Clinical CYP2D6 Genotyping to Personalize Adjuvant Tamoxifen Treatment in ER-Positive Breast Cancer Patients: Current Status of a Controversy
Abstract
:Simple Summary
Abstract
1. Introduction
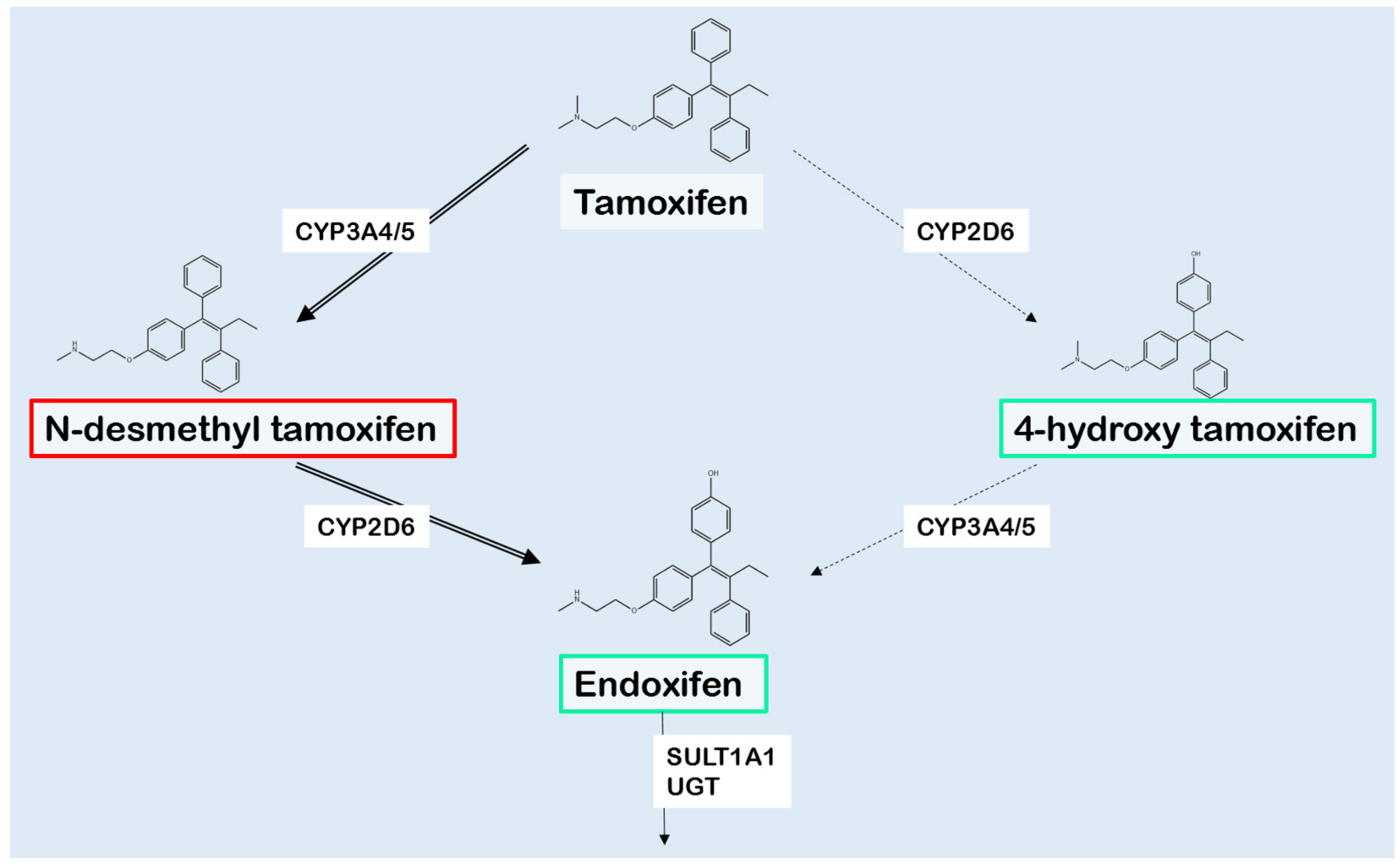
2. Tamoxifen Metabolism
3. Cytochrome P450 2D6
4. Influence of Other Drug Metabolizing Enzymes
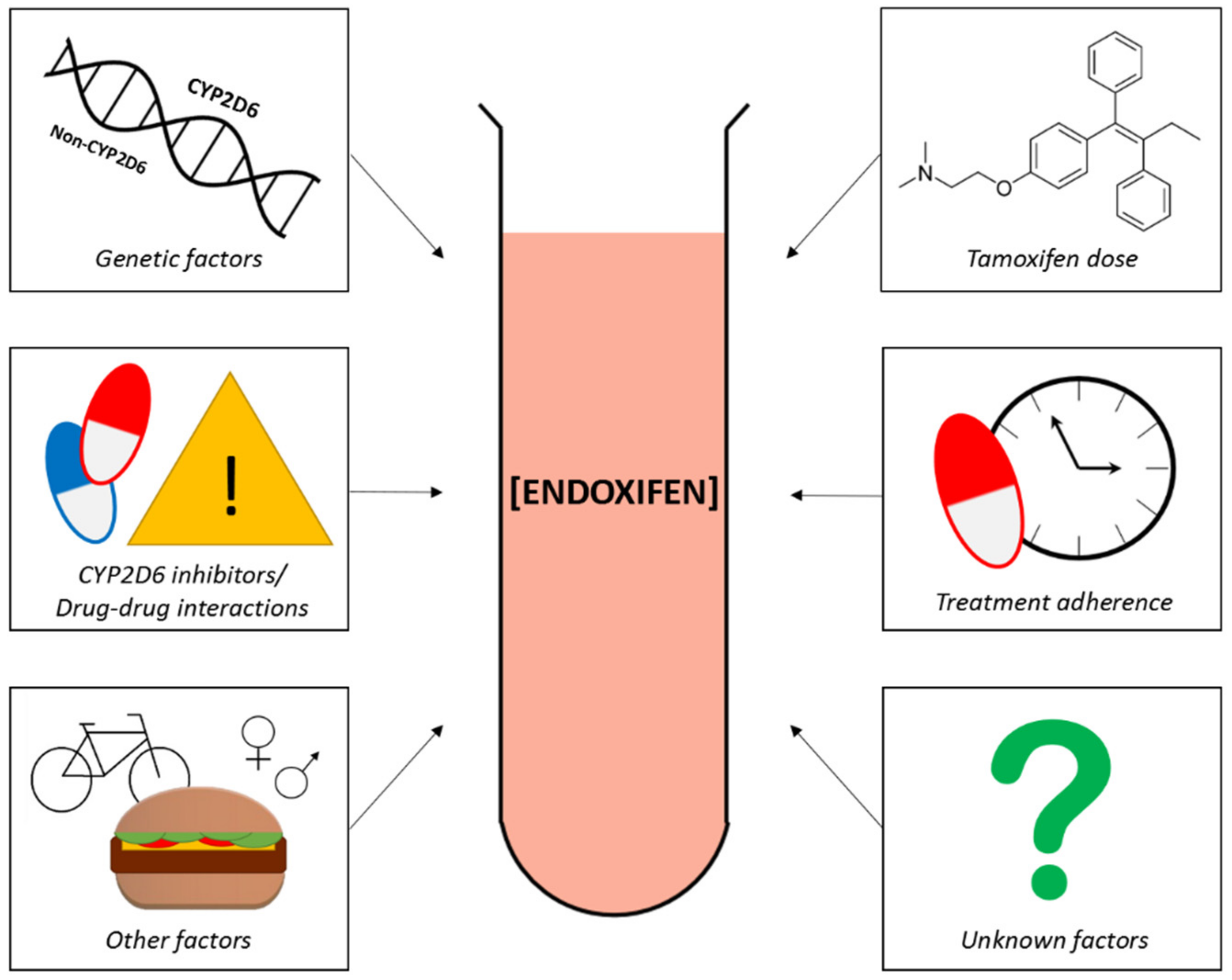
5. Factors Affecting Endoxifen Levels
5.1. Tamoxifen Dose Escalation and Endoxifen Levels
5.2. Patient Therapy Adherence and Endoxifen Levels
5.3. Drug–Drug Interactions
6. CYP2D6 Genotype and Outcome
6.1. Positive Association CYP2D6 Genotype and Outcome
6.2. No Association CYP2D6 Genotype and Outcome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, V.C. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr. Relat. Cancer 2014, 21, R235–R246. [Google Scholar] [CrossRef] [Green Version]
- Briest, S.; Stearns, V. Tamoxifen metabolism and its effect on endocrine treatment of breast cancer. Clin. Adv. Hematol. Oncol. 2009, 7, 185–192. [Google Scholar] [PubMed]
- Jordan, V.C. Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer. Br. J. Pharm. 1993, 110, 507–517. [Google Scholar] [CrossRef]
- Osborne, C.K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Tamoxifen therapy and CYP2D6 genotype. In Medical Genetics Summaries [Internet]; Pratt, V.M., Pirmohamed, M., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2014. [Google Scholar]
- Binkhorst, L.; Bannink, M.; de Bruijn, P.; Ruit, J.; Droogendijk, H.; van Alphen, R.J.; den Boer, T.D.; Lam, M.H.; Jager, A.; van Gelder, T.; et al. Augmentation of endoxifen exposure in tamoxifen-treated women following SSRI switch. Clin. Pharm. 2016, 55, 249–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binkhorst, L.; Kloth, J.S.L.; de Wit, A.S.; de Bruijn, P.; Lam, M.H.; Chaves, I.; Burger, H.; van Alphen, R.J.; Hamberg, P.; van Schaik, R.H.N.; et al. Circadian variation in tamoxifen pharmacokinetics in mice and breast cancer patients. Breast Cancer Res. Treat. 2015, 152, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Braal, C.L.; Hussaarts, K.; Seuren, L.; Oomen-de Hoop, E.; de Bruijn, P.; Buck, S.A.J.; Bos, M.; Thijs-Visser, M.F.; Zuetenhorst, H.J.M.; Mathijssen-van Stein, D.; et al. Influence of green tea consumption on endoxifen steady-state concentration in breast cancer patients treated with tamoxifen. Breast Cancer Res. Treat. 2020, 184, 107–113. [Google Scholar] [CrossRef]
- Hussaarts, K.; Hurkmans, D.P.; Oomen-de Hoop, E.; van Harten, L.J.; Berghuis, S.; van Alphen, R.J.; Spierings, L.E.A.; van Rossum-Schornagel, Q.C.; Vastbinder, M.B.; van Schaik, R.H.N.; et al. Impact of curcumin (with or without piperine) on the pharmacokinetics of tamoxifen. Cancers 2019, 11, 403. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Schoell, A.; Klopp-Schulze, L.; Schroth, W.; Mürdter, T.; Michelet, R.; Brauch, H.; Huisinga, W.; Joerger, M.; Neven, P.; Koolen, S.L.W.; et al. Obesity alters endoxifen plasma levels in young breast cancer patients: A pharmacometric simulation approach. Clin. Pharmacol. Ther. 2020, 108, 661–670. [Google Scholar] [CrossRef]
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; Van Driest, S.L. Pharmacogenomics. Lancet 2019, 394, 521–532. [Google Scholar] [CrossRef]
- FDA. Table of Pharmacogenetic Associations. Available online: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (accessed on 26 October 2020).
- Henricks, L.M.; Lunenburg, C.; de Man, F.M.; Meulendijks, D.; Frederix, G.W.J.; Kienhuis, E.; Creemers, G.J.; Baars, A.; Dezentjé, V.O.; Imholz, A.L.T.; et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: A prospective safety analysis. Lancet Oncol. 2018, 19, 1459–1467. [Google Scholar] [CrossRef]
- Jin, Y.; Desta, Z.; Stearns, V.; Ward, B.; Ho, H.; Lee, K.H.; Skaar, T.; Storniolo, A.M.; Li, L.; Araba, A.; et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. 2005, 97, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Goetz, M.P.; Rae, J.M.; Suman, V.J.; Safgren, S.L.; Ames, M.M.; Visscher, D.W.; Reynolds, C.; Couch, F.J.; Lingle, W.L.; Flockhart, D.A.; et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J. Clin. Oncol. 2005, 23, 9312–9318. [Google Scholar] [CrossRef] [PubMed]
- Rae, J.M.; Goetz, M.P.; Hayes, D.F.; Ingle, J.N.; Li, L.; Storniolo, A.M.; Stearns, V.; Flockhart, D.A. CYP2D6 genotype and tamoxifen response. Breast Cancer Res. 2005, 7, E6. [Google Scholar] [CrossRef] [Green Version]
- Goetz, M.P.; Sangkuhl, K.; Guchelaar, H.J.; Schwab, M.; Province, M.; Whirl-Carrillo, M.; Symmans, W.F.; McLeod, H.L.; Ratain, M.J.; Zembutsu, H.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin. Pharmacol. Ther. 2018, 103, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [Green Version]
- Regan, M.M.; Leyland-Jones, B.; Bouzyk, M.; Pagani, O.; Tang, W.; Kammler, R.; Dell’orto, P.; Biasi, M.O.; Thürlimann, B.; Lyng, M.B.; et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The breast international group 1-98 trial. J. Natl. Cancer Inst. 2012, 104, 441–451. [Google Scholar] [CrossRef]
- Rae, J.M.; Drury, S.; Hayes, D.F.; Stearns, V.; Thibert, J.N.; Haynes, B.P.; Salter, J.; Sestak, I.; Cuzick, J.; Dowsett, M.; et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J. Natl. Cancer Inst. 2012, 104, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Pritchard, K.I. CYP2D6 genotype as a marker for benefit of adjuvant tamoxifen in postmenopausal women: Lessons learned. J. Natl. Cancer Inst. 2012, 104, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Stanton, V., Jr. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial. J. Natl. Cancer Inst. 2012, 104, 1265–1266. [Google Scholar] [CrossRef] [Green Version]
- Pharoah, P.D.; Abraham, J.; Caldas, C. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial and Re: CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J. Natl. Cancer Inst. 2012, 104, 1263–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Ratain, M.J.; Cox, N.J.; McLeod, H.L.; Kroetz, D.L.; Flockhart, D.A. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial. J. Natl. Cancer Inst. 2012, 104, 1264. [Google Scholar] [CrossRef] [Green Version]
- Province, M.A.; Goetz, M.P.; Brauch, H.; Flockhart, D.A.; Hebert, J.M.; Whaley, R.; Suman, V.J.; Schroth, W.; Winter, S.; Zembutsu, H.; et al. CYP2D6 genotype and adjuvant tamoxifen: Meta-analysis of heterogeneous study populations. Clin. Pharmacol. Ther. 2014, 95, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.A.; Lim, H.S. Association between CYP2D6 genotypes and the clinical outcomes of adjuvant tamoxifen for breast cancer: A meta-analysis. Pharmacogenomics 2014, 15, 49–60. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Liu, Z.; You, J.; Chen, Z.; Wang, J.; Peng, Q.; Xie, L.; Li, R.; Li, S.; et al. CYP2D6 polymorphisms influence tamoxifen treatment outcomes in breast cancer patients: A meta-analysis. Cancer Chemother. Pharm. 2013, 72, 287–303. [Google Scholar] [CrossRef]
- Lum, D.W.; Perel, P.; Hingorani, A.D.; Holmes, M.V. CYP2D6 genotype and tamoxifen response for breast cancer: A systematic review and meta-analysis. PLoS ONE 2013, 8, e76648. [Google Scholar] [CrossRef]
- Cronin-Fenton, D.P.; Damkier, P.; Lash, T.L. Metabolism and transport of tamoxifen in relation to its effectiveness: New perspectives on an ongoing controversy. Future Oncol. 2014, 10, 107–122. [Google Scholar] [CrossRef] [Green Version]
- WHO. Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/graphic-isotype?type=0&type_sex=0&mode=population&sex=2&populations=900&cancers=20&age_group=value&apc_male=0&apc_female=0&single_unit=500000&print=0 (accessed on 19 September 2020).
- Saladores, P.H.; Precht, J.C.; Schroth, W.; Brauch, H.; Schwab, M. Impact of metabolizing enzymes on drug response of endocrine therapy in breast cancer. Expert Rev. Mol. Diagn. 2013, 13, 349–365. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 1443, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Bradford, L.D. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002, 3, 229–243. [Google Scholar] [CrossRef] [PubMed]
- McKusick, V.A.; Hamosh, A. Cytochrome P450, Subfamily IID, Polypeptide 6; CYP2D6. Available online: https://www.omim.org/entry/124030 (accessed on 29 September 2020).
- PharmVar. Cyp2d6. Available online: https://www.pharmvar.org/gene/cyp2d6 (accessed on 29 September 2020).
- Sachse, C.; Brockmöller, J.; Bauer, S.; Roots, I. Cytochrome P450 2D6 variants in a Caucasian population: Allele frequencies and phenotypic consequences. Am. J. Hum. Genet. 1997, 60, 284–295. [Google Scholar]
- Hicks, J.K.; Swen, J.J.; Gaedigk, A. Challenges in CYP2D6 phenotype assignment from genotype data: A critical assessment and call for standardization. Curr. Drug Metab. 2014, 15, 218–232. [Google Scholar] [CrossRef] [PubMed]
- CPIC. Final Consensus CYP2D6 Genotype to Phenotype Table-March. 2019. Available online: https://cpicpgx.org/wp-content/uploads/2019/03/Final-Consensus-CYP2D6-genotype-to-phenotype-table_-final_Mar2019.pdf (accessed on 29 September 2020).
- Caudle, K.E.; Sangkuhl, K.; Whirl-Carrillo, M.; Swen, J.J.; Haidar, C.E.; Klein, T.E.; Gammal, R.S.; Relling, M.V.; Scott, S.A.; Hertz, D.L.; et al. Standardizing CYP2D6 genotype to phenotype translation: Consensus recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2020, 13, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.I.; Low, S.K.; Maldonado, R.; Fox, P.; Balakrishnar, B.; Coulter, S.; de Bruijn, P.; Koolen, S.L.W.; Gao, B.; Lynch, J.; et al. Simplified phenotyping of CYP2D6 for tamoxifen treatment using the N-desmethyl-tamoxifen/ endoxifen ratio. Breast 2020, 54, 229–234. [Google Scholar] [CrossRef]
- Binkhorst, L.; Mathijssen, R.H.; Jager, A.; van Gelder, T. Individualization of tamoxifen therapy: Much more than just CYP2D6 genotyping. Cancer Treat. Rev. 2015, 41, 289–299. [Google Scholar] [CrossRef]
- Binkhorst, L.; van Gelder, T.; Mathijssen, R.H. Individualization of tamoxifen treatment for breast carcinoma. Clin. Pharmacol. 2012, 92, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Helland, T.; Henne, N.; Bifulco, E.; Naume, B.; Borgen, E.; Kristensen, V.N.; Kvaløy, J.T.; Lash, T.L.; Alnæs, G.I.G.; van Schaik, R.H.; et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017, 19, 125. [Google Scholar] [CrossRef]
- De Vries Schultink, A.H.M.; Huitema, A.D.R.; Beijnen, J.H. Therapeutic Drug Monitoring of endoxifen as an alternative for CYP2D6 genotyping in individualizing tamoxifen therapy. Breast 2018, 42, 38–40. [Google Scholar] [CrossRef]
- de Graan, A.J.; Teunissen, S.F.; de Vos, F.Y.; Loos, W.J.; van Schaik, R.H.; de Jongh, F.E.; de Vos, A.I.; van Alphen, R.J.; van der Holt, B.; Verweij, J.; et al. Dextromethorphan as a phenotyping test to predict endoxifen exposure in patients on tamoxifen treatment. J. Clin. Oncol. 2011, 29, 3240–3246. [Google Scholar] [CrossRef]
- Opdam, F.L.; Modak, A.S.; Gelderblom, H.; Guchelaar, H.J. Further characterization of a ¹3C-dextromethorphan breath test for CYP2D6 phenotyping in breast cancer patients on tamoxifen therapy. J. Breath Res. 2015, 9, 026003. [Google Scholar] [CrossRef]
- Gusella, M.; Pasini, F.; Corso, B.; Bertolaso, L.; De Rosa, G.; Falci, C.; Modena, Y.; Barile, C.; Da Corte, Z.D.; Fraccon, A.; et al. Predicting steady-state endoxifen plasma concentrations in breast cancer patients by CYP2D6 genotyping or phenotyping. Which approach is more reliable? Pharm. Res. Perspect. 2020, 8, e00646. [Google Scholar] [CrossRef] [PubMed]
- Johänning, J.; Kröner, P.; Thomas, M.; Zanger, U.M.; Nörenberg, A.; Eichelbaum, M.; Schwab, M.; Brauch, H.; Schroth, W.; Mürdter, T.E. The formation of estrogen-like tamoxifen metabolites and their influence on enzyme activity and gene expression of ADME genes. Arch. Toxicol. 2018, 92, 1099–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mürdter, T.E.; Schroth, W.; Bacchus-Gerybadze, L.; Winter, S.; Heinkele, G.; Simon, W.; Fasching, P.A.; Fehm, T.; German, T.; Group, A.I.C.; et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin. Pharmacol. 2011, 89, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Saladores, P.; Mürdter, T.; Eccles, D.; Chowbay, B.; Zgheib, N.K.; Winter, S.; Ganchev, B.; Eccles, B.; Gerty, S.; Tfayli, A.; et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharm. J. 2015, 15, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manish, M.; Lynn, A.M.; Mishra, S. Cytochrome P450 2C9 polymorphism: Effect of amino acid substitutions on protein flexibility in the presence of tamoxifen. Comput. Biol. Chem. 2020, 84, 107166. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Spitman, A.B.; Moes, D.; Gelderblom, H.; Dezentje, V.O.; Swen, J.J.; Guchelaar, H.J. Effect of CYP3A4*22, CYP3A5*3, and CYP3A combined genotypes on tamoxifen metabolism. Eur. J. Clin. Pharm. 2017, 73, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Puszkiel, A.; Arellano, C.; Vachoux, C.; Evrard, A.; Le Morvan, V.; Boyer, J.C.; Robert, J.; Delmas, C.; Dalenc, F.; Debled, M.; et al. Model-based quantification of impact of genetic polymorphisms and co-medications on pharmacokinetics of tamoxifen and six metabolites in breast cancer. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Lim, J.S.; Sutiman, N.; Muerdter, T.E.; Singh, O.; Cheung, Y.B.; Ng, R.C.; Yap, Y.S.; Wong, N.S.; Ang, P.C.; Dent, R.; et al. Association of CYP2C19*2 and associated haplotypes with lower norendoxifen concentrations in tamoxifen-treated Asian breast cancer patients. Br. J. Clin. Pharm. 2016, 81, 1142–1152. [Google Scholar] [CrossRef] [Green Version]
- Ruiter, R.; Bijl, M.J.; van Schaik, R.H.; Berns, E.M.; Hofman, A.; Coebergh, J.W.; van Noord, C.; Visser, L.E.; Stricker, B.H. CYP2C19*2 polymorphism is associated with increased survival in breast cancer patients using tamoxifen. Pharmacogenomics 2010, 11, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Van Schaik, R.H.; Kok, M.; Sweep, F.C.; van Vliet, M.; van Fessem, M.; Meijer-van Gelder, M.E.; Seynaeve, C.; Lindemans, J.; Wesseling, J.; Van ‘t Veer, L.J.; et al. The CYP2C19*2 genotype predicts tamoxifen treatment outcome in advanced breast cancer patients. Pharmacogenomics 2011, 12, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Beelen, K.; Opdam, M.; Severson, T.M.; Koornstra, R.H.; Vincent, A.D.; Hauptmann, M.; van Schaik, R.H.; Berns, E.M.; Vermorken, J.B.; van Diest, P.J.; et al. CYP2C19 2 predicts substantial tamoxifen benefit in postmenopausal breast cancer patients randomized between adjuvant tamoxifen and no systemic treatment. Breast Cancer Res. Treat. 2013, 139, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.L.; Buys, S.S.; Fletcher, D.; Melis, R.; Johnson-Davis, K.L.; Lyon, E.; Malmberg, E.M.; McMillin, G.A. Multigene and drug interaction approach for tamoxifen metabolite patterns reveals possible involvement of CYP2C9, CYP2C19, and ABCB1. J. Clin. Pharm. 2016, 56, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Robinson, R.; Fohner, A.E.; Muzquiz, L.I.; Schilling, B.D.; Beans, J.A.; Olnes, M.J.; Trawicki, L.; Frydenlund, H.; Laukes, C.; et al. Cytochrome P450 genetic variation associated with tamoxifen biotransformation in american indian and alaska native people. Clin. Transl. Sci. 2018, 11, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damkier, P.; Kjærsgaard, A.; Barker, K.A.; Cronin-Fenton, D.; Crawford, A.; Hellberg, Y.; Janssen, E.A.M.; Langefeld, C.; Ahern, T.P.; Lash, T.L. CYP2C19*2 and CYP2C19*17 variants and effect of tamoxifen on breast cancer recurrence: Analysis of the International Tamoxifen Pharmacogenomics Consortium dataset. Sci. Rep. 2017, 7, 7727. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.S.; Chen, X.A.; Singh, O.; Yap, Y.S.; Ng, R.C.; Wong, N.S.; Wong, M.; Lee, E.J.; Chowbay, B. Impact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharmacokinetics in Asian breast cancer patients. Br. J. Clin. Pharm. 2011, 71, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.H.; Makonnen, E.; Fotoohi, A.; Aseffa, A.; Howe, R.; Aklillu, E. CYP2D6 genotype predicts plasma concentrations of tamoxifen metabolites in Ethiopian breast cancer patients. Cancers 2019, 11, 1353. [Google Scholar] [CrossRef] [Green Version]
- Cronin-Fenton, D.P.; Damkier, P. Tamoxifen and CYP2D6: A controversy in pharmacogenetics. Adv. Pharm. 2018, 83, 65–91. [Google Scholar]
- Lazarus, P.; Blevins-Primeau, A.S.; Zheng, Y.; Sun, D. Potential role of UGT pharmacogenetics in cancer treatment and prevention: Focus on tamoxifen. Ann. N. Y. Acad. Sci. 2009, 1155, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutiman, N.; Lim, J.S.L.; Muerdter, T.E.; Singh, O.; Cheung, Y.B.; Ng, R.C.H.; Yap, Y.S.; Wong, N.S.; Ang, P.C.S.; Dent, R.; et al. Pharmacogenetics of UGT1A4, UGT2B7 and UGT2B15 and their influence on tamoxifen disposition in Asian breast cancer patients. Clin. Pharm. 2016, 55, 1239–1250. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Dezentjé, V.O.; Swen, J.J.; Moes, D.; Gelderblom, H.; Guchelaar, H.J. Genetic polymorphisms of 3’-untranslated region of SULT1A1 and their impact on tamoxifen metabolism and efficacy. Breast Cancer Res. Treat. 2018, 172, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Madlensky, L.; Natarajan, L.; Tchu, S.; Pu, M.; Mortimer, J.; Flatt, S.W.; Nikoloff, D.M.; Hillman, G.; Fontecha, M.R.; Lawrence, H.J.; et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin. Pharmacol. 2011, 89, 718–725. [Google Scholar] [CrossRef]
- Dezentjé, V.O.; Opdam, F.L.; Gelderblom, H.; Hartigh den, J.; Van der Straaten, T.; Vree, R.; Maartense, E.; Smorenburg, C.H.; Putter, H.; Dieudonné, A.S.; et al. CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects. Breast Cancer Res. Treat. 2015, 153, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Spitman, A.B.; Moes, D.A.R.; Swen, J.J.; Dezentjé, V.O.; Lambrechts, D.; Neven, P.; Gelderblom, H.; Guchelaar, H.J. Exposure-response analysis of endoxifen serum concentrations in early-breast cancer. Cancer Chemother. Pharm. 2020, 85, 1141–1152. [Google Scholar] [CrossRef]
- Thorén, L.; Lindh, J.D.; Ackehed, G.; Kringen, M.K.; Hall, P.; Bergh, J.; Molden, E.; Margolin, S.; Eliasson, E. Impairment of endoxifen formation in tamoxifen-treated premenopausal breast cancer patients carrying reduced-function CYP2D6 alleles. Br. J. Clin. Pharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nardin, J.M.; Schroth, W.; Almeida, T.A.; Mürdter, T.; Picolotto, S.; Vendramini, E.C.L.; Hoppe, R.; Kogin, J.P.; Miqueleto, D.; de Moraes, S.D.R.; et al. The influences of adherence to tamoxifen and CYP2D6pharmacogenetics on plasma concentrations of the active metabolite (Z)-endoxifen in breast cancer. Clin. Transl. Sci. 2020, 13, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Khalaj, Z.; Baratieh, Z.; Nikpour, P.; Schwab, M.; Schaeffeler, E.; Mokarian, F.; Khanahmad, H.; Salehi, R.; Mürdter, T.E.; Salehi, M. Clinical Trial: CYP2D6 related dose escalation of tamoxifen in breast cancer patients with Iranian ethnic background resulted in increased concentrations of tamoxifen and its metabolites. Front. Pharm. 2019, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Imamura, C.K.; Takano, T.; Saji, S.; Yamanaka, T.; Yonemori, K.; Takahashi, M.; Tsurutani, J.; Nishimura, R.; Sato, K.; et al. CYP2D6 genotype-guided tamoxifen dosing in hormone receptor-positive metastatic breast cancer (TARGET-1): A randomized, open-label, phase II study. J. Clin. Oncol. 2020, 38, 558–566. [Google Scholar] [CrossRef]
- Irvin, W.J., Jr.; Walko, C.M.; Weck, K.E.; Ibrahim, J.G.; Chiu, W.K.; Dees, E.C.; Moore, S.G.; Olajide, O.A.; Graham, M.L.; Canale, S.T.; et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: A multicenter study. J. Clin. Oncol. 2011, 29, 3232–3239. [Google Scholar] [CrossRef] [Green Version]
- Welzen, M.E.; Dezentjé, V.O.; van Schaik, R.H.; Colbers, A.P.; Guchelaar, H.J.; van Erp, N.P.; den Hartigh, J.; Burger, D.M.; van Laarhoven, H.W. The effect of tamoxifen dose increment in patients with impaired CYP2D6 activity. Drug Monit. 2015, 37, 501–507. [Google Scholar] [CrossRef]
- Martinez de Dueñas, E.; Ochoa Aranda, E.; Blancas Lopez-Barajas, I.; Ferrer Magdalena, T.; Bandrés Moya, F.; Chicharro García, L.M.; Gómez Capilla, J.A.; Zafra Ceres, M.; de Haro, T.; Romero Llorens, R.; et al. Adjusting the dose of tamoxifen in patients with early breast cancer and CYP2D6 poor metabolizer phenotype. Breast 2014, 23, 400–406. [Google Scholar] [CrossRef]
- Braal, L.; Jager, A.; Lommen, K.M.; Oomen-de Hoop, E.; De Bruijn, P.; Vastbinder, M.B.; Van Rossum-Schornagel, Q.C.; Thijs-Visser, M.F.; Van Alphen, R.J.; Struik, E.M.; et al. 191P Therapeutic drug monitoring of tamoxifen to improve adjuvant treatment of hormone sensitive breast cancer: The TOTAM study. Ann. Oncol. 2020, 31, S303–S339. [Google Scholar] [CrossRef]
- He, W.; Grassmann, F.; Eriksson, M.; Eliasson, E.; Margolin, S.; Thorén, L.; Hall, P.; Czene, K. CYP2D6 genotype predicts tamoxifen discontinuation and prognosis in patients with breast cancer. J. Clin. Oncol. 2020, 38, 548–557. [Google Scholar] [CrossRef]
- Beijnen, J.H.; Schellens, J.H. Drug interactions in oncology. Lancet Oncol. 2004, 5, 489–496. [Google Scholar] [CrossRef]
- Blower, P.; de Wit, R.; Goodin, S.; Aapro, M. Drug-drug interactions in oncology: Why are they important and can they be minimized? Crit. Rev. Oncol. Hematol. 2005, 55, 117–142. [Google Scholar] [CrossRef]
- Arafah, A.; Yakout, K.; Rehman, M.U.; Mohammed Alsharif, A.; AlJawadi, M.H.; Al-Omar, H.A. Prevalence of the co-prescription of tamoxifen and CYP2D6 inhibitors in Saudi population: A cross sectional study. Saudi Pharm. J. 2020, 28, 440–444. [Google Scholar] [CrossRef]
- Borges, S.; Desta, Z.; Li, L.; Skaar, T.C.; Ward, B.A.; Nguyen, A.; Jin, Y.; Storniolo, A.M.; Nikoloff, D.M.; Wu, L.; et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin. Pharmacol. 2006, 80, 61–74. [Google Scholar] [CrossRef]
- Stearns, V.; Johnson, M.D.; Rae, J.M.; Morocho, A.; Novielli, A.; Bhargava, P.; Hayes, D.F.; Desta, Z.; Flockhart, D.A. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. 2003, 95, 1758–1764. [Google Scholar] [CrossRef]
- Monte, A.A.; West, K.; McDaniel, K.T.; Flaten, H.K.; Saben, J.; Shelton, S.; Abdelmawla, F.; Bushman, L.R.; Williamson, K.; Abbott, D.; et al. CYP2D6 genotype phenotype discordance due to drug-drug interaction. Clin. Pharmacol. 2018, 104, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; Citi, V.; Crucitta, S.; Rofi, E.; Belcari, F.; van Schaik, R.H.; Danesi, R. Pharmacogenetics of CYP2D6 and tamoxifen therapy: Light at the end of the tunnel? Pharm. Res. 2016, 107, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Dezentjé, V.O.; Guchelaar, H.J.; Nortier, J.W.; van de Velde, C.J.; Gelderblom, H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin. Cancer Res. 2009, 15, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, J.D.; Comen, E.A.; Reiner, A.S.; Orlow, I.; Leong, S.F.; Liang, X.; Mellemkjær, L.; Knight, J.A.; Lynch, C.F.; John, E.M.; et al. CYP2D6 phenotype, tamoxifen, and risk of contralateral breast cancer in the WECARE Study. Breast Cancer Res. 2018, 20, 149. [Google Scholar] [CrossRef] [Green Version]
- Malash, I.; Mansour, O.; Shaarawy, S.; Abdellateif, M.S.; Omar, A.; Gaafer, R.; Zekri, A.N.; Ahmed, O.S.; Bahnassy, A. The Role of CYP2D6 Polymorphisms in Determining Response to Tamoxifen in Metastatic Breast Cancer Patients: Review and Egyptian Experience. Asian Pac. J. Cancer Prev. 2020, 21, 3619–3625. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.; Dezentjé, V.; Swen, J.; Moes, D.; Böhringer, S.; Batman, E.; van Druten, E.; Smorenburg, C.; van Bochove, A.; Zeillemaker, A.; et al. Tamoxifen pharmacogenetics and metabolism: Results from the prospective CYPTAM study. J. Clin. Oncol. 2019, 37, 636–646. [Google Scholar] [CrossRef]
- Goetz, M.P.; Suman, V.J.; Nakamura, Y.; Kiyotani, K.; Jordan, V.C.; Ingle, J.N. Tamoxifen metabolism and breast cancer recurrence: A question unanswered by CYPTAM. J. Clin. Oncol. 2019, 37, 1982–1983. [Google Scholar] [CrossRef] [PubMed]
- Brauch, H.; Schroth, W.; Mürdter, T.; Schwab, M. Tamoxifen pharmacogenetics and metabolism: The same is not the same. J. Clin. Oncol. 2019, 37, 1981–1982. [Google Scholar] [CrossRef]
- Braal, C.L.; Beijnen, J.H.; Koolen, S.L.W.; Oomen-de Hoop, E.; Steeghs, N.; Jager, A.; Huitema, A.D.R.; Mathijssen, R.H.J. Relevance of Endoxifen Concentrations: Absence of Evidence Is Not Evidence of Absence. J. Clin. Oncol. 2019, 37, 1980–1981. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Méndez, J.A.; Rubi-Castellanos, R.; Ascencio-Montiel, I.J.; Moo-Puc, R.E. CYP2D6 does not impact on breast cancer-free survival in Southeast Mexican patients under tamoxifen treatment. Pers. Med. 2020, 17, 261–270. [Google Scholar]
- Hertz, D.L.; Kidwell, K.M.; Hilsenbeck, S.G.; Oesterreich, S.; Osborne, C.K.; Philips, S.; Chenault, C.; Hartmaier, R.J.; Skaar, T.C.; Sikora, M.J.; et al. CYP2D6 genotype is not associated with survival in breast cancer patients treated with tamoxifen: Results from a population-based study. Breast Cancer Res. Treat. 2017, 166, 277–287. [Google Scholar] [CrossRef] [PubMed]
| Likely Phenotype | CURRENT CPIC Activity Score Definition | CURRENT DPWG Activity Score Definition | NEW Standardized Activity Score Definition |
|---|---|---|---|
| CYP2D6 UM | >2 | >2.5 | >2.25 |
| CYP2D6 NM | 1–2 | 1.5–2.5 | 1.25–2.25 |
| CYP2D6 IM | 0.5 | 0.5–1.0 | 0.25–1.0 |
| CYP2D6 PM | 0 | 0 | 0 |
| Reference | End-Point | N. PTS. | Material and Methods | Results |
|---|---|---|---|---|
| Thorén et al., 2020 [70] Nardin et al., 2020 [71] | Endoxifen plasma concentrations in PM, IM, NM, and UM patients | 118, 192 | CYP2D6 genotyping; LC-MS/MS | CYP2D6 metabolizer status is a strong determinant of plasma endoxifen concentrations. Increasing CYP2D6 allele activity correlates with increasing endoxifen levels. |
| Khalaj et al., 2019 [72] Tamura et al., [73] | Endoxifen plasma concentrations in PM, IM, NM, and UM patients | 134, 186 | CYP2D6 genotyping; LC-MS/MS | Dose escalation in CYP2D6-compromised patients (PMs and IMs combined) resulted in an increase in endoxifen levels, similar as NMs (using standard dosage of 20 mg/day). No difference in the occurrence of the most common side effect (hot flushes) or severe side effects was found. |
| Nardin et al., 2020 [71] | Endoxifen plasma concentrations in PM, IM, NM and UM patients; Patient adherence behavior | 192 | CYP2D6 genotyping; LC-MS/MS; Morisky, Green, and Levine medication adherence scale | Adherence explained 47% of tamoxifen variability (p < 0.001). Combination of patients adherence and CYP2D6 genotype explained 40% of endoxifen variability at 12 months (p < 0.001). So, endoxifen levels are influenced both by patients’ tamoxifen treatment adherence and CYP2D6 genotype. |
| He et al., 2020 [78] | Tamoxifen discontinuation | 1309 | CYP2D6 genotyping; Self-reported questionnaires | UMs show a significantly higher discontinuation rate at 6 months after start of tamoxifen treatment (18.8%) compared to NMs (6.7). No significant difference in tamoxifen discontinuation was found for PMs (7.1%) or IMs (7.6%). After 6 months, no significant difference in discontinuation rates was found. |
| Monte et al., 2018 [84] | Dextro-methorphan (DM)/dextror-phan (DX) ratio in PM, IM, NM, and UM patients | 39 | CYP2D6 genotyping; Plasma DM and DX assay using LC-MS | Patients with co-ingestion of dextromethorphan (as CYP2D6 enzyme probe drug) and another CYP2D6-dependent drug were 9.5 times more likely to have genotype-phenotype discordance based upon the 3 h DX/DM ratio. |
| Reference | End-Point | N. PTS. | Material and Methods | Results |
|---|---|---|---|---|
| Brooks et al., 2018 [87] | Recurrence risk of contralateral breast cancer | 1514 cases, 2203 controls | CYP2D6 genotyping; population based case-control study. | No significant difference in recurrence risk between metabolizer groups (p = 0.09). However, a trend of decreasing CBC RR associated with tamoxifen treatment, with increasing AS was shown. |
| Malash et al., 2020 [88] | Frequency of CYP2D6 variant alleles in responder vs. refractory group | 157 | CYP2D6 genotyping; Patient assessment for adverse events and tumor response after 4, 8, 16 and 24 weeks | Wild-type CYP2D6 was present in 113 of the patients; 62.0% in the refractory group and 82.1% in responders. In 44 patients, CYP2D6 polymorphisms were detected: 30 of them were refractory (68.2%) and 14 were responders (31.8%). CYP2D6*3 and *4 were the most common CYP2D6 variants detected in the refractory group (86.7%). CYP2D6*10/*10 and *10/*3 were the most common variant diplotypes (85.7%) in the responders group. |
| He et al., 2020 [78] | Breast cancer-specific mortality | 1309 | CYP2D6 genotyping; | Patients genotyped as PM and UM show worse prognosis compared to NMs under standard tamoxifen dosage (20 mg/day) (HR: 2.59, 95% CI 1.01–6.67 and HR: 4.52, 95% CI 1.42–14.37 respectively). |
| Sanchez-Spitman et al., 2019 [89] | Relapse-free survival | 667 | CYP2D6 genotyping; prospective CYPTAM study using Cox regression analysis. | No significant difference in RFS between combined groups of PMs + IMs + hetEMS and NMs + UMs (p = 0.944). Of note: This study was seriously criticized for several reasons. |
| Rangel-Méndez et al., 2020 [93] | Breast cancer-free survival | 71 | CYP2D6 genotyping; retrospective study, using Kaplan–Meier method and log-rank test to estimate BCFS | No difference in BCFS and recurrence risk between combined group of NMs + UMs and PMs (p = 0.45) and IMs (p = 0.55). |
| Tamura et al., 2019 [73] | Progression-free survival rate at 6 months | 186 | CYP2D6 genotyping; A randomized, open-label, multicenter, phase II study. | No significant difference in PFS rate at 6 months between increased dosage arm and regular dosage arm (67,6% vs. 66,7%). Survival curves did not significantly differ (p = 0.15). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulder, T.A.M.; de With, M.; del Re, M.; Danesi, R.; Mathijssen, R.H.J.; van Schaik, R.H.N. Clinical CYP2D6 Genotyping to Personalize Adjuvant Tamoxifen Treatment in ER-Positive Breast Cancer Patients: Current Status of a Controversy. Cancers 2021, 13, 771. https://doi.org/10.3390/cancers13040771
Mulder TAM, de With M, del Re M, Danesi R, Mathijssen RHJ, van Schaik RHN. Clinical CYP2D6 Genotyping to Personalize Adjuvant Tamoxifen Treatment in ER-Positive Breast Cancer Patients: Current Status of a Controversy. Cancers. 2021; 13(4):771. https://doi.org/10.3390/cancers13040771
Chicago/Turabian StyleMulder, Tessa A. M., Mirjam de With, Marzia del Re, Romano Danesi, Ron H. J. Mathijssen, and Ron H. N. van Schaik. 2021. "Clinical CYP2D6 Genotyping to Personalize Adjuvant Tamoxifen Treatment in ER-Positive Breast Cancer Patients: Current Status of a Controversy" Cancers 13, no. 4: 771. https://doi.org/10.3390/cancers13040771
APA StyleMulder, T. A. M., de With, M., del Re, M., Danesi, R., Mathijssen, R. H. J., & van Schaik, R. H. N. (2021). Clinical CYP2D6 Genotyping to Personalize Adjuvant Tamoxifen Treatment in ER-Positive Breast Cancer Patients: Current Status of a Controversy. Cancers, 13(4), 771. https://doi.org/10.3390/cancers13040771






