Circulating Cell-Free DNA Methylation Profiles in the Early Detection of Ovarian Cancer: A Scoping Review of the Literature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Study Characteristics
2.2. Methylation Modification and Analysis
2.3. Target Genes
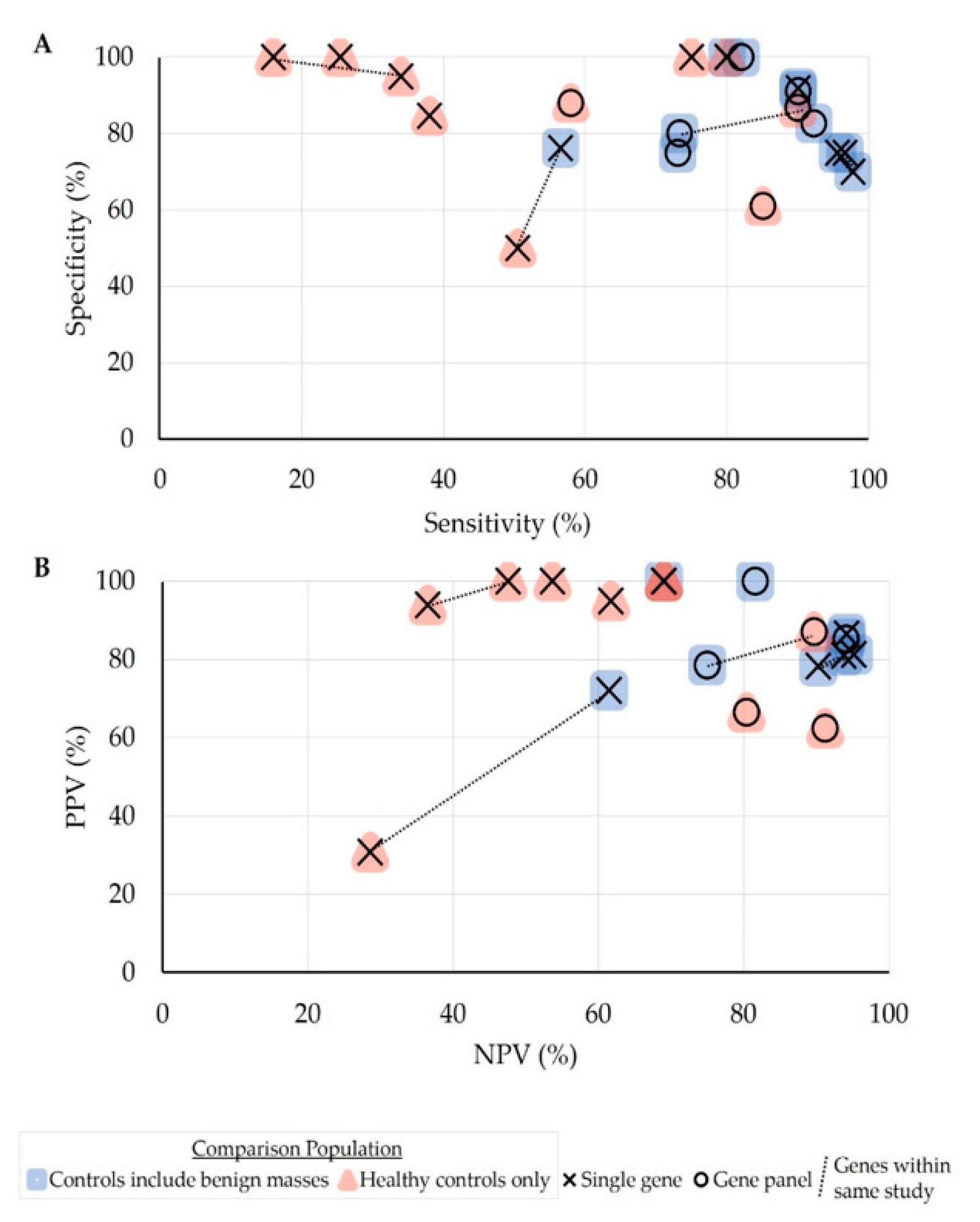
2.4. Diagnostic Performance
3. Discussion
4. Materials and Methods
4.1. Search Strateg
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures. 2020. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 7 July 2020).
- National Cancer Institution Surveillance and End Results Program. Cancer Stat Facts: Ovarian Cancer. SEER Cancer Stat. Rev. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 7 July 2020).
- Zhang, Y.; Luo, G.; Li, M.; Guo, P.; Xiao, Y.; Ji, H.; Hao, Y. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer 2019, 19, 984–1014. [Google Scholar] [CrossRef]
- Nebgen, D.R.; Lu, K.H.; Bast, R.C. Novel Approaches to Ovarian Cancer Screening. Curr. Oncol. Rep. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Ovarian Cancer (Version 1.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 31 August 2020).
- Scholler, N.; Urban, N. CA125 in ovarian cancer. Biomarkers Med. 2007, 1, 513–523. [Google Scholar] [CrossRef]
- Gupta, K.K.; Gupta, V.K.; Naumann, R.W. Ovarian cancer: Screening and future directions. Int. J. Gynecol. Cancer 2019, 29, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Muinao, T.; Boruah, H.P.D.; Pal, M. Multi-biomarker panel signature as the key to diagnosis of ovarian cancer. Heliyon 2019, 5, e02826. [Google Scholar] [CrossRef] [Green Version]
- Elias, K.M.; Guo, J.; Bast, R.C. Early Detection of Ovarian Cancer. Hematol. Clin. N. Am. 2018, 32, 903–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortner, R.T.; Damms-Machado, A.; Kaaks, R. Systematic review: Tumor-associated antigen autoantibodies and ovarian cancer early detection. Gynecol. Oncol. 2017, 147, 465–480. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L.; Chen, Y.; Qing, C. Circulating cell-free DNA and circulating tumor cells, the “liquid biopsies” in ovarian cancer. J. Ovarian Res. 2017, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arneth, B. Update on the types and usage of liquid biopsies in the clinical setting: A systematic review. BMC Cancer 2018, 18, 527. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Li, W.; Leng, B.; Zheng, W.; He, Z.; Zuo, M.; Chen, A. Circulating Cell Free DNA as the Diagnostic Marker for Ovarian Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0155495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Pu, K.; Ge, L.; Wu, X. Diagnostic significance assessment of the circulating cell-free DNA in ovarian cancer: An updated meta-analysis. Gene 2019, 714, 143993. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Ohlsson, R.; Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006, 7, 21–33. [Google Scholar] [CrossRef]
- Paluszczak, J.; Baer-Dubowska, W. Epigenetic diagnostics of cancer—the application of DNA methylation markers. J. Appl. Genet. 2006, 47, 365–375. [Google Scholar] [CrossRef]
- Constâncio, V.; Nunes, S.P.; Henrique, R.; Jerónimo, C. DNA Methylation-Based Testing in Liquid Biopsies as Detection and Prognostic Biomarkers for the Four Major Cancer Types. Cells 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levenson, V.V. DNA methylation as a universal biomarker. Expert Rev. Mol. Diagn. 2010, 10, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Legendre, C.R.; Demeure, M.J.; Whitsett, T.G.; Gooden, G.C.; Bussey, K.J.; Jung, S.; Waibhav, T.; Kim, S.; Salhia, B. Pathway Implications of Aberrant Global Methylation in Adrenocortical Cancer. PLoS ONE 2016, 11, e0150629. [Google Scholar] [CrossRef]
- Elshimali, Y.I.; Khaddour, H.; Sarkissyan, M.; Wu, Y.; Vadgama, J.V. The Clinical Utilization of Circulating Cell Free DNA (CCFDNA) in Blood of Cancer Patients. Int. J. Mol. Sci. 2013, 14, 18925–18958. [Google Scholar] [CrossRef] [PubMed]
- Legendre, C.; Gooden, G.C.; Johnson, K.; Martinez, R.A.; Liang, W.S.; Salhia, B. Whole-genome bisulfite sequencing of cell-free DNA identifies signature associated with metastatic breast cancer. Clin. Epigenetics 2015, 7, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunami, E.; Vu, A.-T.; Nguyen, S.L.; Hoon, D.S. Analysis of Methylated Circulating DNA in Cancer Patients’ Blood. Adv. Struct. Saf. Stud. 2009, 507, 349–356. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, M.H.; Han, Y.J.; Lee, D.E.; Park, S.Y.; Han, J.Y.; Kim, M.Y.; Ryu, H.M. Cell-Free Fetal DNA and Cell-Free Total DNA Levels in Spontaneous Abortion with Fetal Chromosomal Aneuploidy. PLoS ONE 2013, 8, e56787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.Y.; Park, B.H. Plasma tumor DNA: On your markers, get set, go! Ann. Transl. Med. 2014, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Oxnard, G.; Klein, E.; Swanton, C.; Seiden, M.; Smith, D.; Richards, D.; Yeatman, T.J.; Cohn, A.L.; Lapham, R.; et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Hentze, J.L.; Høgdall, C.K.; Høgdall, E.V. Methylation and ovarian cancer: Can DNA methylation be of diagnostic use? (Review). Mol. Clin. Oncol. 2019, 10, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liggett, T.E.; Melnikov, A.; Yi, Q.; Replogle, C.; Hu, W.; Rotmensch, J.; Kamat, A.; Sood, A.K.; Levenson, V. Distinctive DNA methylation patterns of cell-free plasma DNA in women with malignant ovarian tumors. Gynecol. Oncol. 2011, 120, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnikov, A.; Scholtens, D.; Godwin, A.; Levenson, V. Differential Methylation Profile of Ovarian Cancer in Tissues and Plasma. J. Mol. Diagn. 2009, 11, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yu, L.; Yang, G.-Z.; Luo, X.; Huang, L. Application of Multiplex Nested Methylated Specific PCR in Early Diagnosis of Epithelial Ovarian Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 3003–3007. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yu, L.; Luo, X.; Huang, L.; Li, Q.-S.; Shao, X.-S.; Liu, Y.; Fan, Y.; Yang, G.-Z. Detection of OPCML methylation, a possible epigenetic marker, from free serum circulating DNA to improve the diagnosis of early-stage ovarian epithelial cancer. Oncol. Lett. 2017, 14, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Giannopoulou, L.; Chebouti, I.; Pavlakis, K.; Kasimir-Bauer, S.; Lianidou, E.S. RASSF1A promoter methylation in high-grade serous ovarian cancer: A direct comparison study in primary tumors, adjacent morphologically tumor cell-free tissues and paired circulating tumor DNA. Oncotarget 2017, 8, 21429–21443. [Google Scholar] [CrossRef] [Green Version]
- Giannopoulou, L.; Mastoraki, S.; Buderath, P.; Strati, A.; Pavlakis, K.; Kasimir-Bauer, S.; Lianidou, E.S. ESR1 methylation in primary tumors and paired circulating tumor DNA of patients with high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 150, 355–360. [Google Scholar] [CrossRef]
- Bondurant, A.E.; Huang, Z.; Whitaker, R.S.; Simel, L.R.; Berchuck, A.; Murphy, S.K. Quantitative detection of RASSF1A DNA promoter methylation in tumors and serum of patients with serous epithelial ovarian cancer. Gynecol. Oncol. 2011, 123, 581–587. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, G.; Yang, Q.; Dong, R.; Xie, X.; Ma, D.; Shen, K.; Kong, B. A multiplex methylation-specific PCR assay for the detection of early-stage ovarian cancer using cell-free serum DNA. Gynecol. Oncol. 2013, 130, 132–139. [Google Scholar] [CrossRef]
- Widschwendter, M.; Zikan, M.; Wahl, B.; Lempiäinen, H.; Paprotka, T.; Evans, I.; Jones, A.; Ghazali, S.; Reisel, D.; Eichner, J.; et al. The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Med. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Lin, L.; Ma, X.-P.; Ma, Y.-C.; Liu, P.-S. Aberrant methylation of RASSF2A in tumors and plasma of patients with epithelial ovarian cancer. Asian Pac. J. Cancer Prev. 2014, 15, 1171–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caceres, I.I.; Battagli, C.; Esteller, M.; Herman, J.G.; Dulaimi, E.; Edelson, M.I.; Bergman, C.; Ehya, H.; Eisenberg, B.L.; Cairns, P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004, 64, 6476–6481. [Google Scholar] [CrossRef] [Green Version]
- Häfner, N.; Diebolder, H.; Jansen, L.; Hoppe, I.; Dürst, M.; Runnebaum, I.B. Hypermethylated DAPK in serum DNA of women with uterine leiomyoma is a biomarker not restricted to cancer. Gynecol. Oncol. 2011, 121, 224–229. [Google Scholar] [CrossRef]
- Widschwendter, M.; Apostolidou, S.; Jones, A.A.; Fourkala, E.O.; Arora, R.; Pearce, C.L.; Frasco, M.A.; Ayhan, A.; Zikan, M.; Cibula, D.; et al. HOXA methylation in normal endometrium from premenopausal women is associated with the presence of ovarian cancer: A proof of principle study. Int. J. Cancer 2009, 125, 2214–2218. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-Y.; Lai, H.-C.; Lin, Y.-W.; Chou, Y.-C.; Liu, C.-Y.; Yu, M.-H. An epigenetic marker panel for screening and prognostic prediction of ovarian cancer. Int. J. Cancer 2009, 124, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yu, J.; Pu, H.; Zhang, Z.; Xu, X. Frequent SLIT2 promoter methylation in the serum of patients with ovarian cancer. J. Int. Med. Res. 2012, 40, 681–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-Q.; Yan, Q.; Zhang, J.-R.; Li, S.-D.; Yang, Y.-X.; Wan, X.-P. Epigenetic inactivation of BRCA1 through promoter hypermethylation in ovarian cancer progression. J. Obstet. Gynaecol. Res. 2012, 39, 549–554. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, M.; Tao, G.; Chen, X.; Xie, W.; Wang, Y.; Cao, X. Detection of Circulating Methylated Opioid Binding Protein/Cell Adhesion Molecule-Like Gene as a Biomarker for Ovarian Carcinoma. Clin. Lab. 2014, 60, 759–765. [Google Scholar] [CrossRef]
- Zuberi, M.; Mir, R.; Dholariya, S.; Najar, I.; Yadav, P.; Javid, J.; Guru, S.; Mirza, M.; Gandhi, G.; Khurana, N.; et al. RASSF1 and PTEN Promoter Hypermethylation Influences the Outcome in Epithelial Ovarian Cancer. Clin. Ovarian Other Gynecol. Cancer 2014, 7, 33–39. [Google Scholar] [CrossRef]
- Swellam, M.; Ramadan, A.; Mahmoud, M.S.; Hashim, M.; Emam, M. Diagnostic Role of Aberrant DNA Promoter Methylation in Ovarian Cancer. Annu. Res. Rev. Biol. 2017, 19, 1–13. [Google Scholar] [CrossRef]
- Aghajanian, C.; Goff, B.; Nycum, L.R.; Wang, Y.V.; Husain, A.; Blank, S.V. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 2015, 139, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, C.A.; Hacker, N.F.; Clark, S.J.; O’Brien, P.M. DNA methylation changes in ovarian cancer: Implications for early diagnosis, prognosis and treatment. Gynecol. Oncol. 2008, 109, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Tuaeva, N.O.; Falzone, L.; Porozov, Y.B.; Nosyrev, A.E.; Trukhan, V.M.; Kovatsi, L.; Spandidos, D.A.; Drakoulis, N.; Kalogeraki, A.; Mamoulakis, C.; et al. Translational Application of Circulating DNA in Oncology: Review of the Last Decades Achievements. Cells 2019, 8, 1251. [Google Scholar] [CrossRef] [Green Version]
- Taryma-Leśniak, O.; Sokolowska, K.E.; Wojdacz, T.K. Current status of development of methylation biomarkers for in vitro diagnostic IVD applications. Clin. Epigenetics 2020, 12. [Google Scholar] [CrossRef]
- Detect Cancer Early: Liquid Biopsy Based ctDNA Methylation as a Promising Diagnostic Approach-Onco’Zine. Available online: https://www.oncozine.com/detect-cancer-early-liquid-biopsy-based-ctdna-methylation-as-a-promising-diagnostic-approach/ (accessed on 26 September 2020).
- Maradeo, M.E.; Cairns, P. Translational application of epigenetic alterations: Ovarian cancer as a model. FEBS Lett. 2011, 585, 2112–2120. [Google Scholar] [CrossRef] [Green Version]
- Kint, S.; De Spiegelaere, W.; De Kesel, J.; Vandekerckhove, L.; Van Criekinge, W. Evaluation of bisulfite kits for DNA methylation profiling in terms of DNA fragmentation and DNA recovery using digital PCR. PLoS ONE 2018, 13, e0199091. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, M.; Seong, M.-W.; Kim, H.-S.; Lee, Y.K.; Kang, H.J. Plasma vs. serum in circulating tumor DNA measurement: Characterization by DNA fragment sizing and digital droplet polymerase chain reaction. Clin. Chem. Lab. Med. 2019, 58, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Pittella-Silva, F.; Chin, Y.M.; Chan, H.T.; Nagayama, S.; Miyauchi, E.; Low, S.-K.; Nakamura, Y. Plasma or Serum: Which Is Preferable for Mutation Detection in Liquid Biopsy? Clin. Chem. 2020, 66, 946–957. [Google Scholar] [CrossRef]
- Gu, X.; Lu, Y.; Ma, R.; Liu, X.; Guo, S. Diagnostic application of serum DNA quantification and methylation of epithelial ovarian cancer. Chin. J. Obstet. Gynecol. 2009, 44, 295–297. [Google Scholar]
- Tricco, A.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef] [PubMed]

| First Author, Year | Source | Cases | # Controls | Methylation Method | Gene Target(s) | Findings and Study Summary | ||
|---|---|---|---|---|---|---|---|---|
| Histology (%) | Stage (%) | # Cases | ||||||
| De Caceres 2004 [37] | Tissue, serum or plasma, peritoneal fluid | Tissue and serum/plasma: Serous (65), endometroid (12), mucinous (8), clear cell (10), transitional cell (2), undifferentiated (2) Tissue only samples: Unknown | Tissue and serum/ plasma: I (20), III (62), IV (18) Tissue only samples: I (100) | Tissue and serum/ plasma: 40 OC 10 borderline Tissue only archival samples: 21 OC | Tissue and fluid: 10 benign ov mass 10 normal ov/peritoneal fluid Serum/ plasma: 20 healthy controls | Bisulfite modified with NaHSO3; MSP | RASSF1A, BRCA1, APC, p14-ARF, p16-INK4a, DAPK | Tissue: -99% OC and borderline (70/71) had hypermethylation of at least one of the 6 genes, in all stages and histologic types Comparisons: -82% (41/50—OC and borderline) of matched serum/plasma and tumor had identical hypermethylation status, including 76.5% (13/17) stage I and 84.8% (28/33) stage III-IV → Sensitivity 82% -0% non-hypermethylated tumors had hypermethylated serum -0% (0/40) hypermethylation in tissue, peritoneal fluid, or serum for benign ov msas, normal ov, or controls → Specificity 100% -No correlations between methylation and stage or histology ----------------------- Summary for plasma/serum test in differentiating OC from benign/control: -Sensitivity 82% (41/50 for OC and borderline), specificity 100% (40/40 healthy controls, benign ov mass, normal ov) -*PPV 100% (41/41), *NPV 81.6% (40/49), *accuracy 90% (81/90) |
| Melnikov 2009 [28] | Tissue, plasma | Tissue: Serous (70), endometroid (30) Plasma: Serous (100) | Tissue: I-II (13); III-IV (87) Plasma: III–IV (100) | Tissue: 30 OC Plasma: 33 OC | Tissue: 30 healthy ov (RRSO) Plasma: 33 healthy controls | Methylation analysis with MethDet technique | Selected after MethDet analysis For tissue: BRCA1, EP300, NR3C1, MLH1, DNAJC15, CDKN1C, TP73, PGR, THBS1, PYCARD For plasma: BRCA1, H1C1, PAX5, PGR, THBS1 | Tissue: -Sensitivity, specificity, and accuracy of methylation test (averaged after 25 rounds of cross-validation) in detecting OC vs. heathy ov tissue: 69.4% (20.82/30), 70.2% (21.06/30), 69.8% (41.88/60) Plasma: -Sensitivity, specificity, and accuracy of methylation test (averaged after 25 rounds of cross-validation) in detecting OC vs. healthy control serum: 85.1% (28.25/33), 61.1% (20.16/33), 73.1% (48.24/66) ----------------------- Summary for MethDet plasma test in differentiating OC from controls: -Sensitivity 85.1% (28.08/33), specificity 61.1% (20.16/33), -PPV 68.6% (28.02/40.85), NPV 80.4% (20.16/25.08), accuracy 73.1% (48.24/66) |
| Su 2009 [40] | Tissue, serum | Tissue: Serous (66), mucinous (26), endometrial (6), other (2) | Tissue: I (25); II (9); III (56); IV (10) Serum subset: No breakdown | Tissue: 126 OC 14 borderline Serum subset: 26 OC | Tissue: 75 benign ov mass or normal ov Serum subset: 20 benign ov mass | Bisulfite modified with EZ DNA kit; MSP | SFRP1, SFRP2, SFRP4, SFRP5, SOX1, PAX1, LMX1A Plasma: Excluded SFRP4 | Tissue: -Methylation rates -OC: SFRPI (35%), SFRP2 (63%), SFRP4 (2%), SFRP5 (44%), SOX1 (59%), PAX1 (50%), LMX1A (35%) -Lower rates for borderline compared to OC; lowest rates for benign ov mass (p < 0.001 for all comparisons except SFRP4) -No correlations between methylation with stage or grade Plasma (no numbers provided for calculating statistics): -Significant concordance between plasma and tissue methylation for all markers—highest concordance with SFRP1 and SFRP5 (p < 0.001) -Best potential for screening gene combinations with SOX1, PAX1, SFRP1 ----------------------- Summary for serum test in differentiating OC from benign tumor for: -SOX1 + PAX1 + SFRP1: Sensitivity 73.1%, specificity 75% -All 6 genes: Sensitivity 73%, specificity 55% |
| BonDurant 2011 [33] | Tissue, serum | Serous (100) | Full/subset: I/II (4/5), III (83/71), IV (11/14), unk (2,10) | Tissue: 106 OC Serum subset: 21 OC | n/a | Bisulfite modified via NaHSO3; multiplexed real-time MSP assay | RASSF1A | Tissue: -51% (45/106) OC methylated; 25% (1/4) stage I-II Serum: -86% (18/21) OC methylated; 0% (0/1) stage I-II Comparison: -100% (18/18) of methylated serum samples had positive tumor methylation - Methylation correlated with increased age. No correlations between methylation with stage, clinical response, treatment, survival. ----------------------- Extrapolated summary for methylation assay in identifying OC for serum samples compared to tissue samples: -Sensitivity 86% (18/21), 100% concordance of serum with tissue testing for OC methylation status |
| Häfner 2011 [38] | Tissue, serum | Tissue: Serous (81), endometroid (12), papillary (16), clear cell (3), neuroendocrine (3) Serum subset: No breakdown | Tissue: IIc (6), III (78), IV (16) Serum subset: No breakdown | Tissue: 32 OC Serum subset: 23 OC | Tissue: 30 fibroid 20 healthy controls Serum subsets: 21 fibroid 8 healthy controls | Bisulfite modified via MethylAmp kit; MSP with sequencing | DAPK | Tissue: -Aberrant methylation: 50% (14/28) OC, 35.3% (6/17) fibroid Serum: -Aberrant methylation: 56% (13/23) OC, 23.8% (5/21) fibroid, 50% (5/8) controls -No correlations between serum methylation and clinicopathologic characteristics ----------------------- Extrapolated summary for serum test in differentiating OC: -From control: Sensitivity 56.5% (13/23), *specificity 50% (4/8), *PPV 30.9% (4/17), *NPV 28.6% (4/14), *accuracy 54.8% (17/31) -From fibroids: Sensitivity 56.5% (13/23), *specificity 76.2% (16/21), *PPV 72.2% (13/18), *NPV 61.5% (16/26), *accuracy 65.9% (29/44) |
| Liggett 2011 [27] | Plasma | Serous (100) | III (60), IV (40) | 30 OC | 30 benign ov mass 30 healthy controls | Methylation analysis with MethDet technique | Selected genes after analysis: OC vs. Control: CALCA, EP300, and RASSF1A Benign vs. control: BRCA1, CALCA, CDKN1C OC vs. benign: PGR-PROX, RASSF1A | Diagnostic performance of methylation test (uncertain how many runs were performed) in: -Differentiating OC from controls: Sensitivity 90.0%, specificity 86.7% -Differentiating benign ov mass from controls: Sensitivity 90.0%, specificity 76.7% -Differentiating OC from benign ov mass: Sensitivity 73.3%, specificity 80.0% ----------------------- Summary for MethDet plasma test in differentiating OC: -From controls: Sensitivity 90.0%, specificity 86.7%, PPV 87.1%, NPV 89.7% -From benign ovarian masses: Sensitivity 73.3%, specificity 80.0%, PPV 78.6%, NPV 75.0% |
| Dong 2012 [41] | Tissue, serum | Serous (50), endometrioid (17), mucinous (33) | I (8), II (3), III (52), IV (36) | 36 OC | 25 healthy controls | Bisulfite modified via CpGenome kit; MSP | SLIT2 | Tissue: -Aberrant methylation: 80.6% (29/36) OC cases Serum: -Aberrant methylation: 93.1% (27/29) case-matched aberrantly methylated OC tissue; 0% (0/25) controls (p<.0001) -0% (0/7) of remaining non-aberrantly methylated tissue samples had aberrant serum methylation -No correlation between serum methylation with stage, histology, age, CA125 ----------------------- Extrapolated summary for serum test in differentiating OC from control: -Sensitivity 75% (27/36), specificity 100% (25/25) -PPV 100% (27/27), NPV 69% (25/36), accuracy: 85% (52/61) |
| Wang 2013 [42] | Tissue, serum | Serous (60), endometroid (20), clear cell (10), mucinous (10) | I (30), II (23), III (47) | 60 OC | 30 benign ov mass 30 normal ovary/ healthy controls | Bisulfite modified via EpiTect kit; real-time PCR | BRCA1 | Tissue: -Hypermethylation almost 100% of stage II and III OC, higher frequency in stage III -Stage I not differentially methylated from stage II (p > 0.05) -Stage II more methylated than stage III, normal, and benign ov (p < 0.05) -Stage III more methylated than all groups (p < 0.01) Serum: -Hypomethylation in 100% of stage I, benign, and controls -Stage I not differentially methylated from stage II (p > 0.05) -Hypermethylation frequency higher in stage III OC than all groups (p < 0.05) -No correlations for methylation between controls with benign and stage I, stage I and stage II, or with histology ----------------------- Summary for serum test in identifying OC methylation compared to tissue test (no data for comparative statistics): -Serum less sensitive than tissue for methylation analysis -Higher methylation status in higher tumor stages |
| Zhang 2013 [34] | Serum | Serous (71), mucinous (12), clear cell (12), endometrioid (14), mixed (7), other (5) Screening: Serous (77), mucinous (10), endometrioid (8), other (5) | I (56) II (10) III (52) IV (1) Screening: I (15), II (10), III (74) | 73 OC Screening: 69 pelvic masses (39 OC, 29 benign) | 53 benign ov mass 62 healthy controls | Bisulfite modified via EpiTect kit; multiplex-PCR | APC, RASSF1A, CDH1, RUNX3, TFPI2, SFRP5, OPCML | -Diagnostic performance of methylation in differentiating OC from benign (unclear which numbers used for calculation): sensitivity 90.57%, specificity 89.66%, AUC 0.9126 -AUC early stage vs. benign: 0.8916; advanced stage vs. benign: 0.9313 -Prospective diagnosis with screening cohort in differentiating OC from benign (unclear which numbers for calculation): sensitivity 92.3%, specificity 89.9%, AUC 89.9% -AUC early stage vs. benign: 0.8218, advanced stage vs. benign: 0.9127 -No correlations between methylation status with stage or histology -Methylation status had stronger diagnostic performance than CA125 for early-stage OC patients (p = 0.004) but not advanced-stage OC patients (p = 0.6) ----------------------- Summary for serum test in differentiating OC from benign ov mass in prospective screening sample: -For all OC samples: Sensitivity 92.3%, specificity 82.7%, AUC 89.9% -For early-stage OC samples: Sensitivity 83.3%, AUC 82.2%, no specificity data -For advanced-stage OC samples: Sensitivity 93.9%, AUC 91.3%, no specificity data |
| Wu 2014 [36] | Tissue, plasma | Serous (49), mucinous (32), endometrioid (19) | Early (47), advanced (53) | 47 OC | 14 benign ov mass 10 normal ov | Bisulfite modified with NaHSO3 and hydroquinone; MSP | RASSF1A | Tissue: -51% (24/47) OC methylated, 0% (0/20) benign and normal Plasma: -36% (17/47) OC methylated, 0% (0/20) benign and normal -“Positive correlation” between serum and tissue methylation profiles -No correlations between methylation with clinicopathologic characteristics ----------------------- Extrapolated summary for plasma test in differentiating OC from benign ov mass or control: -Sensitivity 36.2% (17/47), specificity 100% (20/20) -PPV 100% (17/17), NPV 40% (20/50), accuracy 55.2% (37/67) |
| Zhou 2014 [43] | Tissue, serum | Serous (44), endometroid (22), mucinous (13), clear cell (11), undifferentiated (9) | I (16), II (7), III (62), IV (16) | Tissue and serum: 45 OC | Tissue: 40 normal ov Serum: 20 healthy controls | Bisulfite modified via CpGenome DNA Modification kit; MSP | OPCML | Tissue: -Hypermethylation in 87% (39/45) OC and 0% (0/40) normal ov Serum: -Hypermethylation in 80% (36/45) OC and 0% (0/20) healthy control -Correlation between methylation with increasing stage (p < 0.05). No correlations with histology. ----------------------- Extrapolated summary for serum test in differentiating OC from control: -Sensitivity 80% (36/45), specificity 100% (20/20) -PPV 100% (36/36), NPV 69% (20/29), accuracy 86.1% (56/65) |
| Zuberi 2014 [44] | Serum | Serous (46), mucinous (46), endometrioid (4), clear cell (2), undifferentiated (2) | Early (20), advanced (80) | 50 OC | 20 healthy controls | Bisulfite modified via BisfulFlash DNA Modification kit; MSP | RASSF1A, PTEN | -RASSF1A methylated in 34% (17/50) OC and 5% (1/20) healthy control 1/20 -PTEN methylated in 16% (8/50) OC and 0% (0/20) healthy control -Correlations between methylation with: menopausal status (p = 0.03) and histology (p = 0.03; highest correlation with serous) for RASSF1; higher stages for both gene targets (NS) ----------------------- Extrapolated summary for serum test in differentiating OC from control for: -RASSF1A: Sensitivity 34% (17/50), specificity 95% (19/20), PPV 94% (17/18), NPV 36.5% (19/52), accuracy 51.4% (36/70) -PTEN: Sensitivity 16% (8/50), specificity 100% (20/20), PPV 100% (8/8), NPV 47.6% (20/42), accuracy 40% (28/70) |
| Wang 2015 [29] | Tissue, serum | Serous (58), endometrioid (11), mucinous (10), clear cell (10), other (11) | I (46), II (8), III (44), IV (1) | 71 OC | 43 benign ov mass 80 healthy controls | Methylation modification via unknown technique; multiplex-nested MSP | RUNX3, TFPI2, OPCML | Diagnostic performance of methylation status in detecting all OC, early OC, and advanced OC: -Sensitivity: 90.14% (64/71), 84.62% (33/39), 93.75% (30/32) -Specificity: 91.06% (112/123) -PPV: 85.33% (64/75), 75%, 73.17% (30/41) -Methylation status had stronger PPV than CA125 -No correlation between methylation and stage ----------------------- Summary for serum test in differentiating: -All OC from controls/benign ov mass: Sensitivity 90.1% (64/71), specificity 91.1% (112/123), PPV 85.3% (64/75), *NPV 94.1% (112/119), accuracy 90.7% (176/194) -Early OC from controls/benign ov mass: Sensitivity 84.6% (33/39), specificity 91.1% (112/123), PPV 75% (33/44), *NPV 94.9% (112/118), accuracy 89.5% (145/162) -Advanced OC from controls/benign ov mass: Sensitivity 93.8% (30/32), specificity 91.1% (112/123), PPV 73.2% (30/41), *NPV 98.2% (112/114), accuracy 91.6% (142/155) |
| Giannopoulou 2017 [31] | Tissue, plasma | Serous (100) | Group A, B: I (19,2), II (57,3), III (18,64), IV (-,13) unk (6,23) | Tissue: Group A: 67 OC Group B: 61 OC Group B: 58 matched adjacent cell-free tissue Plasma: Group B: Subset of 59 OC | Tissue: 16 normal fallopian tube Plasma: 51 healthy controls | Bisulfite modified via EZ-DNA Methylation Gold kit 200; real-time MSP and methylation-sensitive high-resolution melting analysis (ms-HRMA) | RASSF1A | Tissue: -41% (52/128) OC methylated via MSP, 43% (55/128) OC methylated via ms-HRMA -29.3% (17/58) adjacent cf tissue methylated via MSP, 36% (21/58) adjacent methylated via ms-HRMA -0% (0/16) normal fallopian tube methylated via MSP and ms-HRMA Plasma: -25.4% (15/59) OC methylated via MSP -0% (0/51) healthy control methylated In matched OC, adjacent tissue, and serum (n = 53): -84.9% (45/53) matched OC and tissue methylation with strong agreement -62.3% (33/53) matched OC and serum methylation with slight agreement Correlation between methylation with tumor grade (p = 0.04), lymph node metastasis (p = 0.04), overall survival (p =0.02 with ms-HRMA method on tissue). No correlation between methylation and overall survival for plasma samples. ----------------------- Extrapolated summary for plasma test in differentiating OC from healthy controls: -Sensitivity 25.4% (15/59), specificity 100% (51/51) -PPV 100% (15/15), NPV 53.7% (51/95), accuracy 60% (66/110) |
| Swellam 2017 [45] | Serum | Serous (60), endometrioid (21), mucinous (19) | I-II (47), III-IV (53) | 90 OC | 50 benign ov mass 30 healthy controls | Bisulfite modified via EpiTect Fast Bisulfite kit; MSP | DAPK, OPCML, DLEC1 | DAPK methylated in 96.7% (87/90) OC, 40% (20/50) benign, 0% (0/30) controls -Differentiating benign and controls from OC: AUC 0.858 OPCML methylated in 97.8% (88/90) OC, 48% (24/50) benign, 0% (0/30) controls -Differentiating benign and controls from OC: AUC 0.839 DLEC1 methylated in 95.6% (86/90), 40% (20/50) benign, 0% (0/30) controls -Differentiating benign and controls from OC: AUC 0.841 Correlation between methylation with: higher stage for DAPK, OPCML (p = 0.006, p < 0.0001 respectively), higher grade (p < 0.0001, p < 0.001), and serous pathology (p = 0.034, p = 0.001); higher stage (p = 0.03) and grade (p < 0.0001) for DLEC1 -For early stage, sensitivity 95.2-97.6%, specificity 70–75% for all targets -Methylation markers outperformed CA125 and CEA in sensitivity and specificity ----------------------- Summary for plasma test in differentiating OC from healthy controls/benign ov mass for: -DAPK: Sensitivity 96.7% (87/90), specificity 75% (60/80), * PPV 81.3% (87/107), * NPV 95.2% (60/63), accuracy 86.5% (147/170), AUC 0.858 -OPCML: Sensitivity 97.8% (88/90), specificity 70% (56/80), * PPV 78.4% (87/111), * NPV 90.3% (56/62), accuracy 84.7% (144/170), AUC 0.839 -DLEC1: Sensitivity 95.6% (86/90), specificity 75% (60/80), *PPV 81.1% (86/106), *NPV 93.8% (60/64), accuracy 85.8% (146/170), AUC 0.841 |
| Wang 2017 [30] | Tissue, plasma | Data unavailablea but extrapolated as: Serous (58), endometrioid (11), mucinous (10), clear cell (10), other (11) | I-II (55), III-IV (45) | 71 OC | 43 benign ov mass 80 healthy controls | Bisulfite modified via EpiTect kit; nested MSP | Initial investigation with RUNX3, TFPI2, OPCML; final analysis with OPCML | Plasma: -Diagnostic performance of methylation status to detect overall, early, advanced OC -Sensitivity 90%, 87%, 94%, -Specificity 92%, 92%, 92% -Accuracy 91%, 91%, 92% -All values superior to CA125 -Methylation difference between healthy controls and early, advanced, and overall OC (p < 0.0001) -No correlations between methylation with healthy controls and benign tumors -Methylation markers outperformed CA125 in detection of early-stage OC ----------------------- Summary for plasma test in differentiating: -All OC from controls/benign ov mass: Sensitivity 90.1% (64/71), specificity 91.9% (113/123), PPV 86% (64/74), *NPV 94.2% (113/120), accuracy 91.2% (177/194) -Early OC from controls/benign ov mass: Sensitivity 87.2% (34/39), specificity 91.9% (113/123), PPV 77.3% (34/44), ^NPV 95.8% (113/118), accuracy 90.7% (147/162) -Advanced OC from controls/benign ov mass: Sensitivity 93.8% (30/32), specificity 91.9 (113/123), PPV 75% (30/40), *NPV 98.3% (113/115), accuracy 92.3% (143/155) |
| Widschwendter 2017 [35] | Tissue, serum | Marker discovery Array: Data unavailable RRBS: Serous (82), endometrioid (9), mucinous (9) Assay development: Serous (75), endometrioid (11), mucinous (4), clear cell (9) Assay validation: Data incomplete | Marker discovery: Data unavailable Assay development: I-II (44), III-IV (56) Assay validation: Data incomplete | Marker discovery with 2 tissue sets: Array: 218 OC, 10 benign pelvic mass, 55 fallopian tube, 96 endometrium, 107 WBC, 170 other organs RRBS: 11 OC, 1 benign pelvic mass, 18 normal, 2 endometrium, 23 WBC Assay development with 2 serum sets: 45 OC, 11 borderline, 56 benign pelvic mass, 39 healthy controls Assay validation with 3 serum sets: 164 OC (including 5 non-epithelial), 27 borderline, 119 benign pelvic mass, 37 other cancer, 150 healthy controls | Bisulfite modified at GATC Biotech; RRBS performed at GATC Biotech and GWAS methylation analysis via Infinium Human Methylation 450 K array; PCR with ultra-high coverage bisulfite sequencing via Illumina MiSeq or HiSeq 2500 | Four candidate markers for differentiating high-grade serous patients, specifically; narrowed to 3 due to limited serum volume: COL23A1, C2CD4D, WNT6 | Diagnostic performance of methylation assay in: -Differentiating HGS OC from benign/controls in validation set: Sensitivity 41.4% (12/29), specificity 90.7% (127/140) -Early detection in screened healthy participants (after reducing threshold for regions and evaluating only samples with less than the median amount of DNA): Sensitivity 58%, specificity 88% -Early detection only for CA125-normal healthy participants: Sensitivity 64%, specificity 87.5% -Identifying chemotherapy responders and non-responders, respectively: Sensitivity 78%, 86% Key limitation for screening sample: Leukocyte DNA leakage into serum samples due to delayed processing time ----------------------- Summary for serum test in differentiating HGS OC from: -Benign/controls in validation sample: Sensitivity 41.4% (12/29), specificity 90.7% (127/140), * PPV 48% (12/25), * NPV 88.2% (127/144), * accuracy 82.2% (139/169) -Healthy controls in screening sample: Sensitivity 58% * (25/43), specificity 88% * (114/129), * PPV 62.5% (25/40), * NPV 91.2% (114/125), * accuracy 80.8% (139/172) -Healthy controls in screening sample with normal CA125: Sensitivity 64%, specificity 87.5%, no further data provided for extrapolation | |
| Giannopoulou 2018 [32] | Tissue, plasma | Serous (100) | Group A, B: I (20, 6); II (56, 5); III (21, 76); IV (-,13) | Tissue: Group A: 66 OC Group B: 63 OC Plasma: Group B: Subset of 50 OC (chemo-treated) | Tissue: 16 normal fallopian tube Plasma: 51 healthy controls | Bisulfite modified via EZ-DNA Methylation Gold kit 200; real-time MSP | ESR1 | Tissue: -39% (47/119) OC methylated -94% (15/16) normal fallopian tube methylated Plasma: -38% (19/50) OC methylated -2% (1/51) healthy control methylated In matched OC and serum: -75% (36/48) matched OC and serum methylation with moderate agreement - No correlations between serum methylation with clinicopathologic features, survival ----------------------- Extrapolated summary for plasma test in differentiating OC from healthy controls: -Sensitivity 38% (19/50), specificity 84.7% (50/51) -PPV 95% (19/20), NPV 61.7% (50/81), accuracy 68.3% (69/101) |
| Gene Target | Type | Description | Ref. | |
|---|---|---|---|---|
| RASSF1A | Ras association domain-containing protein 1 | Tumor suppressor | Modulates multiple apoptotic and cell cycle checkpoint functions | [27,28,31,33,34,36,37,44] |
| OPCML | Opioid binding protein/cell adhesion molecule-like gene | Tumor suppressor | Involved in cell adhesion and cell–cell recognition | [29,30,34,43,45] |
| BRCA1 | Breast cancer type 1 susceptibility protein | Tumor suppressor | Maintains genomic stability via DNA repair, cell cycle regulation, and others | [28,37,42] |
| DAPK | Death-associated protein kinase 1 | Tumor suppressor | Involved in multiple cell death-associated signaling pathways | [37,38,45] |
| APC | Adenomatous polyposis coli | Tumor suppressor | Controls cell division, motility, and adhesion | [34,37] |
| RUNX3 | Runt-related transcription factor 3 | Tumor suppressor | Involved in cell differentiation and DNA repair | [29,34] |
| TFP12 | Tissue factor pathway inhibitor 2 | Tumor suppressor | Involved in regulation of extracellular matrix digestion and remodeling | [29,34] |
| SFRP5, 1, 2 | Secreted frizzled-related protein 5,1,2 | Tumor suppressor | Regulates cell growth and differentiation | [34,40] |
| PAX1, 5 | Paired box 1, 5 | Oncogene; tumor suppressor | Involved in cell development | [28,40] |
| PGR, PGR-PROX | Progesterone receptor; Progesterone receptor, proximal promotor | Oncogene; tumor suppressor | Regulates cell proliferation and differentiation | [27,28] |
| p14-ARF | Alternate reading frame protein product | Tumor suppressor | Involved in cell cycle regulation and apoptosis | [37] |
| p16-INK4a | Inhibitors of CDK4 | Tumor suppressor | Regulates cell cycle progression | [37] |
| H1C1 | Hypermethylated in cancer 1 | Tumor suppressor | Regulates cell growth and apoptosis | [28] |
| THBS1 | Thrombospondin 1 | Tumor suppressor | Modulates cell motility, adhesion, and growth | [28] |
| EP300 | Adenovirus early region 1A-associated protein p300 | Tumor suppressor | Regulates cell growth and differentiation | [27] |
| CALCA | Calcitonin related polypeptide alpha | Tumor suppressor | Regulates calcium and phosphorous metabolism; vasodilation | [27] |
| SLIT2 | Slit guidance ligand 2 | Tumor suppressor | Involved in cell migration processes | [41] |
| SOX1 | Sex determining region Y-box 1 | Tumor suppressor | Regulates embryonic development and stem cell function | [40] |
| LMX1A | LIM homeobox transcription factor 1, alpha | Tumor suppressor | Regulates cell growth and differentiation | [40] |
| CDH1 | Cadherin-1 | Tumor suppressor | Involved with cell adhesion and motility | [34] |
| PTEN | Phosphatase and tensin homolog | Tumor suppressor | Regulates cell proliferation | [44] |
| ESR1 | Estrogen receptor, alpha 1 | Oncogene; tumor suppressor | Regulates cell proliferation and differentiation | [32] |
| WNT6 | Wingless-type MMTV integration site family, member 6 | Oncogene | Involved with cell proliferation, differentiation, adhesion | [35] |
| COL23A1 | Collagen, type XIII, alpha 1 | Oncogene | Likely involved with cell adhesion | [35] |
| C2CD4D | C2 calcium-dependent domain containing 4D | Oncogene | Unknown | [35] |
| DLEC1 | Deleted in lung and esophageal cancer1 | Tumor suppressor | Regulates cell proliferation | [45] |
| Search Number | Early Diagnosis | Ovarian Cancer | Biomarkers | Type of Marker |
|---|---|---|---|---|
| 1 | Early diagnosis [MeSH] | Ovarian neoplasms [MeSH] | Biomarkers, Tumor [MeSH] | DNA methylation |
| Early diagnosis | Ovarian neoplasms/diagnosis [MeSH] | Biomarker* | ctDNA* | |
| Early detection of cancer [MeSH] | Ovarian neoplasms | Tumor marker* | Circulating tumor DNA | |
| Cancer screening | Ovarian cancer | Tumor biomarkers | miRNA* | |
| High-grade serous carcinoma | Cancer biomarkers | microRNA | ||
| HGSC | Neoplasm markers | Cytolog* | ||
| Cystadenocarcinoma, Serous [MeSH] | Carcinogen markers | |||
| Serous cystadenoma | ||||
| Serous epithelial | ||||
| 2 | Early diagnosis [MeSH] | Ovarian neoplasms [MeSH] | ctDNA* | |
| Early diagnosis | Ovarian neoplasms/diagnosis [MeSH] | Circulating tumor DNA | ||
| Early detection of cancer [MeSH] | Ovarian neoplasms | cfDNA* | ||
| Cancer screening | Ovarian cancer | Cell free DNA | ||
| High-grade serous carcinoma | Liquid biopsy | |||
| HGSC | Liquid biopsies | |||
| Cystadenocarcinoma, Serous [MeSH] | Methylation | |||
| Serous cystadenoma | Epigenetic | |||
| Serous epithelial |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.M.; Miller, H.; Matsuo, K.; Roman, L.D.; Salhia, B. Circulating Cell-Free DNA Methylation Profiles in the Early Detection of Ovarian Cancer: A Scoping Review of the Literature. Cancers 2021, 13, 838. https://doi.org/10.3390/cancers13040838
Guo XM, Miller H, Matsuo K, Roman LD, Salhia B. Circulating Cell-Free DNA Methylation Profiles in the Early Detection of Ovarian Cancer: A Scoping Review of the Literature. Cancers. 2021; 13(4):838. https://doi.org/10.3390/cancers13040838
Chicago/Turabian StyleGuo, Xiaoyue M., Heather Miller, Koji Matsuo, Lynda D. Roman, and Bodour Salhia. 2021. "Circulating Cell-Free DNA Methylation Profiles in the Early Detection of Ovarian Cancer: A Scoping Review of the Literature" Cancers 13, no. 4: 838. https://doi.org/10.3390/cancers13040838







