortality. Cancer Epidemiol. Biomed. Prev. 2010, 19, 2869–2876. [Google Scholar] [CrossRef]
- Wiklund, F.E.; Adami, H.O.; Zheng, S.L.; Stattin, P.; Isaacs, W.B.; Gronberg, H.; Xu, J. Established prostate cancer susceptibility variants are not associated with disease outcome. Cancer Epidemiol. Biomed. Prev. 2009, 18, 1659–1662. [Google Scholar] [CrossRef]
- Berndt, S.I.; Wang, Z.; Yeager, M.; Alavanja, M.C.; Albanes, D.; Amundadottir, L.; Andriole, G.; Beane Freeman, L.; Campa, D.; Cancel-Tassin, G.; et al. Two susceptibility loci identified for prostate cancer aggressiveness. Nat. Commun. 2015, 6, 6889. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, S.L.; Isaacs, S.D.; Wiley, K.E.; Wiklund, F.; Sun, J.; Kader, A.K.; Li, G.; Purcell, L.D.; Kim, S.T.; et al. Inherited genetic variant predisposes to aggressive but not indolent prostate cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 2136–2140. [Google Scholar] [CrossRef]
- Li, W.; Middha, M.; Bicak, M.; Sjoberg, D.D.; Vertosick, E.; Dahlin, A.; Haggstrom, C.; Hallmans, G.; Ronn, A.C.; Stattin, P.; et al. Genome-wide scan identifies role for aox1 in prostate cancer survival. Eur. Urol. 2018, 74, 710–719. [Google Scholar] [CrossRef]
- Shui, I.M.; Wong, C.J.; Zhao, S.; Kolb, S.; Ebot, E.M.; Geybels, M.S.; Rubicz, R.; Wright, J.L.; Lin, D.W.; Klotzle, B.; et al. Prostate tumor DNA methylation is associated with cigarette smoking and adverse prostate cancer outcomes. Cancer 2016, 122, 2168–2177. [Google Scholar] [CrossRef]
- Haldrup, C.; Mundbjerg, K.; Vestergaard, E.M.; Lamy, P.; Wild, P.; Schulz, W.A.; Arsov, C.; Visakorpi, T.; Borre, M.; Hoyer, S.; et al. DNA methylation signatures for prediction of biochemical recurrence after radical prostatectomy of clinically localized prostate cancer. J. Clin. Oncol. 2013, 31, 3250–3258. [Google Scholar] [CrossRef]
- Li, H.; Fei, X.; Shen, Y.; Wu, Z. Association of gene polymorphisms of klk3 and prostate cancer: A meta-analysis. Adv. Clin. Exp. Med. 2020, 29, 1001–1009. [Google Scholar] [CrossRef]
- He, Y.; Gu, J.; Strom, S.; Logothetis, C.J.; Kim, J.; Wu, X. The prostate cancer susceptibility variant rs2735839 near klk3 gene is associated with aggressive prostate cancer and can stratify gleason score 7 patients. Clin. Cancer Res. 2014, 20, 5133–5139. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Werner, L.; Xie, W.; Regan, M.M.; Lee, G.S.; Sun, T.; Evan, C.; Petrozziello, G.; Nakabayashi, M.; Oh, W.K.; et al. Association of prostate cancer risk loci with disease aggressiveness and prostate cancer-specific mortality. Cancer Prev. Res. (Phila) 2011, 4, 719–728. [Google Scholar] [CrossRef]
- Kader, A.K.; Sun, J.; Isaacs, S.D.; Wiley, K.E.; Yan, G.; Kim, S.T.; Fedor, H.; DeMarzo, A.M.; Epstein, J.I.; Walsh, P.C.; et al. Individual and cumulative effect of prostate cancer risk-associated variants on clinicopathologic variables in 5895 prostate cancer patients. Prostate 2009, 69, 1195–1205. [Google Scholar] [CrossRef]
- Xu, J.; Isaacs, S.D.; Sun, J.; Li, G.; Wiley, K.E.; Zhu, Y.; Hsu, F.C.; Wiklund, F.; Turner, A.R.; Adams, T.S.; et al. Association of prostate cancer risk variants with clinicopathologic characteristics of the disease. Clin. Cancer Res. 2008, 14, 5819–5824. [Google Scholar] [CrossRef][Green Version]
- Helfand, B.T.; Roehl, K.A.; Cooper, P.R.; McGuire, B.B.; Fitzgerald, L.M.; Cancel-Tassin, G.; Cornu, J.N.; Bauer, S.; Van Blarigan, E.L.; Chen, X.; et al. Associations of prostate cancer risk variants with disease aggressiveness: Results of the nci-spore genetics working group analysis of 18,343 cases. Hum. Genet. 2015, 134, 439–450. [Google Scholar] [CrossRef]
- Amin Al Olama, A.; Benlloch, S.; Antoniou, A.C.; Giles, G.G.; Severi, G.; Neal, D.E.; Hamdy, F.C.; Donovan, J.L.; Muir, K.; Schleutker, J.; et al. Risk analysis of prostate cancer in practical, a multinational consortium, using 25 known prostate cancer susceptibility loci. Cancer Epidemiol. Biomed. Prev. 2015, 24, 1121–1129. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, F.; Lin, X.; Wang, X.; Ding, Q.; Jiang, H.; Chen, H.; Lu, D.; Jin, G.; Hsing, A.W.; et al. Predictive performance of prostate cancer risk in chinese men using 33 reported prostate cancer risk-associated snps. Prostate 2012, 72, 577–583. [Google Scholar] [CrossRef]
- Macinnis, R.J.; Antoniou, A.C.; Eeles, R.A.; Severi, G.; Al Olama, A.A.; McGuffog, L.; Kote-Jarai, Z.; Guy, M.; O’Brien, L.T.; Hall, A.L.; et al. A risk prediction algorithm based on family history and common genetic variants: Application to prostate cancer with potential clinical impact. Genet. Epidemiol. 2011, 35, 549–556. [Google Scholar] [CrossRef]
- Machiela, M.J.; Chen, C.Y.; Chen, C.; Chanock, S.J.; Hunter, D.J.; Kraft, P. Evaluation of polygenic risk scores for predicting breast and prostate cancer risk. Genet. Epidemiol. 2011, 35, 506–514. [Google Scholar] [CrossRef]
- Lecarpentier, J.; Silvestri, V.; Kuchenbaecker, K.B.; Barrowdale, D.; Dennis, J.; McGuffog, L.; Soucy, P.; Leslie, G.; Rizzolo, P.; Navazio, A.S.; et al. Prediction of breast and prostate cancer risks in male brca1 and brca2 mutation carriers using polygenic risk scores. J. Clin. Oncol. 2017, 35, 2240–2250. [Google Scholar] [CrossRef]
- Conran, C.A.; Na, R.; Chen, H.; Jiang, D.; Lin, X.; Zheng, S.L.; Brendler, C.B.; Xu, J. Population-standardized genetic risk score: The snp-based method of choice for inherited risk assessment of prostate cancer. Asian J. Androl. 2016, 18, 520–524. [Google Scholar]
- Szulkin, R.; Whitington, T.; Eklund, M.; Aly, M.; Eeles, R.A.; Easton, D.; Kote-Jarai, Z.S.; Amin Al Olama, A.; Benlloch, S.; Muir, K.; et al. Prediction of individual genetic risk to prostate cancer using a polygenic score. Prostate 2015, 75, 1467–1474. [Google Scholar] [CrossRef]
- Akamatsu, S.; Takahashi, A.; Takata, R.; Kubo, M.; Inoue, T.; Morizono, T.; Tsunoda, T.; Kamatani, N.; Haiman, C.A.; Wan, P.; et al. Reproducibility, performance, and clinical utility of a genetic risk prediction model for prostate cancer in japanese. PLoS ONE 2012, 7, e46454. [Google Scholar] [CrossRef]
- Sipeky, C.; Talala, K.M.; Tammela, T.L.J.; Taari, K.; Auvinen, A.; Schleutker, J. Prostate cancer risk prediction using a polygenic risk score. Sci. Rep. 2020, 10, 17075. [Google Scholar] [CrossRef]
- Pashayan, N.; Duffy, S.W.; Neal, D.E.; Hamdy, F.C.; Donovan, J.L.; Martin, R.M.; Harrington, P.; Benlloch, S.; Amin Al Olama, A.; Shah, M.; et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet. Med. 2015, 17, 789–795. [Google Scholar] [CrossRef]
- Callender, T.; Emberton, M.; Morris, S.; Eeles, R.; Kote-Jarai, Z.; Pharoah, P.D.P.; Pashayan, N. Polygenic risk-tailored screening for prostate cancer: A benefit-harm and cost-effectiveness modelling study. PLoS Med. 2019, 16, e1002998. [Google Scholar] [CrossRef]
- Tasa, T.; Puustusmaa, M.; Tonisson, N.; Kolk, B.; Padrik, P. Precision prostate cancer screening with a polygenic risk score. medRxiv 2008, 20180570. [Google Scholar]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genom. Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- Martin, A.R.; Kanai, M.; Kamatani, Y.; Okada, Y.; Neale, B.M.; Daly, M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019, 51, 584–591. [Google Scholar] [CrossRef]
- Huynh-Le, M.P.; Fan, C.C.; Karunamuni, R.; Walsh, E.I.; Turner, E.L.; Lane, J.A.; Martin, R.M.; Neal, D.E.; Donovan, J.L.; Hamdy, F.C.; et al. A genetic risk score to personalize prostate cancer screening, applied to population data. Cancer Epidemiol. Biomed. Prev. 2020, 29, 1731–1738. [Google Scholar] [CrossRef]
- Seibert, T.M.; Fan, C.C.; Wang, Y.; Zuber, V.; Karunamuni, R.; Parsons, J.K.; Eeles, R.A.; Easton, D.F.; Kote-Jarai, Z.; Al Olama, A.A.; et al. Polygenic hazard score to guide screening for aggressive prostate cancer: Development and validation in large scale cohorts. BMJ 2018, 360, j5757. [Google Scholar] [CrossRef]
- Huynh-Le, M.-P.; Fan, C.C.; Karunamuni, R.; Thompson, W.K.; Martinez, M.E.; Eeles, R.A.; Kote-Jarai, Z.; Muir, K.; Schleutker, J.; Pashayan, N.; et al. Polygenic hazard score is associated with prostate cancer in multi-ethnic populations. medRxiv 2020, 19012237. [Google Scholar]
- MacInnis, R.J.; Antoniou, A.C.; Eeles, R.A.; Severi, G.; Guy, M.; McGuffog, L.; Hall, A.L.; O’Brien, L.T.; Wilkinson, R.A.; Dearnaley, D.P.; et al. Prostate cancer segregation analyses using 4390 families from uk and australian population-based studies. Genet. Epidemiol. 2010, 34, 42–50. [Google Scholar] [CrossRef]
- Mancuso, N.; Rohland, N.; Rand, K.A.; Tandon, A.; Allen, A.; Quinque, D.; Mallick, S.; Li, H.; Stram, A.; Sheng, X.; et al. The contribution of rare variation to prostate cancer heritability. Nat. Genet. 2016, 48, 30–35. [Google Scholar] [CrossRef]
- Sham, P.C.; Purcell, S.M. Statistical power and significance testing in large-scale genetic studies. Nat. Rev. Genet. 2014, 15, 335–346. [Google Scholar] [CrossRef]
- Lee, S.; Abecasis, G.R.; Boehnke, M.; Lin, X. Rare-variant association analysis: Study designs and statistical tests. Am. J. Hum. Genet. 2014, 95, 5–23. [Google Scholar] [CrossRef]
- Li, D.; Lewinger, J.P.; Gauderman, W.J.; Murcray, C.E.; Conti, D. Using extreme phenotype sampling to identify the rare causal variants of quantitative traits in association studies. Genet. Epidemiol. 2011, 35, 790–799. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Schrader, K.A.; Cheng, D.T.; Joseph, V.; Prasad, M.; Walsh, M.; Zehir, A.; Ni, A.; Thomas, T.; Benayed, R.; Ashraf, A.; et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016, 2, 104–111. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Huang, K.L.; Mashl, R.J.; Wu, Y.; Ritter, D.I.; Wang, J.; Oh, C.; Paczkowska, M.; Reynolds, S.; Wyczalkowski, M.A.; Oak, N.; et al. Pathogenic germline variants in 10,389 adult cancers. Cell 2018, 173, 355–370. [Google Scholar] [CrossRef]
- Hart, S.N.; Ellingson, M.S.; Schahl, K.; Vedell, P.T.; Carlson, R.E.; Sinnwell, J.P.; Barman, P.; Sicotte, H.; Eckel-Passow, J.E.; Wang, L.; et al. Determining the frequency of pathogenic germline variants from exome sequencing in patients with castrate-resistant prostate cancer. BMJ Open 2016, 6, e010332. [Google Scholar] [CrossRef]
- Lu, C.; Xie, M.; Wendl, M.C.; Wang, J.; McLellan, M.D.; Leiserson, M.D.; Huang, K.L.; Wyczalkowski, M.A.; Jayasinghe, R.; Banerjee, T.; et al. Patterns and functional implications of rare germline variants across 12 cancer types. Nat. Commun. 2015, 6, 10086. [Google Scholar] [CrossRef]
- Nicolosi, P.; Ledet, E.; Yang, S.; Michalski, S.; Freschi, B.; O’Leary, E.; Esplin, E.D.; Nussbaum, R.L.; Sartor, O. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019, 5, 523–528. [Google Scholar] [CrossRef]
- Leongamornlert, D.; Saunders, E.; Dadaev, T.; Tymrakiewicz, M.; Goh, C.; Jugurnauth-Little, S.; Kozarewa, I.; Fenwick, K.; Assiotis, I.; Barrowdale, D.; et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br. J. Cancer 2014, 110, 1663–1672. [Google Scholar] [CrossRef]
- Paulo, P.; Maia, S.; Pinto, C.; Pinto, P.; Monteiro, A.; Peixoto, A.; Teixeira, M.R. Targeted next generation sequencing identifies functionally deleterious germline mutations in novel genes in early-onset/familial prostate cancer. PLoS Genet. 2018, 14, e1007355. [Google Scholar] [CrossRef]
- Pilie, P.G.; Johnson, A.M.; Hanson, K.L.; Dayno, M.E.; Kapron, A.L.; Stoffel, E.M.; Cooney, K.A. Germline genetic variants in men with prostate cancer and one or more additional cancers. Cancer 2017, 123, 3925–3932. [Google Scholar] [CrossRef]
- Mijuskovic, M.; Saunders, E.J.; Leongamornlert, D.A.; Wakerell, S.; Whitmore, I.; Dadaev, T.; Cieza-Borrella, C.; Govindasami, K.; Brook, M.N.; Haiman, C.A.; et al. Rare germline variants in DNA repair genes and the angiogenesis pathway predispose prostate cancer patients to develop metastatic disease. Br. J. Cancer 2018, 119, 96–104. [Google Scholar] [CrossRef]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.; Jiang, D.; Shah, S.; Ewing, C.M.; Zhang, L.; Novakovic, K.; Petkewicz, J.; et al. Germline mutations in atm and brca1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur. Urol. 2017, 71, 740–747. [Google Scholar] [CrossRef]
- Leongamornlert, D.A.; Saunders, E.J.; Wakerell, S.; Whitmore, I.; Dadaev, T.; Cieza-Borrella, C.; Benafif, S.; Brook, M.N.; Donovan, J.L.; Hamdy, F.C.; et al. Germline DNA repair gene mutations in young-onset prostate cancer cases in the uk: Evidence for a more extensive genetic panel. Eur. Urol. 2019, 76, 329–337. [Google Scholar] [CrossRef]
- Darst, B.F.; Dadaev, T.; Saunders, E.; Sheng, X.; Wan, P.; Pooler, L.; Xia, L.Y.; Chanock, S.; Berndt, S.I.; Gapstur, S.M.; et al. Germline sequencing DNA repair genes in 5,545 men with aggressive and non-aggressive prostate cancer. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Matejcic, M.; Patel, Y.; Lilyquist, J.; Hu, C.; Lee, K.Y.; Gnanaolivu, R.D.; Hart, S.N.; Polley, E.C.; Yadav, S.; Boddicker, N.J.; et al. Pathogenic variants in cancer predisposition genes and prostate cancer risk in men of african ancestry. JCO Precis. Oncol. 2020, 4, 32–43. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Dadaev, T.; Govindasami, K.; Guy, M.; Ellis, S.; Frost, D.; et al. Effect of brca mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur. Urol. 2015, 68, 186–193. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline brca mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef]
- Taylor, R.A.; Fraser, M.; Livingstone, J.; Espiritu, S.M.; Thorne, H.; Huang, V.; Lo, W.; Shiah, Y.J.; Yamaguchi, T.N.; Sliwinski, A.; et al. Germline brca2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat. Commun. 2017, 8, 13671. [Google Scholar] [CrossRef]
- Castro, E.; Jugurnauth-Little, S.; Karlsson, Q.; Al-Shahrour, F.; Pineiro-Yanez, E.; Van de Poll, F.; Leongamornlert, D.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. High burden of copy number alterations and c-myc amplification in prostate cancer from brca2 germline mutation carriers. Ann. Oncol. 2015, 26, 2293–2300. [Google Scholar] [CrossRef]
- Akbari, M.R.; Wallis, C.J.; Toi, A.; Trachtenberg, J.; Sun, P.; Narod, S.A.; Nam, R.K. The impact of a brca2 mutation on mortality from screen-detected prostate cancer. Br. J. Cancer 2014, 111, 1238–1240. [Google Scholar] [CrossRef]
- Thorne, H.; Willems, A.J.; Niedermayr, E.; Hoh, I.M.; Li, J.; Clouston, D.; Mitchell, G.; Fox, S.; Hopper, J.L.; Kathleen Cunningham Consortium for Research in Familial Breast Cancer Consortium; et al. Decreased prostate cancer-specific survival of men with brca2 mutations from multiple breast cancer families. Cancer Prev. Res. (Phila) 2011, 4, 1002–1010. [Google Scholar] [CrossRef]
- Lang, S.H.; Swift, S.L.; White, H.; Misso, K.; Kleijnen, J.; Quek, R.G.W. A systematic review of the prevalence of DNA damage response gene mutations in prostate cancer. Int. J. Oncol. 2019, 55, 597–616. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Li, S.; Wiley, K.; Zheng, S.L.; LaDuca, H.; Gielzak, M.; Na, R.; Sarver, B.A.J.; Helfand, B.T.; et al. Rare germline pathogenic mutations of DNA repair genes are most strongly associated with grade group 5 prostate cancer. Eur. Urol. Oncol. 2020, 3, 224–230. [Google Scholar] [CrossRef]
- Carter, H.B.; Helfand, B.; Mamawala, M.; Wu, Y.; Landis, P.; Yu, H.; Wiley, K.; Na, R.; Shi, Z.; Petkewicz, J.; et al. Germline mutations in atm and brca1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur. Urol. 2019, 75, 743–749. [Google Scholar] [CrossRef]
- Karlsson, Q.; Brook, M.N.; Dadaev, T.; Wakerell, S.; Saunders, E.J.; Muir, K.; Neal, D.E.; Giles, G.G.; MacInnis, R.J.; Thibodeau, S.N.; et al. Rare germline variants in atm predispose to prostate cancer: A practical consortium study. Eur. Urol. Oncol. 2021. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Oak, N.; Cherniack, A.D.; Mashl, R.J.; Network, T.A.; Hirsch, F.R.; Ding, L.; Beroukhim, R.; Gumus, Z.H.; Plon, S.E.; Huang, K.L. Ancestry-specific predisposing germline variants in cancer. Genom. Med. 2020, 12, 51. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Kanchi, K.L.; Gui, B.; Larson, D.E.; Fulton, R.S.; Isaacs, W.B.; Kraja, A.; Borecki, I.B.; Jia, L.; Wilson, R.K.; et al. Rare variation in tet2 is associated with clinically relevant prostate carcinoma in african americans. Cancer Epidemiol. Biom. Prev. 2016, 25, 1456–1463. [Google Scholar] [CrossRef]
- Nyberg, T.; Frost, D.; Barrowdale, D.; Evans, D.G.; Bancroft, E.; Adlard, J.; Ahmed, M.; Barwell, J.; Brady, A.F.; Brewer, C.; et al. Prostate cancer risks for male brca1 and brca2 mutation carriers: A prospective cohort study. Eur. Urol. 2020, 77, 24–35. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Ott, J.; Wang, J.; Leal, S.M. Genetic linkage analysis in the age of whole-genome sequencing. Nat. Rev. Genet. 2015, 16, 275–284. [Google Scholar] [CrossRef]
- Morgans, A.K.; Szymaniak, B.M. Genetically-informed treatment for advanced and metastatic prostate cancer. Can. J. Urol. 2019, 26, 54–56. [Google Scholar]
- Giri, V.N.; Knudsen, K.E.; Kelly, W.K.; Cheng, H.H.; Cooney, K.A.; Cookson, M.S.; Dahut, W.; Weissman, S.; Soule, H.R.; Petrylak, D.P.; et al. Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus conference 2019. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Prostate Cancer (Version 3.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 14 December 2020).
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; McGovern, U.B.; Elliott, T.; Jones, R.J.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.A.; Parikh, O.; et al. Toparp-b: A phase ii randomized trial of the poly(adp)-ribose polymerase (parp) inhibitor olaparib for metastatic castration resistant prostate cancers (mcrpc) with DNA damage repair (ddr) alterations. J. Clin. Oncol. 2019, 37, 5005. [Google Scholar] [CrossRef]
- Mota, J.M.; Barnett, E.; Nauseef, J.T.; Nguyen, B.; Stopsack, K.H.; Wibmer, A.; Flynn, J.R.; Heller, G.; Danila, D.C.; Rathkopf, D.; et al. Platinum-based chemotherapy in metastatic prostate cancer with DNA repair gene alterations. JCO Precis. Oncol. 2020, 4, 355–366. [Google Scholar] [CrossRef]
- Hager, S.; Ackermann, C.J.; Joerger, M.; Gillessen, S.; Omlin, A. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann. Oncol. 2016, 27, 975–984. [Google Scholar] [CrossRef]
- Graff, J.N.; Alumkal, J.J.; Thompson, R.F.; Moran, A.; Thomas, G.V.; Wood, M.A.; Drake, C.G.; Slottke, R.; Beer, T.M. Pembrolizumab (pembro) plus enzalutamide (enz) in metastatic castration resistant prostate cancer (mcrpc): Extended follow up. J. Clin. Oncol. 2018, 36, 5047. [Google Scholar] [CrossRef]
- Tucker, M.D.; Zhu, J.; Marin, D.; Gupta, R.T.; Gupta, S.; Berry, W.R.; Ramalingam, S.; Zhang, T.; Harrison, M.; Wu, Y.; et al. Pembrolizumab in men with heavily treated metastatic castrate-resistant prostate cancer. Cancer Med. 2019, 8, 4644–4655. [Google Scholar] [CrossRef]
- Tan, S.H.; Petrovics, G.; Srivastava, S. Prostate cancer genomics: Recent advances and the prevailing underrepresentation from racial and ethnic minorities. Int. J. Mol. Sci. 2018, 19, 1255. [Google Scholar] [CrossRef]
- Bentley, A.R.; Callier, S.; Rotimi, C.N. Diversity and inclusion in genomic research: Why the uneven progress? J. Commun. Genet. 2017, 8, 255–266. [Google Scholar] [CrossRef]
- Haga, S.B. Impact of limited population diversity of genome-wide association studies. Genet. Med. 2010, 12, 81–84. [Google Scholar] [CrossRef]
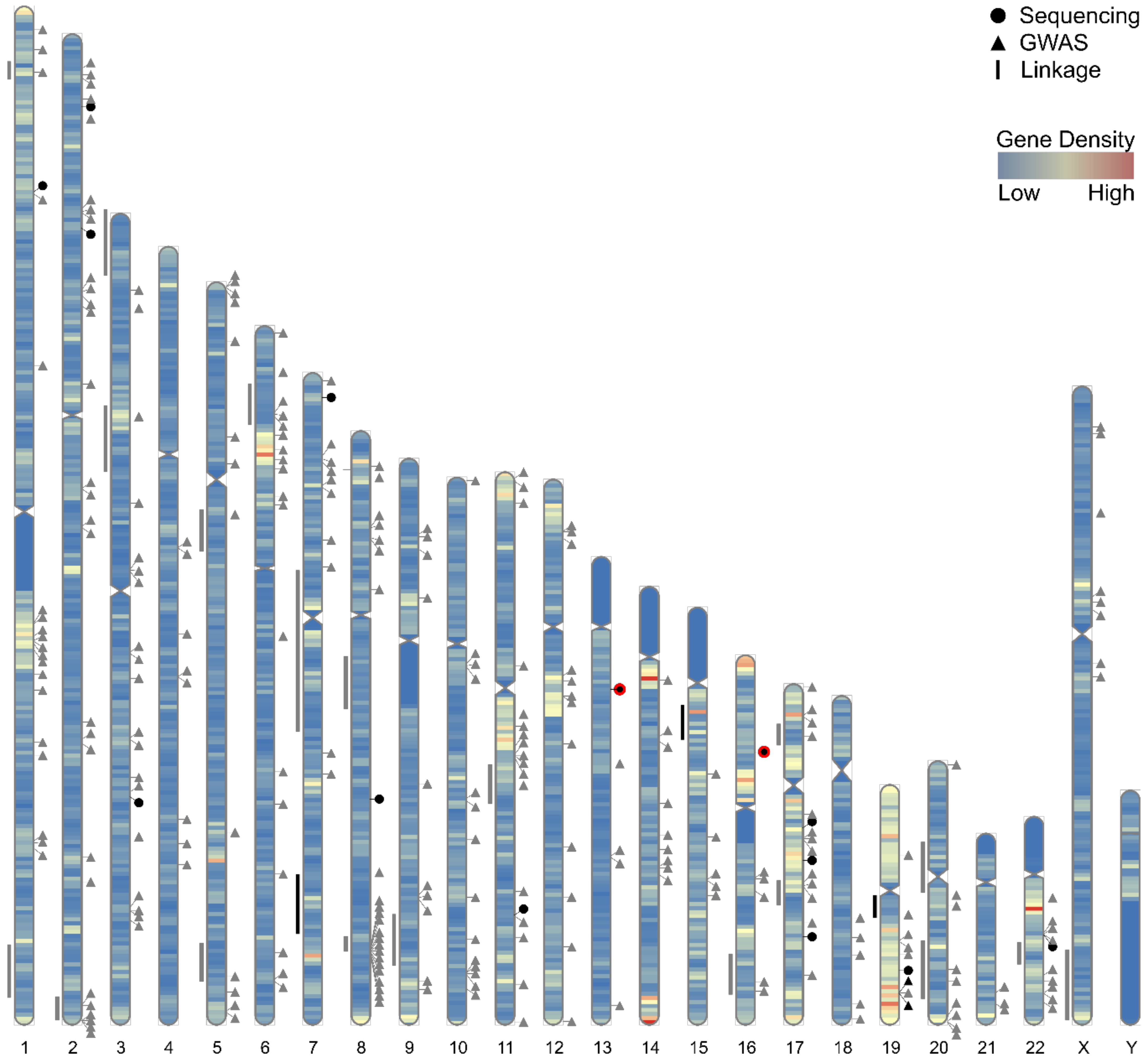

| Gene | Chromosome | Reporting Studies (Reference Number) |
|---|---|---|
| ATM | 11 | [144,271,275,276,277,278,281,282,283,284,285,295,296] |
| ATR | 3 | [271,275,281] |
| BRCA1 | 17 | [271,277,278,282,283] |
| BRCA2 | 13 | [144,271,275,277,278,281,282,283,284,285,296] |
| BRIP1 | 17 | [271,278] |
| CHEK2 | 22 | [271,277,278,283] |
| ERCC2 | 19 | [281,283] |
| GEN1 | 2 | [271,283] |
| MSH2 | 2 | [271,277,283] |
| MUTYH | 1 | [275,277,278] |
| NBN | 8 | [271,281,283,285] |
| PALB2 | 16 | [271,277,278,281,284,285] |
| PMS2 | 7 | [271,277,278,281] |
| RAD51D | 17 | [271,281] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saunders, E.J.; Kote-Jarai, Z.; Eeles, R.A. Identification of Germline Genetic Variants that Increase Prostate Cancer Risk and Influence Development of Aggressive Disease. Cancers 2021, 13, 760. https://doi.org/10.3390/cancers13040760
Saunders EJ, Kote-Jarai Z, Eeles RA. Identification of Germline Genetic Variants that Increase Prostate Cancer Risk and Influence Development of Aggressive Disease. Cancers. 2021; 13(4):760. https://doi.org/10.3390/cancers13040760
Chicago/Turabian StyleSaunders, Edward J., Zsofia Kote-Jarai, and Rosalind A. Eeles. 2021. "Identification of Germline Genetic Variants that Increase Prostate Cancer Risk and Influence Development of Aggressive Disease" Cancers 13, no. 4: 760. https://doi.org/10.3390/cancers13040760
APA StyleSaunders, E. J., Kote-Jarai, Z., & Eeles, R. A. (2021). Identification of Germline Genetic Variants that Increase Prostate Cancer Risk and Influence Development of Aggressive Disease. Cancers, 13(4), 760. https://doi.org/10.3390/cancers13040760






