Serum CYFRA 21.1 Level Predicts Disease Course in Thyroid Cancer with Distant Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Serum Cyfra 21.1 and Thyroglobulin (Tg) Measurements
2.3. Pathologic Review
2.4. 131Iodine Therapy Refractoriness
- Absence of radioiodine uptake by malignant/metastatic tissue outside the thyroid bed during the initial therapeutic whole-body scan;
- Loss of ability to concentrate radioiodine in the tumor tissue after previous evidence of radioiodine-avid disease (in the absence of stable iodine contamination);
- Concentration of radioiodine in some lesions but not in others;
- Progressive metastatic disease despite significant concentration of radioiodine [19].
2.5. BRAF Mutation
2.6. One-Year Follow-Up of Serum Cyfra 21.1 Levels
- Complete response defined by disappearance of all target lesions;
- Partial response defined by decrease in the number of metastatic nodules or 30% decrease in the sum of the longest diameter of target lesions;
- Progressive disease involving increased number of metastatic nodules or 20% increase in the sum of the longest diameter of target lesions;
- Stable disease with neither progression nor regression.
2.7. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics of Study Subjects and Their Serum Cyfra 21.1 Levels
3.2. Subgroup Analysis of Serum Cyfra 21.1 in Thyroid Cancer Patients with Distant Metastasis (DM-TC) Based on the Metastatic Site, Braf Mutation Status, Radioiodine Refractoriness, and Treatment with Tyrosine Kinase Inhibitor
3.3. Serial Follow-Up of Serum Cyfra 21.1 Level
3.4. Representative Patients with Serum Cyfra 21.1 as a Prognostic Biomarker for Disease Progression in Thyroid Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coulombe, P.A.; Wong, P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat. Cell Biol. 2004, 6, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Yasuchika, K.; Ishii, T.; Katayama, H.; Yoshitoshi, E.Y.; Ogiso, S.; Kita, S.; Yasuda, K.; Fukumitsu, K.; Mizumoto, M.; et al. Keratin 19, a Cancer Stem Cell Marker in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2015, 21, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.-H.; Oh, S.; Lee, K.-M.; Yang, W.; Nam, K.S.; Moon, H.-G.; Noh, D.-Y.; Kim, C.G.; Park, G.; Park, J.B.; et al. Cytokeratin19 induced by HER2/ERK binds and stabilizes HER2 on cell membranes. Cell Death Differ. 2015, 22, 665–676. [Google Scholar] [CrossRef]
- Asfaha, S.; Hayakawa, Y.; Muley, A.; Stokes, S.; Graham, T.A.; Ericksen, R.E.; Westphalen, C.B.; Von Burstin, J.; Mastracci, T.L.; Worthley, D.L.; et al. Krt19+/Lgr5− Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell 2015, 16, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Ezzat, S.; Freeman, J.L.; Rosen, I.B.; Asa, S.L. Immunohistochemical Diagnosis of Papillary Thyroid Carcinoma. Mod. Pathol. 2001, 14, 338–342. [Google Scholar] [CrossRef]
- Rosai, J. Immunohistochemical Markers of Thyroid Tumors Significance and Diagnostic Applications. Tumori J. 2003, 89, 517–519. [Google Scholar] [CrossRef]
- Brown, L.M.; Helmke, S.M.; Hunsucker, S.W.; Netea-Maier, R.T.; Chiang, S.A.; Heinz, D.E.; Shroyer, K.R.; Duncan, M.W.; Haugen, B.R. Quantitative and qualitative differences in protein expression between papillary thyroid carcinoma and normal thyroid tissue. Mol. Carcinog. 2006, 45, 613–626. [Google Scholar] [CrossRef]
- Rodríguez, C.A.; Cruz, J.J.; Martin, T.; Gómez, A.; Olaverri, A.; Hernandez, M. Serum CYFRA 21-1 is one of the most reliable tumor markers for breast carcinoma. Cancer 2002, 95, 670–671. [Google Scholar] [CrossRef]
- Sánchez-Carbayo, M.; Espasa, A.; Chinchilla, V.; Herrero, E.; Megías, J.; Mira, A.; Soria, F. New Electrochemiluminescent Immunoassay for the Determination of CYFRA 21-1: Analytical Evaluation and Clinical Diagnostic Performance in Urine Samples of Patients with Bladder Cancer. Clin. Chem. 1999, 45, 1944–1953. [Google Scholar] [CrossRef]
- Boeck, S.; Wittwer, C.; Heinemann, V.; Haas, M.; Kern, C.; Stieber, P.; Nagel, D.; Holdenrieder, S. Cytokeratin 19-fragments (CYFRA 21-1) as a novel serum biomarker for response and survival in patients with advanced pancreatic cancer. Br. J. Cancer 2013, 108, 1684–1694. [Google Scholar] [CrossRef]
- Pujol, J.L.; Grenier, J.; Daurès, J.P.; Daver, A.; Pujol, H.; Michel, F.B. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993, 53, 61–66. [Google Scholar]
- Li, Y.; Tang, Z.-Y.; Tian, B.; Ye, S.-L.; Qin, L.-X.; Xue, Q.; Sun, R.-X. Serum CYFRA 21-1 level reflects hepatocellular carcinoma metastasis: Study in nude mice model and clinical patients. J. Cancer Res. Clin. Oncol. 2006, 132, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Alarcon, J.L.; Carrillo-Vico, A.; Santotoribio, J.D.; Leon-Justel, A.; Sanchez-Gil, R.; Gonzalez-Castro, A.; Guerrero, J.M. CYFRA 21-1 as a tool for distant metastasis detection in lung cancer. Clin. Lab. 2011, 57, 1011–1014. [Google Scholar]
- Išić, T.; Savin, S.; Cvejić, D.; Marečko, I.; Tatić, S.; Havelka, M.; Paunović, I. Serum Cyfra 21.1 and galectin-3 protein levels in relation to immunohistochemical cytokeratin 19 and galectin-3 expression in patients with thyroid tumors. J. Cancer Res. Clin. Oncol. 2010, 136, 1805–1812. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, H.; Yuan, Z.; Zhu, R. Tumor Markers in Thyroid Carcinoma With Pulmonary Metastases After Thyroidectomy. Lab. Med. 2009, 40, 30–34. [Google Scholar] [CrossRef][Green Version]
- Giovanella, L.; Imperiali, M.; Trimboli, P. Role of serum cytokeratin 19 fragment (Cyfra 21.1) as a prognostic biomarker in patients with differentiated thyroid cancer. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Böttger, V.; Stasiak, P.C.; Harrison, D.L.; Mellerick, D.M.; Lane, E.B. Epitope Mapping of Monoclonal Antibodies to Keratin 19 Using Keratin Fragments, Synthetic Peptides and Phage Peptide Libraries. JBIC J. Biol. Inorg. Chem. 1995, 231, 475–485. [Google Scholar] [CrossRef]
- Sarwar, M.; Tomiyoshi, K.; Inoue, T.; Fukazawa, K.; Endo, K. CYFRA 21-1 as a tumor marker used in measuring the serum fragment of cytokeratin subunit 19 by immunoradiometric assay. Ann. Nucl. Med. 1994, 8, 301–306. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- den Dunnen, J.T.; Antonarakis, S.E. Mutation nomenclature extensions and suggestions to describe complex mutations: A discussion. Hum. Mutat. 2000, 15, 7–12. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/NG_007873.1 (accessed on 13 December 2020).
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/NM_004333.4 (accessed on 13 December 2020).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, H.L.; Jeong, C.-W.; Ha, J.; Jo, K.; Kim, M.-H.; Han, J.-S.; Lee, S.; Bae, J.; Jung, C.K.; et al. CYFRA 21-1 in Lymph Node Fine Needle Aspiration Washout Improves Diagnostic Accuracy for Metastatic Lymph Nodes of Differentiated Thyroid Cancer. Cancers 2019, 11, 487. [Google Scholar] [CrossRef]
- Sheard, M.A.; Vojtesek, B.; Šimíčková, M.; Valik, D. Release of cytokeratin-18 and -19 fragments (TPS and CYFRA 21-1) into the extracellular space during apoptosis. J. Cell. Biochem. 2002, 85, 670–677. [Google Scholar] [CrossRef]
- Giovanella, L.; Ghelfo, A.; Maffioli, M.; Ceriani, L. Circulating cytokeratin 19 fragments in patients with benign nodules and carcinomas of the thyroid gland. Int. J. Biol. Markers 2008, 23, 54–57. [Google Scholar] [CrossRef]
- Guerra, A.; Marotta, V.; Deandrea, M.; Motta, M.; Limone, P.P.; Caleo, A.; Zeppa, P.; Esposito, S.; Fulciniti, F.; Vitale, M. BRAF V600E associates with cytoplasmatic localization of p27kip1 and higher cytokeratin 19 expression in papillary thyroid carcinoma. Endocrine 2012, 44, 165–171. [Google Scholar] [CrossRef]
- Guerra, A.; Fugazzola, L.; Marotta, V.; Cirillo, M.; Rossi, S.; Cirello, V.; Forno, I.; Moccia, T.; Budillon, A.; Vitale, M. A High Percentage of BRAFV600E Alleles in Papillary Thyroid Carcinoma Predicts a Poorer Outcome. J. Clin. Endocrinol. Metab. 2012, 97, 2333–2340. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Peng, C.; Qin, Y.; Gao, T.; Jing, J.; Zhao, H. BRAFV600E-induced KRT19 expression in thyroid cancer promotes lymph node metastasis via EMT. Oncol. Lett. 2019, 18, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Dohmoto, K.; Hojo, S.; Fujita, J.; Yang, Y.; Ueda, Y.; Bandoh, S.; Yamaji, Y.; Ohtsuki, Y.; Dobashi, N.; Ishida, T.; et al. The role of caspase 3 in producing cytokeratin 19 fragment (CYFRA21-1) in human lung cancer cell lines. Int. J. Cancer 2001, 91, 468–473. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.-Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; Megeney, L.A. Rehabilitation of a contract killer: Caspase-3 directs stem cell differentiation. Cell Stem Cell 2008, 2, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Luo, K.-J.; Bella, A.E.; Bu, S.-S.; Wen, J.; Zhang, S.-S.; Hu, Y. Caspase-3 expression in metastatic lymph nodes of esophageal squamous cell carcinoma is prognostic of survival. World J. Gastroenterol. 2014, 20, 4414–4420. [Google Scholar] [CrossRef]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Carpi, A.; Berti, P.; Materazzi, G.; Minuto, M.N.; Guastalli, M.; Miccoli, P. Dedifferentiated thyroid cancer: A therapeutic challenge. Biomed. Pharmacother. 2008, 62, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef]
- Giovanella, L.; Treglia, G.; A Verburg, F.; Salvatori, M.; Ceriani, L. Serum cytokeratin 19 fragments: A dedifferentiation marker in advanced thyroid cancer. Eur. J. Endocrinol. 2012, 167, 793–797. [Google Scholar] [CrossRef]
- Campennì, A.; Giovanella, L.; Pignata, S.A.; Vento, A.; Alibrandi, A.; Sturiale, L.; Laudicella, R.; Comis, A.D.; Filice, R.; Giuffrida, G.; et al. Undetectable or low (<1 ng/mL) postsurgical thyroglobulin values do not rule out metastases in early stage differentiated thyroid cancer patients. Oncotarget 2018, 9, 17491–17500. [Google Scholar] [CrossRef]
- Broecker-Preuss, M.; Müller, S.; Britten, M.; Worm, K.; Schmid, K.W.; Mann, K.; Fuhrer, D. Sorafenib inhibits intracellular signaling pathways and induces cell cycle arrest and cell death in thyroid carcinoma cells irrespective of histological origin or BRAF mutational status. BMC Cancer 2015, 15, 184. [Google Scholar] [CrossRef] [PubMed]





| Clinical Variables | Metastasis n = 51 | No Metastasis n = 26 | Healthy Controls n = 50 | p-Value |
|---|---|---|---|---|
| Age (years) | 53.6 ± 12.8 | 55.6 ± 9.0 | 58.2 ± 13.4 | 0.27 |
| Gender (M/F) | 19/32 | 15/11 | 14/36 | 0.04 |
| Cyfra21.1 (ng/mL) | 1.86 ± 1.58 | 1.30 ± 0.85 | 1.15 ± 0.71 | 0.01 |
| Thyroglobulin (ng/mL) | 258.85 ± 635.5 | 12.69 ± 16.78 | NA | 0.02 |
| Anti-thyroglobulin Ab (IU/mL) | 48.49 ± 114.57 | 14.79 ± 15.84 | NA | 0.03 |
| Subtypes | ||||
| Papillary | 33 | 25 | - | - |
| Follicular | 7 | 1 | - | - |
| Papillary + Follicular | 3 | 0 | - | - |
| Poorly differentiated | 5 | 0 | - | - |
| Anaplastic | 3 | 0 | - | - |
| Distant metastasis site | ||||
| Lung only | 40 | - | - | - |
| Bone only | 6 | - | - | - |
| Both lung and bone | 5 | - | - | - |
| BRAF mutation | ||||
| Positive | 16 | - | - | - |
| Negative | 23 | - | - | - |
| Unknown | 12 | - | - | - |
| Response to radioiodine therapy | ||||
| Avid | 12 | - | - | - |
| Refractory | 13 | - | - | - |
| Unknown | 26 | - | - | - |
| No. | Sex | Age | Subtype | Initial Tg (TgAb) | 1 Year Follow-Up Tg (TgAb) | Initial Cyfra 21.1 | 1 Year Follow-Up Cyfra 21.1 | Metastasis | State | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 57 | PDTC | 0.4 (14.2) | 0.36 (16.1) | 1.87 | 5.24 | Lung | Progression | TKI (LE) |
| 2 | F | 60 | FTC | 741 | 1671.4 | 1.05 | 2.16 | Lung | Progression | Observation |
| 3 | F | 74 | PTC | 28.0 | 68.9 | 3.06 | 6.32 | Lung | Progression | Observation |
| 4 | F | 62 | PTC | 98.4 | 33.1 | 0.9 | 2.49 | Lung | Progression | TKI * (SO)/LN excision |
| 5 | M | 65 | PTC | 140.7 | 183.8 | 1.98 | 2.3 | Lung | Progression | Observation |
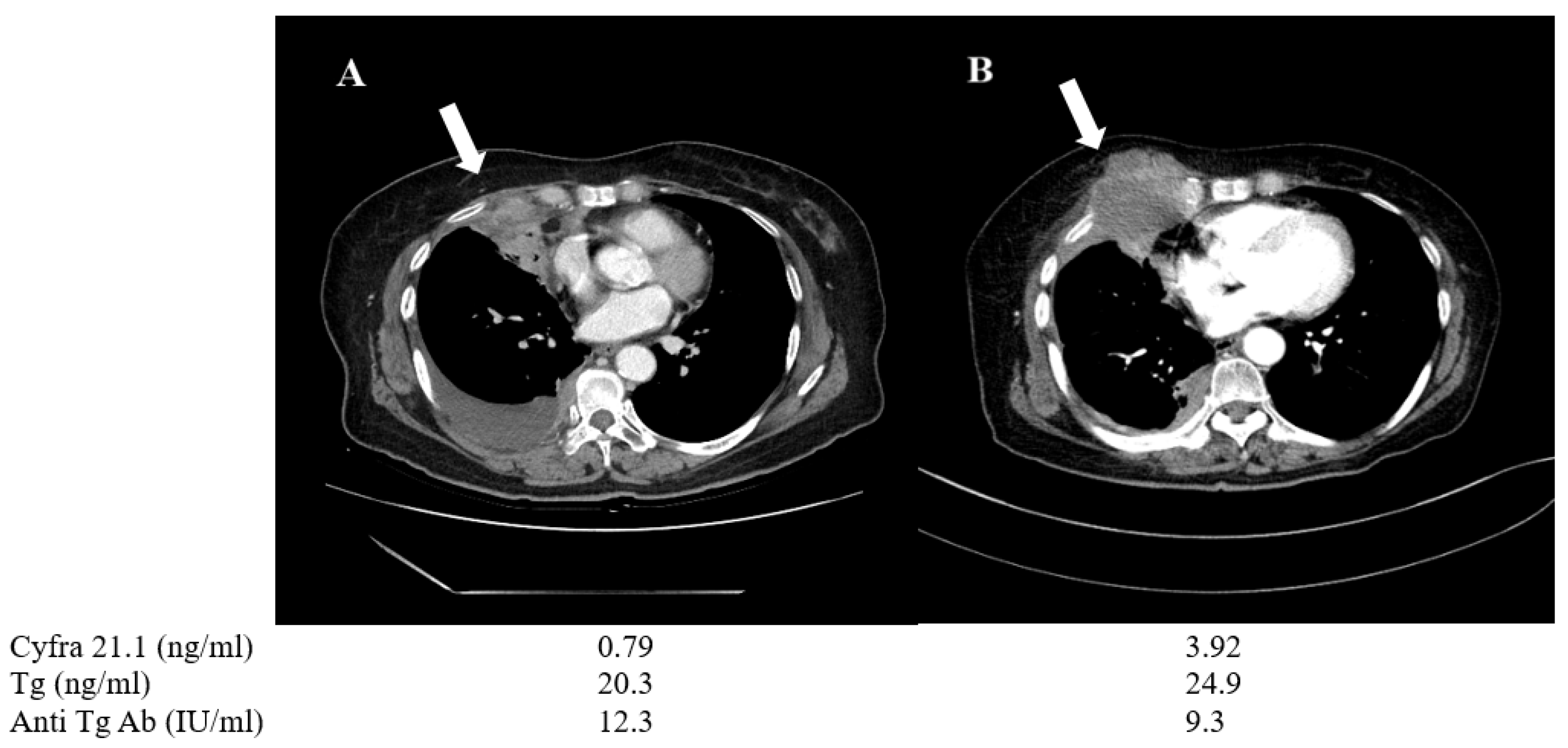
| 6 | F | 70 | PTC > ATC | 20.3 | 24.9 | 0.79 | 3.92 | Lung | Progression/ Change to anaplastic feature | TKI*(LE + SO) |
| 7 | F | 59 | PTC + FTC | 2519 | 964 | 2.07 | 0.87 | Lung + Bone | Stable | TKI * (LE) |
| 8 | F | 50 | PTC | 0.2 (400.6) | 0.2 (333.9) | 9.42 | 7.61 | Lung | Stable | TKI (SO) |
| 9 | F | 60 | PTC + FTC | 58.0 | 0.36 | 0.95 | 2.78 | Lung | Stable | TKI * (L E) |
| 10 | M | 62 | PTC | 11.3 | 17.6 | 1.87 | 0.78 | Lung | Stable | Observation |
| 11 | F | 57 | PTC | 4.1 | 3.2 | 0.6 | 0.81 | Lung | Stable | Observation/LN excision |
| 12 | F | 55 | PTC | 21.9 | 32.7 | 1.14 | 1.06 | Lung | Stable | Observation |
| 13 | M | 55 | PDTC | 58.8 | 2.6 | 0.5 | 0.66 | Bone | Stable | Resection of metastatic site/RT |
| 14 | M | 62 | PTC + FTC | 19.2 | 39.9 | 2.3 | 1.08 | Bone | Stable | RAI |
| 15 | F | 55 | PTC | 0.88 (495.2) | 0.36 (246.0) | 1.5 | 0.8 | Lung | Stable | Observation |
| 16 | M | 62 | PTC | 1.8 | 2.0 | 2.11 | 0.65 | Lung | Stable | Observation |
| 17 | F | 78 | PTC | 86.6 | 37.3 | 1.05 | 2.65 | Lung | Stable | TKI (LE) |
| 18 | F | 73 | PTC | 34.6 | 35.7 | 1.61 | 0.91 | Lung | Stable | Observation |
| 19 | F | 55 | PTC | 20.5 | 20.2 | 0.99 | 0.52 | Lung | Stable | Observation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.; Lee, J.; Yoon, H.; Ha, J.; Kim, M.-H.; Bae, J.-S.; Jung, C.-K.; Kim, J.-S.; Kang, M.-I.; Lim, D.-J. Serum CYFRA 21.1 Level Predicts Disease Course in Thyroid Cancer with Distant Metastasis. Cancers 2021, 13, 811. https://doi.org/10.3390/cancers13040811
Jeong C, Lee J, Yoon H, Ha J, Kim M-H, Bae J-S, Jung C-K, Kim J-S, Kang M-I, Lim D-J. Serum CYFRA 21.1 Level Predicts Disease Course in Thyroid Cancer with Distant Metastasis. Cancers. 2021; 13(4):811. https://doi.org/10.3390/cancers13040811
Chicago/Turabian StyleJeong, Chaiho, Jeongmin Lee, Hyukjin Yoon, Jeonghoon Ha, Min-Hee Kim, Ja-Seong Bae, Chan-Kwon Jung, Jeong-Soo Kim, Moo-Il Kang, and Dong-Jun Lim. 2021. "Serum CYFRA 21.1 Level Predicts Disease Course in Thyroid Cancer with Distant Metastasis" Cancers 13, no. 4: 811. https://doi.org/10.3390/cancers13040811
APA StyleJeong, C., Lee, J., Yoon, H., Ha, J., Kim, M.-H., Bae, J.-S., Jung, C.-K., Kim, J.-S., Kang, M.-I., & Lim, D.-J. (2021). Serum CYFRA 21.1 Level Predicts Disease Course in Thyroid Cancer with Distant Metastasis. Cancers, 13(4), 811. https://doi.org/10.3390/cancers13040811







