Pathogenetic Features and Current Management of Glioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathogenetic Features
2.1. Cellular Heterogeneity, Tumor Vascularity, and Extracellular Matrix
2.2. Molecular Heterogeneity
2.3. Immunosuppressive Features
3. Current Treatment
3.1. Standard of Care and Other FDA Approved Treatments
3.2. Hurdles with Current Treatments
4. Treatments in Development
4.1. Targeted Therapies
4.1.1. Targeting the p53 Pathway
4.1.2. Targeting the Rb Pathway
4.1.3. Targeting the RTK Pathway
Epidermal Growth Factor Receptor (EGFR) Inhibitors
Phosphatidylinositol-3-Kinase/AKT/Mammalian Target of Rapamycin (PI3K/AKT/mTOR) Inhibitors
Hepatocyte Growth Factor Receptor (HGFR/c-MET) Inhibitors
Fibroblast Growth Factor Receptor (FGFR) Inhibitors
Vascular Endothelial Growth Factor Receptor (VEGFR) Inhibitors
Platelet-Derived Growth Factor Receptor (PDGFR) Inhibitors
4.1.4. Targeting Other Pathways
Transforming Growth Factor-Beta (TGF-β) Inhibitors
Proteasome Inhibitors
DNA Damage Response (DDR) Inhibitors
4.2. Immunotherapy
4.2.1. Immune Checkpoint Inhibitors (ICIs)
Anti-PD-1/PD-L1 Antibodies
Anti-CTLA-4 Antibody
IDO Inhibition
4.2.2. Oncolytic Viruses (OVs)
4.2.3. Therapeutic Vaccines
4.2.4. Adoptive Cell Therapies (ACTs)
4.2.5. Macrophage and NK Cell-Based Immunotherapy
4.3. Nanomedicine
4.4. Photodynamic Therapy (PDT)
4.5. Inhibition of Extracellular Vesicles (EVs) and Micro RNA (miRNA)-Based Therapies
4.6. Targeting Vessel Co-Option and Vascular Mimicry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado-Lopez, P.D.; Corrales-Garcia, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Witthayanuwat, S.; Pesee, M.; Supaadirek, C.; Supakalin, N.; Thamronganantasakul, K.; Krusun, S. Survival Analysis of Glioblastoma Multiforme. Asian Pac. J. Cancer Prev. 2018, 19, 2613–2617. [Google Scholar] [CrossRef]
- Lyon, J.G.; Mokarram, N.; Saxena, T.; Carroll, S.L.; Bellamkonda, R.V. Engineering challenges for brain tumor immunotherapy. Adv. Drug Deliv. Rev. 2017, 114, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.; Bankhead, A., 3rd; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Lieberman, F. Glioblastoma update: Molecular biology, diagnosis, treatment, response assessment, and translational clinical trials. F1000Research 2017, 6, 1892. [Google Scholar] [CrossRef] [Green Version]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Melin, B.S.; Barnholtz-Sloan, J.S.; Wrensch, M.R.; Johansen, C.; Il’yasova, D.; Kinnersley, B.; Ostrom, Q.T.; Labreche, K.; Chen, Y.; Armstrong, G.; et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat. Genet. 2017, 49, 789–794. [Google Scholar] [CrossRef]
- Yang, W.; Warrington, N.M.; Taylor, S.J.; Whitmire, P.; Carrasco, E.; Singleton, K.W.; Wu, N.; Lathia, J.D.; Berens, M.E.; Kim, A.H.; et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019, 11, eaao5253. [Google Scholar] [CrossRef] [Green Version]
- Vigneswaran, K.; Neill, S.; Hadjipanayis, C.G. Beyond the World Health Organization grading of infiltrating gliomas: Advances in the molecular genetics of glioma classification. Ann. Transl. Med. 2015, 3, 95. [Google Scholar] [CrossRef]
- Delgado-Martin, B.; Medina, M.A. Advances in the Knowledge of the Molecular Biology of Glioblastoma and Its Impact in Patient Diagnosis, Stratification, and Treatment. Adv. Sci (Weinh) 2020, 7, 1902971. [Google Scholar] [CrossRef]
- Li, R.; Li, H.; Yan, W.; Yang, P.; Bao, Z.; Zhang, C.; Jiang, T.; You, Y. Genetic and clinical characteristics of primary and secondary glioblastoma is associated with differential molecular subtype distribution. Oncotarget 2015, 6, 7318–7324. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Ohgaki, H.; Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Ryu, H.W.; Won, H.R.; Kwon, S.H. Advances in epigenetic glioblastoma therapy. Oncotarget 2017, 8, 18577–18589. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K.L. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, H.; Gu, L.; Ye, B.; Jian, Z.; Stary, C.; Xiong, X. Advances in Immunotherapy for Glioblastoma Multiforme. J. Immunol. Res. 2017, 2017, 3597613. [Google Scholar] [CrossRef]
- Tivnan, A.; Heilinger, T.; Lavelle, E.C.; Prehn, J.H. Advances in immunotherapy for the treatment of glioblastoma. J. Neurooncol. 2017, 131, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedmann-Morvinski, D. Glioblastoma heterogeneity and cancer cell plasticity. Crit. Rev. Oncog. 2014, 19, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef] [Green Version]
- Blissitt, P.A.; American Association of Neuroscience Nurses. Clinical practice guideline series update: Care of the adult patient with a brain tumor. J. Neurosci. Nurs. 2014, 46, 367–368. [Google Scholar] [CrossRef]
- Yao, M.; Li, S.; Wu, X.; Diao, S.; Zhang, G.; He, H.; Bian, L.; Lu, Y. Cellular origin of glioblastoma and its implication in precision therapy. Cell Mol. Immunol. 2018, 15, 737–739. [Google Scholar] [CrossRef] [Green Version]
- Matarredona, E.R.; Pastor, A.M. Neural Stem Cells of the Subventricular Zone as the Origin of Human Glioblastoma Stem Cells. Therapeutic Implications. Front. Oncol. 2019, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Alcantara Llaguno, S.; Chen, J.; Kwon, C.H.; Jackson, E.L.; Li, Y.; Burns, D.K.; Alvarez-Buylla, A.; Parada, L.F. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 2009, 15, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.Y.; Kim, W.K.; Lee, J.K.; Park, J.; et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef]
- Alcantara Llaguno, S.; Sun, D.; Pedraza, A.M.; Vera, E.; Wang, Z.; Burns, D.K.; Parada, L.F. Cell-of-origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat. Neurosci. 2019, 22, 545–555. [Google Scholar] [CrossRef]
- Urbanska, K.; Sokolowska, J.; Szmidt, M.; Sysa, P. Glioblastoma multiforme—An overview. Contemp. Oncol. (Pozn) 2014, 18, 307–312. [Google Scholar] [CrossRef]
- Marfia, G.; Navone, S.E.; Fanizzi, C.; Tabano, S.; Pesenti, C.; Abdel Hadi, L.; Franzini, A.; Caroli, M.; Miozzo, M.; Riboni, L.; et al. Prognostic value of preoperative von Willebrand factor plasma levels in patients with Glioblastoma. Cancer Med. 2016, 5, 1783–1790. [Google Scholar] [CrossRef] [Green Version]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Wang, X.; Prager, B.C.; Wu, Q.; Kim, L.J.Y.; Gimple, R.C.; Shi, Y.; Yang, K.; Morton, A.R.; Zhou, W.; Zhu, Z.; et al. Reciprocal Signaling between Glioblastoma Stem Cells and Differentiated Tumor Cells Promotes Malignant Progression. Cell Stem. Cell 2018, 22, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Huang, Z.; Zhou, W.; Wu, Q.; Donnola, S.; Liu, J.K.; Fang, X.; Sloan, A.E.; Mao, Y.; Lathia, J.D.; et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013, 153, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Gong, T.; Heng, B.C.; Zhang, C.F. A systematic review: Differentiation of stem cells into functional pericytes. FASEB J. 2017, 31, 1775–1786. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.; Chen, Y.-S.; Chen, F.-R.; Xi, S.-Y.; Chen, Z.-P. Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro-Oncology 2017, 19, 1109–1118. [Google Scholar] [CrossRef]
- Beier, C.P.; Rasmussen, T.; Dahlrot, R.H.; Tenstad, H.B.; Aarø, J.S.; Sørensen, M.F.; Heimisdóttir, S.B.; Sørensen, M.D.; Svenningsen, P.; Riemenschneider, M.J.; et al. Aberrant neuronal differentiation is common in glioma but is associated neither with epileptic seizures nor with better survival. Sci. Rep. 2018, 8, 14965. [Google Scholar] [CrossRef] [PubMed]
- Bien-Moller, S.; Balz, E.; Herzog, S.; Plantera, L.; Vogelgesang, S.; Weitmann, K.; Seifert, C.; Fink, M.A.; Marx, S.; Bialke, A.; et al. Association of Glioblastoma Multiforme Stem Cell Characteristics, Differentiation, and Microglia Marker Genes with Patient Survival. Stem. Cells Int. 2018, 2018, 9628289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guelfi, S.; Duffau, H.; Bauchet, L.; Rothhut, B.; Hugnot, J.P. Vascular Transdifferentiation in the CNS: A Focus on Neural and Glioblastoma Stem-Like Cells. Stem. Cells Int. 2016, 2016, 2759403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnier, D.; Renoult, O.; Alves-Guerra, M.C.; Paris, F.; Pecqueur, C. Glioblastoma Stem-Like Cells, Metabolic Strategy to Kill a Challenging Target. Front. Oncol. 2019, 9, 118. [Google Scholar] [CrossRef]
- Das, S.; Marsden, P.A. Angiogenesis in glioblastoma. N. Engl. J. Med. 2013, 369, 1561–1563. [Google Scholar] [CrossRef] [Green Version]
- Kane, J.R. The Role of Brain Vasculature in Glioblastoma. Mol. Neurobiol. 2019, 56, 6645–6653. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef]
- Seano, G.; Jain, R.K. Vessel co-option in glioblastoma: Emerging insights and opportunities. Angiogenesis 2020, 23, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnem, T.; Hu, J.; Ferguson, M.; Adighibe, O.; Snell, C.; Harris, A.L.; Gatter, K.C.; Pezzella, F. Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Med. 2013, 2, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, G.J.; Yadav, V.N.; Motsch, S.; Koschmann, C.; Calinescu, A.A.; Mineharu, Y.; Camelo-Piragua, S.I.; Orringer, D.; Bannykh, S.; Nichols, W.S.; et al. Mechanisms of glioma formation: Iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia 2014, 16, 543–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegand, S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284, 1994–1998. [Google Scholar] [CrossRef] [Green Version]
- Sakariassen, P.; Prestegarden, L.; Wang, J.; Skaftnesmo, K.O.; Mahesparan, R.; Molthoff, C.; Sminia, P.; Sundlisaeter, E.; Misra, A.; Tysnes, B.B.; et al. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16466–16471. [Google Scholar] [CrossRef] [Green Version]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef] [Green Version]
- Ayuso, J.M.; Monge, R.; Martinez-Gonzalez, A.; Virumbrales-Munoz, M.; Llamazares, G.A.; Berganzo, J.; Hernandez-Lain, A.; Santolaria, J.; Doblare, M.; Hubert, C.; et al. Glioblastoma on a microfluidic chip: Generating pseudopalisades and enhancing aggressiveness through blood vessel obstruction events. Neuro-Oncology 2017, 19, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.; Durden, D.L.; Van Meir, E.G.; Brat, D.J. ‘Pseudopalisading’ necrosis in glioblastoma: A familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 529–539. [Google Scholar] [CrossRef]
- Brat, D.J.; Van Meir, E.G. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Investig. 2004, 84, 397–405. [Google Scholar] [CrossRef]
- Machein, M.R.; Renninger, S.; de Lima-Hahn, E.; Plate, K.H. Minor contribution of bone marrow-derived endothelial progenitors to the vascularization of murine gliomas. Brain Pathol. 2003, 13, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, Q.P.; Mu, Y.G.; Zhang, X.H.; Sai, K.; Pang, J.C.; Ng, H.K.; Chen, Z.P. Clinical significance of vasculogenic mimicry in human gliomas. J. Neurooncol. 2011, 105, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Ke, Y.Q.; Lu, G.H.; Song, Z.H.; Yu, L.; Xiao, S.; Sun, X.L.; Jiang, X.D.; Yang, Z.L.; Hu, C.C. Vasculogenic mimicry is a prognostic factor for postoperative survival in patients with glioblastoma. J. Neurooncol. 2013, 112, 339–345. [Google Scholar] [CrossRef]
- Baisiwala, S.; Auffinger, B.; Caragher, S.P.; Shireman, J.M.; Ahsan, R.; Lee, G.; Hasan, T.; Park, C.; Saathoff, M.R.; Christensen, A.C.; et al. Chemotherapeutic Stress Induces Transdifferentiation of Glioblastoma Cells to Endothelial Cells and Promotes Vascular Mimicry. Stem. Cells Int. 2019, 2019, 6107456. [Google Scholar] [CrossRef]
- Deshors, P.; Toulas, C.; Arnauduc, F.; Malric, L.; Siegfried, A.; Nicaise, Y.; Lemarie, A.; Larrieu, D.; Tosolini, M.; Cohen-Jonathan Moyal, E.; et al. Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway. Cell Death Dis. 2019, 10, 816. [Google Scholar] [CrossRef]
- Lu, K.V.; Bergers, G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol. 2013, 2, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Angara, K.; Borin, T.F.; Rashid, M.H.; Lebedyeva, I.; Ara, R.; Lin, P.C.; Iskander, A.; Bollag, R.J.; Achyut, B.R.; Arbab, A.S. CXCR2-Expressing Tumor Cells Drive Vascular Mimicry in Antiangiogenic Therapy-Resistant Glioblastoma. Neoplasia 2018, 20, 1070–1082. [Google Scholar] [CrossRef]
- Zamecnik, J. The extracellular space and matrix of gliomas. Acta Neuropathol. 2005, 110, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Lal, B.; Tung, B.; Wang, S.; Goodwin, C.R.; Laterra, J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro-Oncology 2016, 18, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Serres, E.; Debarbieux, F.; Stanchi, F.; Maggiorella, L.; Grall, D.; Turchi, L.; Burel-Vandenbos, F.; Figarella-Branger, D.; Virolle, T.; Rougon, G.; et al. Fibronectin expression in glioblastomas promotes cell cohesion, collective invasion of basement membrane in vitro and orthotopic tumor growth in mice. Oncogene 2014, 33, 3451–3462. [Google Scholar] [CrossRef]
- Chen, J.E.; Pedron, S.; Shyu, P.; Hu, Y.; Sarkaria, J.N.; Harley, B.A.C. Influence of Hyaluronic Acid Transitions in Tumor Microenvironment on Glioblastoma Malignancy and Invasive Behavior. Front. Mater. 2018, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, J.; Schwab, E.I.; Sperveslage, J.; Tatagiba, M.; Meyermann, R.; Fend, F.; Goodman, S.L.; Sipos, B. Longitudinal expression analysis of alphav integrins in human gliomas reveals upregulation of integrin alphavbeta3 as a negative prognostic factor. J. Neuropathol. Exp. Neurol. 2013, 72, 194–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wang, T.; Liu, S.; Yoshida, D.; Teramoto, A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor. Pathol. 2003, 20, 65–72. [Google Scholar] [CrossRef]
- Roth, P.; Silginer, M.; Goodman, S.L.; Hasenbach, K.; Thies, S.; Maurer, G.; Schraml, P.; Tabatabai, G.; Moch, H.; Tritschler, I.; et al. Integrin control of the transforming growth factor-beta pathway in glioblastoma. Brain 2013, 136, 564–576. [Google Scholar] [CrossRef] [Green Version]
- Malric, L.; Monferran, S.; Gilhodes, J.; Boyrie, S.; Dahan, P.; Skuli, N.; Sesen, J.; Filleron, T.; Kowalski-Chauvel, A.; Cohen-Jonathan Moyal, E.; et al. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: An update. Oncotarget 2017, 8, 86947–86968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janouskova, H.; Maglott, A.; Leger, D.Y.; Bossert, C.; Noulet, F.; Guerin, E.; Guenot, D.; Pinel, S.; Chastagner, P.; Plenat, F.; et al. Integrin alpha5beta1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012, 72, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Hallal, S.; Mallawaaratchy, D.M.; Wei, H.; Ebrahimkhani, S.; Stringer, B.W.; Day, B.W.; Boyd, A.W.; Guillemin, G.J.; Buckland, M.E.; Kaufman, K.L. Extracellular Vesicles Released by Glioblastoma Cells Stimulate Normal Astrocytes to Acquire a Tumor-Supportive Phenotype Via p53 and MYC Signaling Pathways. Mol. Neurobiol. 2019, 56, 4566–4581. [Google Scholar] [CrossRef] [Green Version]
- Abels, E.R.; Maas, S.L.N.; Nieland, L.; Wei, Z.; Cheah, P.S.; Tai, E.; Kolsteeg, C.J.; Dusoswa, S.A.; Ting, D.T.; Hickman, S.; et al. Glioblastoma-Associated Microglia Reprogramming Is Mediated by Functional Transfer of Extracellular miR-21. Cell Rep. 2019, 28, 3105–3119. [Google Scholar] [CrossRef] [Green Version]
- Treps, L.; Perret, R.; Edmond, S.; Ricard, D.; Gavard, J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J. Extracell Vesicles 2017, 6, 1359479. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.; Wang, J.; Zhao, Y.; Wang, Y.; Bihl, J.C.; Chen, Y.; Jiang, C. Glioma stem cells-derived exosomes promote the angiogenic ability of endothelial cells through miR-21/VEGF signal. Oncotarget 2017, 8, 36137–36148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.F.; Liao, F.; Wu, H.; Dai, J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. J. Exp. Clin. Cancer Res. 2019, 38, 201. [Google Scholar] [CrossRef] [Green Version]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringner, M.; Morgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef] [Green Version]
- Virga, J.; Szivos, L.; Hortobagyi, T.; Chalsaraei, M.K.; Zahuczky, G.; Steiner, L.; Toth, J.; Remenyi-Puskar, J.; Bognar, L.; Klekner, A. Extracellular matrix differences in glioblastoma patients with different prognoses. Oncol. Lett. 2019, 17, 797–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [Green Version]
- Park, A.K.; Kim, P.; Ballester, L.Y.; Esquenazi, Y.; Zhao, Z. Subtype-specific signaling pathways and genomic aberrations associated with prognosis of glioblastoma. Neuro-Oncology 2019, 21, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, M.; Proschel, C.; Mayer-Proschel, M. Getting a GR(i)P on oligodendrocyte development. Dev. Biol. 2004, 265, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Behnan, J.; Finocchiaro, G.; Hanna, G. The landscape of the mesenchymal signature in brain tumours. Brain 2019, 142, 847–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef] [Green Version]
- Dick, F.A.; Goodrich, D.W.; Sage, J.; Dyson, N.J. Non-canonical functions of the RB protein in cancer. Nat. Rev. Cancer 2018, 18, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.R.D.; Regad, T. Targeting cellular pathways in glioblastoma multiforme. Signal. Transduct Target. Ther. 2017, 2, 17040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clin. Cancer Res. 2018, 24, 3792–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8(+)CD28(-) Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019, 20, 2810. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mo, W.; Ye, J.; Li, L.; Zhang, Y.; Hsueh, E.C.; Hoft, D.F.; Peng, G. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat. Commun. 2018, 9, 249. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Balyasnikova, I.V.; Chang, A.L.; Ahmed, A.U.; Moon, K.S.; Auffinger, B.; Tobias, A.L.; Han, Y.; Lesniak, M.S. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 2012, 18, 6110–6121. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.F.; Kong, L.Y.; Jordan, J.; Conrad, C.; Madden, T.; Fokt, I.; Priebe, W.; Heimberger, A.B. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007, 67, 9630–9636. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Huang, J.; Liu, X.; Cheng, Q.; Luo, C.; Liu, Z. CTLA-4 correlates with immune and clinical characteristics of glioma. Cancer Cell Int. 2020, 20, 7. [Google Scholar] [CrossRef]
- Davidson, T.B.; Lee, A.; Hsu, M.; Sedighim, S.; Orpilla, J.; Treger, J.; Mastall, M.; Roesch, S.; Rapp, C.; Galvez, M.; et al. Expression of PD-1 by T Cells in Malignant Glioma Patients Reflects Exhaustion and Activation. Clin. Cancer Res. 2019, 25, 1913–1922. [Google Scholar] [CrossRef]
- Litak, J.; Mazurek, M.; Grochowski, C.; Kamieniak, P.; Rolinski, J. PD-L1/PD-1 Axis in Glioblastoma Multiforme. Int. J. Mol. Sci. 2019, 20, 5347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostofa, A.G.; Punganuru, S.R.; Madala, H.R.; Al-Obaide, M.; Srivenugopal, K.S. The Process and Regulatory Components of Inflammation in Brain Oncogenesis. Biomolecules 2017, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nduom, E.K.; Weller, M.; Heimberger, A.B. Immunosuppressive mechanisms in glioblastoma. Neuro-Oncology 2015, 17 (Suppl. 7), vii9–vii14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, C.A.; Ahn, B.J.; Han, S.J.; Parsa, A.T. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: Implications for immunotherapy. Neuro-Oncology 2012, 14, 584–595. [Google Scholar] [CrossRef]
- Qi, L.; Yu, H.; Zhang, Y.; Zhao, D.; Lv, P.; Zhong, Y.; Xu, Y. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget 2016, 7, 71673–71685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Kuang, W.; Zhou, Q.; Zhang, Y. TGF-beta1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int. J. Mol. Med. 2018, 42, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, L.; Masetto, E.; Vettore, M.; Solito, S.; Magri, S.; D’Andolfi, M.; Del Bianco, P.; Lollo, G.; Benoit, J.-P.; Okada, H.; et al. The immune suppressive microenvironment of human gliomas depends on the accumulation of bone marrow-derived macrophages in the center of the lesion. J. Immun.Ther. Cancer 2019, 7, 58. [Google Scholar] [CrossRef]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Paolillo, M.; Boselli, C.; Schinelli, S. Glioblastoma under Siege: An Overview of Current Therapeutic Strategies. Brain Sci. 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Jiapaer, S.; Furuta, T.; Tanaka, S.; Kitabayashi, T.; Nakada, M. Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma. Neurol. Med. Chir. (Tokyo) 2018, 58, 405–421. [Google Scholar] [CrossRef] [Green Version]
- Rosen, L.S.; Jacobs, I.A.; Burkes, R.L. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target. Oncol. 2017, 12, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.E.; Priolo, D.; Antonelli, G.; Libra, M.; McCubrey, J.A.; Ferrau, F. Bevacizumab in the treatment of NSCLC: Patient selection and perspectives. Lung Cancer (Auckl.) 2017, 8, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinzel, A.; Ambrogi, M.; Varshaver, M.; Kirson, E.D. Tumor Treating Fields for Glioblastoma Treatment: Patient Satisfaction and Compliance With the Second-Generation Optune((R)) System. Clin. Med. Insights Oncol. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Fabian, D.; Guillermo Prieto Eibl, M.D.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Burri, S.H.; Gondi, V.; Brown, P.D.; Mehta, M.P. The Evolving Role of Tumor Treating Fields in Managing Glioblastoma: Guide for Oncologists. Am. J. Clin. Oncol. 2018, 41, 191–196. [Google Scholar] [CrossRef]
- Bokstein, F.; Blumenthal, D.; Limon, D.; Harosh, C.B.; Ram, Z.; Grossman, R. Concurrent Tumor Treating Fields (TTFields) and Radiation Therapy for Newly Diagnosed Glioblastoma: A Prospective Safety and Feasibility Study. Front. Oncol. 2020, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, J.P.; Nahed, B.V.; Madden, M.W.; Oliveira, S.M.; Springer, S.; Bhere, D.; Chi, A.S.; Wakimoto, H.; Rothenberg, S.M.; Sequist, L.V.; et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014, 4, 1299–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.D.; Chow, D.S.; Yang, P.H.; Filippi, C.G.; Lignelli, A. Predicting Glioblastoma Recurrence by Early Changes in the Apparent Diffusion Coefficient Value and Signal Intensity on FLAIR Images. AJR Am. J. Roentgenol. 2017, 208, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Muscat, A.M.; Wong, N.C.; Drummond, K.J.; Algar, E.M.; Khasraw, M.; Verhaak, R.; Field, K.; Rosenthal, M.A.; Ashley, D.M. The evolutionary pattern of mutations in glioblastoma reveals therapy-mediated selection. Oncotarget 2018, 9, 7844–7858. [Google Scholar] [CrossRef]
- Razavi, S.M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune Evasion Strategies of Glioblastoma. Front. Surg. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Perng, P.; Lim, M. Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Front. Oncol. 2015, 5, 153. [Google Scholar] [CrossRef] [Green Version]
- Preusser, M.; Lim, M.; Hafler, D.A.; Reardon, D.A.; Sampson, J.H. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat. Rev. Neurol. 2015, 11, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffart, N.; Lombard, A.; Lallemand, F.; Kroonen, J.; Nassen, J.; Di Valentin, E.; Berendsen, S.; Dedobbeleer, M.; Willems, E.; Robe, P.; et al. CXCL12 mediates glioblastoma resistance to radiotherapy in the subventricular zone. Neuro-Oncology 2017, 19, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventero, M.P.; Fuentes-Baile, M.; Quereda, C.; Perez-Valeciano, E.; Alenda, C.; Garcia-Morales, P.; Esposito, D.; Dorado, P.; Manuel Barbera, V.; Saceda, M. Radiotherapy resistance acquisition in Glioblastoma. Role of SOCS1 and SOCS3. PLoS ONE 2019, 14, e0212581. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Piao, Y.; Liang, J.; Holmes, L.; Zurita, A.J.; Henry, V.; Heymach, J.V.; de Groot, J.F. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro-Oncology 2012, 14, 1379–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beier, D.; Rohrl, S.; Pillai, D.R.; Schwarz, S.; Kunz-Schughart, L.A.; Leukel, P.; Proescholdt, M.; Brawanski, A.; Bogdahn, U.; Trampe-Kieslich, A.; et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008, 68, 5706–5715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clement, V.; Sanchez, P.; de Tribolet, N.; Radovanovic, I.; Ruiz i Altaba, A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007, 17, 165–172. [Google Scholar] [CrossRef]
- Tezcan, G.; Tunca, B.; Bekar, A.; Preusser, M.; Berghoff, A.S.; Egeli, U.; Cecener, G.; Ricken, G.; Budak, F.; Taskapilioglu, M.O.; et al. microRNA expression pattern modulates temozolomide response in GBM tumors with cancer stem cells. Cell Mol. Neurobiol. 2014, 34, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.T.; Zhang, X.Q.; Zhuang, J.T.; Chan, H.L.; Li, C.H.; Leung, G.K. MicroRNA-21 inhibition enhances in vitro chemosensitivity of temozolomide-resistant glioblastoma cells. Anticancer Res. 2012, 32, 2835–2841. [Google Scholar]
- Liau, B.B.; Sievers, C.; Donohue, L.K.; Gillespie, S.M.; Flavahan, W.A.; Miller, T.E.; Venteicher, A.S.; Hebert, C.H.; Carey, C.D.; Rodig, S.J.; et al. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell 2017, 20, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codony-Servat, J.; Rosell, R. Cancer stem cells and immunoresistance: Clinical implications and solutions. Transl. Lung Cancer Res. 2015, 4, 689–703. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Curing glioblastoma: Oncolytic HSV-IL12 and checkpoint blockade. Oncoscience 2017, 4, 67–69. [Google Scholar] [CrossRef]
- Fadul, C.E.; Fisher, J.L.; Gui, J.; Hampton, T.H.; Cote, A.L.; Ernstoff, M.S. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro-Oncology 2011, 13, 393–400. [Google Scholar] [CrossRef]
- Wang, S.; Yao, F.; Lu, X.; Li, Q.; Su, Z.; Lee, J.H.; Wang, C.; Du, L. Temozolomide promotes immune escape of GBM cells via upregulating PD-L1. Am. J. Cancer Res. 2019, 9, 1161–1171. [Google Scholar] [PubMed]
- Cenciarini, M.; Valentino, M.; Belia, S.; Sforna, L.; Rosa, P.; Ronchetti, S.; D’Adamo, M.C.; Pessia, M. Dexamethasone in Glioblastoma Multiforme Therapy: Mechanisms and Controversies. Front. Mol. Neurosci. 2019, 12, 65. [Google Scholar] [CrossRef]
- Canon, J.; Osgood, T.; Olson, S.H.; Saiki, A.Y.; Robertson, R.; Yu, D.; Eksterowicz, J.; Ye, Q.; Jin, L.; Chen, A.; et al. The MDM2 Inhibitor AMG 232 Demonstrates Robust Antitumor Efficacy and Potentiates the Activity of p53-Inducing Cytotoxic Agents. Mol. Cancer Ther. 2015, 14, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Schulz-Heddergott, R.; Moll, U.M. Gain-of-Function (GOF) Mutant p53 as Actionable Therapeutic Target. Cancers 2018, 10, 188. [Google Scholar] [CrossRef] [Green Version]
- Walerych, D.; Lisek, K.; Del Sal, G. Mutant p53: One, No One, and One Hundred Thousand. Front. Oncol 2015, 5, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patyka, M.; Sharifi, Z.; Petrecca, K.; Mansure, J.; Jean-Claude, B.; Sabri, S. Sensitivity to PRIMA-1MET is associated with decreased MGMT in human glioblastoma cells and glioblastoma stem cells irrespective of p53 status. Oncotarget 2016, 7, 60245–60269. [Google Scholar] [CrossRef] [Green Version]
- Perdrix, A.; Najem, A.; Saussez, S.; Awada, A.; Journe, F.; Ghanem, G.; Krayem, M. PRIMA-1 and PRIMA-1(Met) (APR-246): From Mutant/Wild Type p53 Reactivation to Unexpected Mechanisms Underlying Their Potent Anti-Tumor Effect in Combinatorial Therapies. Cancers 2017, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Zache, N.; Lambert, J.M.; Wiman, K.G.; Bykov, V.J. PRIMA-1MET inhibits growth of mouse tumors carrying mutant p53. Cell. Oncol. 2008, 30, 411–418. [Google Scholar] [CrossRef]
- Pal, S.; Kozono, D.; Yang, X.; Fendler, W.; Fitts, W.; Ni, J.; Alberta, J.A.; Zhao, J.; Liu, K.X.; Bian, J.; et al. Dual HDAC and PI3K Inhibition Abrogates NFkappaB- and FOXM1-Mediated DNA Damage Response to Radiosensitize Pediatric High-Grade Gliomas. Cancer Res. 2018, 78, 4007–4021. [Google Scholar] [CrossRef] [Green Version]
- Staberg, M.; Michaelsen, S.R.; Rasmussen, R.D.; Villingshoj, M.; Poulsen, H.S.; Hamerlik, P. Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell Oncol. (Dordr.) 2017, 40, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Liffers, K.; Kolbe, K.; Westphal, M.; Lamszus, K.; Schulte, A. Histone Deacetylase Inhibitors Resensitize EGFR/EGFRvIII-Overexpressing, Erlotinib-Resistant Glioblastoma Cells to Tyrosine Kinase Inhibition. Target. Oncol. 2016, 11, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.M.; Johnson, B.; Venkatarayan, A.; Flores, E.R.; Zhang, J.; Su, X.; Barton, M.; Lang, F.; Chandra, J. Preclinical activity of combined HDAC and KDM1A inhibition in glioblastoma. Neuro-Oncology 2015, 17, 1463–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.; Murphy, B.; Miller, R.; Menon, V.; Banik, N.L.; Giglio, P.; Lindhorst, S.M.; Varma, A.K.; Vandergrift, W.A., 3rd; Patel, S.J.; et al. Mechanisms and clinical significance of histone deacetylase inhibitors: Epigenetic glioblastoma therapy. Anticancer Res. 2015, 35, 615–625. [Google Scholar] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.; Madani, D.; Joshi, S.; Chung, S.A.; Johns, T.; Day, B.; Khasraw, M.; McDonald, K.L. Combination of palbociclib and radiotherapy for glioblastoma. Cell Death Discov. 2017, 3, 17033. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Parikh, M.; Phillips, J.J.; James, C.D.; Molinaro, A.M.; Butowski, N.A.; Clarke, J.L.; Oberheim-Bush, N.A.; Chang, S.M.; Berger, M.S.; et al. Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J. Neurooncol. 2018, 140, 477–483. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Olmez, I.; Brenneman, B.; Xiao, A.; Serbulea, V.; Benamar, M.; Zhang, Y.; Manigat, L.; Abbas, T.; Lee, J.; Nakano, I.; et al. Combined CDK4/6 and mTOR Inhibition Is Synergistic against Glioblastoma via Multiple Mechanisms. Clin. Cancer Res. 2017, 23, 6958–6968. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liang, R.; Song, C.; Xiang, Y.; Liu, Y. Prognostic significance of epidermal growth factor receptor expression in glioma patients. Onco Targets Ther. 2018, 11, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Furnari, F.B.; Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 2015, 15, 302–310. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Eskilsson, E.; Rosland, G.V.; Solecki, G.; Wang, Q.; Harter, P.N.; Graziani, G.; Verhaak, R.G.W.; Winkler, F.; Bjerkvig, R.; Miletic, H. EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro-Oncology 2018, 20, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Kwatra, M.M. A Rational Approach to Target the Epidermal Growth Factor Receptor in Glioblastoma. Curr. Cancer Drug Targets 2017, 17, 290–296. [Google Scholar] [CrossRef]
- Kim, M.; Laramy, J.K.; Mohammad, A.S.; Talele, S.; Fisher, J.; Sarkaria, J.N.; Elmquist, W.F. Brain Distribution of a Panel of Epidermal Growth Factor Receptor Inhibitors Using Cassette Dosing in Wild-Type and Abcb1/Abcg2-Deficient Mice. Drug Metab. Dispos. 2019, 47, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, X.; Shi, L.; Shan, Q.; Cao, Q.; Yue, C.; Li, H.; Li, S.; Wang, J.; Gao, S.; et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 219. [Google Scholar] [CrossRef]
- Li, L.; Quang, T.S.; Gracely, E.J.; Kim, J.H.; Emrich, J.G.; Yaeger, T.E.; Jenrette, J.M.; Cohen, S.C.; Black, P.; Brady, L.W. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 2010, 113, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Achenbach, C.; Silginer, M.; Blot, V.; Weiss, W.A.; Weller, M. Depatuxizumab Mafodotin (ABT-414)-induced Glioblastoma Cell Death Requires EGFR Overexpression, but not EGFR(Y1068) Phosphorylation. Mol. Cancer Ther. 2020, 19, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.F.; Wang, J.; Shao, W.; Wu, C.P.; Chen, Z.P.; To, S.T.; Li, W.P. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: Current preclinical and clinical development. Mol. Cancer 2017, 16, 100. [Google Scholar] [CrossRef] [Green Version]
- Djuzenova, C.S.; Fiedler, V.; Memmel, S.; Katzer, A.; Sisario, D.; Brosch, P.K.; Gohrung, A.; Frister, S.; Zimmermann, H.; Flentje, M.; et al. Differential effects of the Akt inhibitor MK-2206 on migration and radiation sensitivity of glioblastoma cells. BMC Cancer 2019, 19, 299. [Google Scholar] [CrossRef] [PubMed]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Targeting mTOR in Glioblastoma: Rationale and Preclinical/Clinical Evidence. Dis. Markers 2018, 2018, 9230479. [Google Scholar] [CrossRef] [Green Version]
- Daniele, S.; Costa, B.; Zappelli, E.; Da Pozzo, E.; Sestito, S.; Nesi, G.; Campiglia, P.; Marinelli, L.; Novellino, E.; Rapposelli, S.; et al. Combined inhibition of AKT/mTOR and MDM2 enhances Glioblastoma Multiforme cell apoptosis and differentiation of cancer stem cells. Sci. Rep. 2015, 5, 9956. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Ciotti, G.M.P.; Chiavari, M.; Pizzoferrato, M.; Mangiola, A.; Kalinin, S.; Feinstein, D.L.; Navarra, P. Phospho-mTOR expression in human glioblastoma microglia-macrophage cells. Neurochem. Int. 2019, 129, 104485. [Google Scholar] [CrossRef]
- Geissler, E.K. The influence of mTOR inhibitors on immunity and the relationship to post-transplant malignancy. Transplant. Res. 2013, 2, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bak, S.; Tischer, S.; Dragon, A.; Ravens, S.; Pape, L.; Koenecke, C.; Oelke, M.; Blasczyk, R.; Maecker-Kolhoff, B.; Eiz-Vesper, B. Selective Effects of mTOR Inhibitor Sirolimus on Naive and CMV-Specific T Cells Extending Its Applicable Range Beyond Immunosuppression. Front. Immunol. 2018, 9, 2953. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef]
- Petterson, S.A.; Dahlrot, R.H.; Hermansen, S.K.; Sune, K.A.M.; Gundesen, M.T.; Wohlleben, H.; Rasmussen, T.; Beier, C.P.; Hansen, S.; Kristensen, B.W. High levels of c-Met is associated with poor prognosis in glioblastoma. J. Neurooncol. 2015, 122, 517–527. [Google Scholar] [CrossRef]
- Kong, D.S.; Song, S.Y.; Kim, D.H.; Joo, K.M.; Yoo, J.S.; Koh, J.S.; Dong, S.M.; Suh, Y.L.; Lee, J.I.; Park, K.; et al. Prognostic significance of c-Met expression in glioblastomas. Cancer 2009, 115, 140–148. [Google Scholar] [CrossRef]
- Cloughesy, T.; Finocchiaro, G.; Belda-Iniesta, C.; Recht, L.; Brandes, A.A.; Pineda, E.; Mikkelsen, T.; Chinot, O.L.; Balana, C.; Macdonald, D.R.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O(6)-Methylguanine-DNA Methyltransferase Biomarker Analyses. J. Clin. Oncol. 2017, 35, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducassou, A.; Uro-Coste, E.; Verrelle, P.; Filleron, T.; Benouaich-Amiel, A.; Lubrano, V.; Sol, J.C.; Delisle, M.B.; Favre, G.; Ken, S.; et al. alphavbeta3 Integrin and Fibroblast growth factor receptor 1 (FGFR1): Prognostic factors in a phase I-II clinical trial associating continuous administration of Tipifarnib with radiotherapy for patients with newly diagnosed glioblastoma. Eur. J. Cancer 2013, 49, 2161–2169. [Google Scholar] [CrossRef]
- Day, E.K.; Sosale, N.G.; Xiao, A.; Zhong, Q.; Purow, B.; Lazzara, M.J. Glioblastoma Cell Resistance to EGFR and MET Inhibition Can Be Overcome via Blockade of FGFR-SPRY2 Bypass Signaling. Cell Rep. 2020, 30, 3383–3396. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.; Storkebaum, E.; de Almodovar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef]
- Xiao, Q.; Yang, S.; Ding, G.; Luo, M. Anti-vascular endothelial growth factor in glioblastoma: A systematic review and meta-analysis. Neurol. Sci. 2018, 39, 2021–2031. [Google Scholar] [CrossRef]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Nazarenko, I.; Hede, S.M.; He, X.; Hedren, A.; Thompson, J.; Lindstrom, M.S.; Nister, M. PDGF and PDGF receptors in glioma. Ups. J. Med. Sci. 2012, 117, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y. Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol. Med. 2013, 19, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Cantanhede, I.G.; de Oliveira, J.R.M. PDGF Family Expression in Glioblastoma Multiforme: Data Compilation from Ivy Glioblastoma Atlas Project Database. Sci. Rep. 2017, 7, 15271. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Yuan, Y.; Lin, Y.; Wang, Y.X.; Zhou, J.; Gai, Q.J.; Zhang, L.; Mao, M.; Yao, X.X.; Qin, Y.; et al. ERBB3, IGF1R, and TGFBR2 expression correlate with PDGFR expression in glioblastoma and participate in PDGFR inhibitor resistance of glioblastoma cells. Am. J. Cancer Res. 2018, 8, 792–809. [Google Scholar] [PubMed]
- Pastorino, S.; Langley, E.J.; Chao, Y.; Jiang, P.; Mukthavaram, R.; Pingle, S.C.; Kim, P.S.; Singh, S.; Kesari, S. Mechanisms of resistance to PDGFR inhibition in glioblastoma. J. Clin. Oncol. 2014, 32, e13030. [Google Scholar] [CrossRef]
- Roy, L.O.; Poirier, M.B.; Fortin, D. Differential Expression and Clinical Significance of Transforming Growth Factor-Beta Isoforms in GBM Tumors. Int. J. Mol. Sci. 2018, 19, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, K. Signaling Cross Talk between TGF-beta/Smad and Other Signaling Pathways. Cold Spring Harb. Perspect Biol. 2017, 9, a022137. [Google Scholar] [CrossRef] [Green Version]
- Wick, A.; Desjardins, A.; Suarez, C.; Forsyth, P.; Gueorguieva, I.; Burkholder, T.; Cleverly, A.L.; Estrem, S.T.; Wang, S.; Lahn, M.M.; et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Investig. New Drugs 2020. [Google Scholar] [CrossRef] [Green Version]
- Areeb, Z.; Stylli, S.S.; Ware, T.M.; Harris, N.C.; Shukla, L.; Shayan, R.; Paradiso, L.; Li, B.; Morokoff, A.P.; Kaye, A.H.; et al. Inhibition of glioblastoma cell proliferation, migration and invasion by the proteasome antagonist carfilzomib. Med. Oncol. 2016, 33, 53. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Voutsadakis, I.A.; Papandreou, C.N. The shaping of invasive glioma phenotype by the ubiquitin-proteasome system. Cell Commun. Adhes. 2013, 20, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Rajendra, J.; Datta, K.K.; Ud Din Farooqee, S.B.; Thorat, R.; Kumar, K.; Gardi, N.; Kaur, E.; Nair, J.; Salunkhe, S.; Patkar, K.; et al. Enhanced proteasomal activity is essential for long term survival and recurrence of innately radiation resistant residual glioblastoma cells. Oncotarget 2018, 9, 27667–27681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, P.; Mason, W.P.; Richardson, P.G.; Weller, M. Proteasome inhibition for the treatment of glioblastoma. Expert Opin. Investig Drugs 2020, 29, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, P.; Lequesne, J.; Grellard, J.M.; Dugue, A.; Coquan, E.; Brachet, P.E.; Geffrelot, J.; Kao, W.; Emery, E.; Berro, D.H.; et al. Phase I/IIa study of concomitant radiotherapy with olaparib and temozolomide in unresectable or partially resectable glioblastoma: OLA-TMZ-RTE-01 trial protocol. BMC Cancer 2019, 19, 198. [Google Scholar] [CrossRef] [PubMed]
- Ferri, A.; Stagni, V.; Barila, D. Targeting the DNA Damage Response to Overcome Cancer Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 4910. [Google Scholar] [CrossRef]
- Frosina, G.; Marubbi, D.; Marcello, D.; Vecchio, D.; Daga, A. The efficacy and toxicity of ATM inhibition in glioblastoma initiating cells-driven tumor models. Crit. Rev. Oncol. Hematol. 2019, 138, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Fisusi, F.A.; Schatzlein, A.G.; Uchegbu, I.F. Nanomedicines in the treatment of brain tumors. Nanomedicine (Lond.) 2018, 13, 579–583. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma. Immunotherapy 2018, 10, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, D.; Dietrich, P.Y.; Stupp, R.; Linette, G.P.; Posey, A.D., Jr.; June, C.H. CAR T-Cell Therapies in Glioblastoma: A First Look. Clin. Cancer Res. 2018, 24, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Kong, Z.; Wang, Y.; Ma, W. Vaccination in the immunotherapy of glioblastoma. Hum. Vaccin. Immunother. 2018, 14, 255–268. [Google Scholar] [CrossRef]
- Caccese, M.; Indraccolo, S.; Zagonel, V.; Lombardi, G. PD-1/PD-L1 immune-checkpoint inhibitors in glioblastoma: A concise review. Crit. Rev. Oncol. Hematol. 2019, 135, 128–134. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Patel, M.A.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 2017, 23, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.Y.; Zhan, Y.P.; Zong, W.J.; Yu, C.J.; Li, J.F.; Qu, Y.M.; Han, S. The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS ONE 2015, 10, e0134715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, N.; Talat, H.; Alonso, A.; Saha, D.; Curry, W.T. Triple combination immunotherapy with GVAX, anti-PD-1 monoclonal antibody, and agonist anti-OX40 monoclonal antibody is highly effective against murine intracranial glioma. Oncoimmunology 2019, 8, e1577108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filley, A.C.; Henriquez, M.; Dey, M. Recurrent glioma clinical trial, CheckMate-143: The game is not over yet. Oncotarget 2017, 8, 91779–91794. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chen, A.X.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019, 25, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Hu, P.; Liu, Q.; Deng, G.; Zhang, J.; Liang, N.; Xie, J.; Zhang, J. The prognostic value of cytotoxic T-lymphocyte antigen 4 in cancers: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 42913. [Google Scholar] [CrossRef]
- Grauer, O.M.; Nierkens, S.; Bennink, E.; Toonen, L.W.; Boon, L.; Wesseling, P.; Sutmuller, R.P.; Adema, G.J. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int. J. Cancer 2007, 121, 95–105. [Google Scholar] [CrossRef]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Vom Berg, J.; Vrohlings, M.; Haller, S.; Haimovici, A.; Kulig, P.; Sledzinska, A.; Weller, M.; Becher, B. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J. Exp. Med. 2013, 210, 2803–2811. [Google Scholar] [CrossRef] [Green Version]
- Carter, T.; Shaw, H.; Cohn-Brown, D.; Chester, K.; Mulholland, P. Ipilimumab and Bevacizumab in Glioblastoma. Clin. Oncol. (R. Coll. Radiol.) 2016, 28, 622–626. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, D.A.; Dey, M.; Chang, A.; Lesniak, M.S. Targeting Tregs in Malignant Brain Cancer: Overcoming IDO. Front. Immunol. 2013, 4, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordillo, P.P.; Sordillo, L.A.; Helson, L. The Kynurenine Pathway: A Primary Resistance Mechanism in Patients with Glioblastoma. Anticancer Res. 2017, 37, 2159–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Lauing, K.L.; Chang, A.L.; Dey, M.; Qian, J.; Cheng, Y.; Lesniak, M.S.; Wainwright, D.A. The role of IDO in brain tumor immunotherapy. J. Neurooncol. 2015, 123, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.; Barakeh, D.; Foshay, K.M. Immunogenetics of glioblastoma: The future of personalized patient management. NPJ Precis Oncol. 2018, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Ladomersky, E.; Lauing, K.L.; Wu, M.; Genet, M.; Gritsina, G.; Gyorffy, B.; Brastianos, P.K.; Binder, D.C.; Sosman, J.A.; et al. Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clin. Cancer Res. 2017, 23, 6650–6660. [Google Scholar] [CrossRef] [Green Version]
- Mitsuka, K.; Kawataki, T.; Satoh, E.; Asahara, T.; Horikoshi, T.; Kinouchi, H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery 2013, 72, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef] [Green Version]
- Ladomersky, E.; Zhai, L.; Lenzen, A.; Lauing, K.L.; Qian, J.; Scholtens, D.M.; Gritsina, G.; Sun, X.; Liu, Y.; Yu, F.; et al. IDO1 Inhibition Synergizes with Radiation and PD-1 Blockade to Durably Increase Survival Against Advanced Glioblastoma. Clin. Cancer Res. 2018, 24, 2559–2573. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bolduc, A.R.; Hoda, M.N.; Gamble, D.N.; Dolisca, S.B.; Bolduc, A.K.; Hoang, K.; Ashley, C.; McCall, D.; Rojiani, A.M.; et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J. Immunother. Cancer 2014, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Eissa, I.R.; Bustos-Villalobos, I.; Ichinose, T.; Matsumura, S.; Naoe, Y.; Miyajima, N.; Morimoto, D.; Mukoyama, N.; Zhiwen, W.; Tanaka, M.; et al. The Current Status and Future Prospects of Oncolytic Viruses in Clinical Trials against Melanoma, Glioma, Pancreatic, and Breast Cancers. Cancers 2018, 10, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’bryan, S.M.; Mathis, J.M. Oncolytic Virotherapy for Breast Cancer Treatment. Curr. Gene Ther. 2018, 18, 192–205. [Google Scholar] [CrossRef]
- Ghouse, S.M.; Nguyen, H.M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bommareddy, P.K.; Peters, C.; Saha, D.; Rabkin, S.D.; Kaufman, H.L. Oncolytic Herpes Simplex Viruses as a Paradigm for the Treatment of Cancer. Annu. Rev. Cancer Biol. 2018, 2, 155–173. [Google Scholar] [CrossRef]
- Peters, C.; Paget, M.; Tshilenge, K.T.; Saha, D.; Antoszczyk, S.; Baars, A.; Frost, T.; Martuza, R.L.; Wakimoto, H.; Rabkin, S.D. Restriction of Replication of Oncolytic Herpes Simplex Virus with a Deletion of gamma34.5 in Glioblastoma Stem-Like Cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, D.; Ahmed, S.S.; Rabkin, S.D. Exploring the Antitumor Effect of Virus in Malignant Glioma. Drugs Future 2015, 40, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Martuza, R.L.; Curry, W.T. Viral oncolysis of glioblastoma. In Neurotropic Viral Infections, 2nd ed.; Reiss, C.S., Ed.; Springer: New York, NY, USA, 2016; Volume 2, pp. 481–517. [Google Scholar]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Rabkin, S.D.; Martuza, R.L. Temozolomide antagonizes oncolytic immunovirotherapy in glioblastoma. J. Immunother. Cancer 2020, 8, e000345. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Wakimoto, H.; Peters, C.W.; Antoszczyk, S.J.; Rabkin, S.D.; Martuza, R.L. Combinatorial Effects of VEGFR Kinase Inhibitor Axitinib and Oncolytic Virotherapy in Mouse and Human Glioblastoma Stem-Like Cell Models. Clin. Cancer Res. 2018, 24, 3409–3422. [Google Scholar] [CrossRef] [Green Version]
- Siurala, M.; Havunen, R.; Saha, D.; Lumen, D.; Airaksinen, A.J.; Tahtinen, S.; Cervera-Carrascon, V.; Bramante, S.; Parviainen, S.; Vaha-Koskela, M.; et al. Adenoviral Delivery of Tumor Necrosis Factor-alpha and Interleukin-2 Enables Successful Adoptive Cell Therapy of Immunosuppressive Melanoma. Mol. Ther. 2016, 24, 1435–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahtinen, S.; Blattner, C.; Vaha-Koskela, M.; Saha, D.; Siurala, M.; Parviainen, S.; Utikal, J.; Kanerva, A.; Umansky, V.; Hemminki, A. T-Cell Therapy Enabling Adenoviruses Coding for IL2 and TNFalpha Induce Systemic Immunomodulation in Mice With Spontaneous Melanoma. J. Immunother. 2016, 39, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Vaha-Koskela, M.; Tahtinen, S.; Gronberg-Vaha-Koskela, S.; Taipale, K.; Saha, D.; Merisalo-Soikkeli, M.; Ahonen, M.; Rouvinen-Lagerstrom, N.; Hirvinen, M.; Veckman, V.; et al. Overcoming tumor resistance by heterologous adeno-poxvirus combination therapy. Mol. Ther. Oncol. 2015, 1, 14006. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Wakimoto, H.; Rabkin, S.D. Oncolytic herpes simplex virus interactions with the host immune system. Curr. Opin. Virol. 2016, 21, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Rabkin, S.D. Immunohistochemistry for Tumor-Infiltrating Immune Cells After Oncolytic Virotherapy. Methods Mol. Biol. 2020, 2058, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, F.; Menotti, L.; Avitabile, E.; Appolloni, I.; Ceresa, D.; Marubbi, D.; Campadelli-Fiume, G.; Malatesta, P. Eradication of glioblastoma by immuno-virotherapy with a retargeted oncolytic HSV in a preclinical model. Oncogene 2019, 38, 4467–4479. [Google Scholar] [CrossRef] [PubMed]
- Alayo, Q.A.; Ito, H.; Passaro, C.; Zdioruk, M.; Mahmoud, A.B.; Grauwet, K.; Zhang, X.; Lawler, S.E.; Reardon, D.A.; Goins, W.F.; et al. Glioblastoma infiltration of both tumor- and virus-antigen specific cytotoxic T cells correlates with experimental virotherapy responses. Sci. Rep. 2020, 10, 5095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todo, T. ATIM-14. RESULTS OF PHASE II CLINICAL TRIAL OF ONCOLYTIC HERPES VIRUS G47Δ IN PATIENTS WITH GLIOBLASTOMA. Neuro-Oncology 2019, 21, vi4. [Google Scholar] [CrossRef]
- Gesundheit, B.; Ben-David, E.; Posen, Y.; Ellis, R.; Wollmann, G.; Schneider, E.M.; Aigner, K.; Brauns, L.; Nesselhut, T.; Ackva, I.; et al. Effective Treatment of Glioblastoma Multiforme With Oncolytic Virotherapy: A Case-Series. Front. Oncol. 2020, 10, 702. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Guz-Montgomery, K.; Saha, D. Oncolytic Virus Encoding a Master Pro-Inflammatory Cytokine Interleukin 12 in Cancer Immunotherapy. Cells 2020, 9, 400. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.M.; Gomez-Manzano, C.; Jiang, H.; Bekele, N.B.; Piao, Y.; Yung, W.K.; Alemany, R.; Fueyo, J. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007, 14, 756–761. [Google Scholar] [CrossRef]
- Gomez-Gutierrez, J.G.; Nitz, J.; Sharma, R.; Wechman, S.L.; Riedinger, E.; Martinez-Jaramillo, E.; Sam Zhou, H.; McMasters, K.M. Combined therapy of oncolytic adenovirus and temozolomide enhances lung cancer virotherapy in vitro and in vivo. Virology 2016, 487, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanai, R.; Rabkin, S.D.; Yip, S.; Sgubin, D.; Zaupa, C.M.; Hirose, Y.; Louis, D.N.; Wakimoto, H.; Martuza, R.L. Oncolytic virus-mediated manipulation of DNA damage responses: Synergy with chemotherapy in killing glioblastoma stem cells. J. Natl. Cancer Inst. 2012, 104, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Kleijn, A.; van den Bossche, W.; Haefner, E.S.; Belcaid, Z.; Burghoorn-Maas, C.; Kloezeman, J.J.; Pas, S.D.; Leenstra, S.; Debets, R.; de Vrij, J.; et al. The Sequence of Delta24-RGD and TMZ Administration in Malignant Glioma Affects the Role of CD8(+)T Cell Anti-tumor Activity. Mol. Ther. Oncol. 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garza-Morales, R.; Gonzalez-Ramos, R.; Chiba, A.; Montes de Oca-Luna, R.; McNally, L.R.; McMasters, K.M.; Gomez-Gutierrez, J.G. Temozolomide Enhances Triple-Negative Breast Cancer Virotherapy In Vitro. Cancers 2018, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Pisklakova, A.; McKenzie, B.; Zemp, F.; Lun, X.; Kenchappa, R.S.; Etame, A.B.; Rahman, M.M.; Reilly, K.; Pilon-Thomas, S.; McFadden, G.; et al. M011L-deficient oncolytic myxoma virus induces apoptosis in brain tumor-initiating cells and enhances survival in a novel immunocompetent mouse model of glioblastoma. Neuro-Oncology 2016, 18, 1088–1098. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Y.; Hong, X.; Liu, X.; Su, X.; Li, S.; Dong, X.; Zhao, G.; Li, Y. Newcastle disease virus enhances the growth-inhibiting and proapoptotic effects of temozolomide on glioblastoma cells in vitro and in vivo. Sci. Rep. 2018, 8, 11470. [Google Scholar] [CrossRef] [Green Version]
- Sivanandam, V.; LaRocca, C.J.; Chen, N.G.; Fong, Y.; Warner, S.G. Oncolytic Viruses and Immune Checkpoint Inhibition: The Best of Both Worlds. Mol. Ther. Oncol. 2019, 13, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Dejaegher, J.; Van Gool, S.; De Vleeschouwer, S. Dendritic cell vaccination for glioblastoma multiforme: Review with focus on predictive factors for treatment response. Immunotargets Ther. 2014, 3, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, T.; Sayegh, E.T.; Fakurnejad, S.; Oyon, D.; Lamano, J.B.; DiDomenico, J.D.; Bloch, O.; Parsa, A.T. Vaccine therapies in malignant glioma. Curr. Neurol. Neurosci. Rep. 2015, 15, 508. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Schuster, J.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; Desjardins, A.; Ashby, L.S.; et al. ReACT: Overall survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. J. Clin. Oncol. 2015, 33, 2009. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Binder, D.C.; Ladomersky, E.; Lenzen, A.; Zhai, L.; Lauing, K.L.; Otto-Meyer, S.D.; Lukas, R.V.; Wainwright, D.A. Lessons learned from rindopepimut treatment in patients with EGFRvIII-expressing glioblastoma. Transl. Cancer Res. 2018, 7, S510–S513. [Google Scholar] [CrossRef] [Green Version]
- Khansur, E.M.; Shah, A.H.; Lacy, K.; Kuchakulla, M.; Komotar, R.J. Novel Immunotherapeutics for the Treatment of Glioblastoma: The Last Decade of Research. Cureus 2018, 10, e2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, N.; Zhang, Y.; Liu, Y.; Xie, J.; Wang, Y.; Hao, S.; Gao, Z. Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: A phase I, single-arm trial. JCI Insight 2018, 3, e99145. [Google Scholar] [CrossRef] [Green Version]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Dossena, M.; Pogliani, S.; Pellegatta, S.; Antozzi, C.; Baggi, F.; Gellera, C.; Pollo, B.; Parati, E.A.; Finocchiaro, G.; et al. An optimized method for manufacturing a clinical scale dendritic cell-based vaccine for the treatment of glioblastoma. PLoS ONE 2012, 7, e52301. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weenink, B.; French, P.J.; Sillevis Smitt, P.A.E.; Debets, R.; Geurts, M. Immunotherapy in Glioblastoma: Current Shortcomings and Future Perspectives. Cancers 2020, 12, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Maus, M.V.; June, C.H.; Sampson, J.H. Immunotherapy for Glioblastoma: Adoptive T-cell Strategies. Clin. Cancer Res. 2019, 25, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, F.; Yao, Y.; Wang, L.L.; Zhu, Y.; Hu, J. Adoptive Cell Therapy: A Novel and Potential Immunotherapy for Glioblastoma. Front. Oncol. 2020, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Meng, Q.; Bartek, J., Jr.; Poiret, T.; Persson, O.; Rane, L.; Rangelova, E.; Illies, C.; Peredo, I.H.; Luo, X.; et al. Tumor-infiltrating lymphocytes (TILs) from patients with glioma. Oncoimmunology 2017, 6, e1252894. [Google Scholar] [CrossRef] [Green Version]
- Duhen, T.; Duhen, R.; Montler, R.; Moses, J.; Moudgil, T.; de Miranda, N.F.; Goodall, C.P.; Blair, T.C.; Fox, B.A.; McDermott, J.E.; et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018, 9, 2724. [Google Scholar] [CrossRef] [Green Version]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Salinas, R.D.; Durgin, J.S.; O’Rourke, D.M. Potential of Glioblastoma-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy. CNS Drugs 2020, 34, 127–145. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Orrego, E.; Castaneda, C.A.; Castillo, M.; Bernabe, L.A.; Casavilca, S.; Chakravarti, A.; Meng, W.; Garcia-Corrochano, P.; Villa-Robles, M.R.; Zevallos, R.; et al. Distribution of tumor-infiltrating immune cells in glioblastoma. CNS Oncol. 2018, 7, CNS21. [Google Scholar] [CrossRef] [Green Version]
- Morisse, M.C.; Jouannet, S.; Dominguez-Villar, M.; Sanson, M.; Idbaih, A. Interactions between tumor-associated macrophages and tumor cells in glioblastoma: Unraveling promising targeted therapies. Expert Rev. Neurother. 2018, 18, 729–737. [Google Scholar] [CrossRef]
- Di Martile, M.; Farini, V.; Consonni, F.M.; Trisciuoglio, D.; Desideri, M.; Valentini, E.; D’Aguanno, S.; Tupone, M.G.; Buglioni, S.; Ercolani, C.; et al. Melanoma-specific bcl-2 promotes a protumoral M2-like phenotype by tumor-associated macrophages. J. Immunother. Cancer 2020, 8, e000489. [Google Scholar] [CrossRef]
- Cho, H.R.; Kumari, N.; Thi Vu, H.; Kim, H.; Park, C.K.; Choi, S.H. Increased Antiangiogenic Effect by Blocking CCL2-dependent Macrophages in a Rodent Glioblastoma Model: Correlation Study with Dynamic Susceptibility Contrast Perfusion MRI. Sci. Rep. 2019, 9, 11085. [Google Scholar] [CrossRef]
- Shi, L.; Bian, Z.; Liu, Y. Dual role of SIRPα in macrophage activation: Inhibiting M1 while promoting M2 polarization via selectively activating SHP-1 and SHP-2 signal. J. Immunol. 2017, 198, 67.12. [Google Scholar]
- Khalil, D.N.; Suek, N.; Campesato, L.F.; Budhu, S.; Redmond, D.; Samstein, R.M.; Krishna, C.; Panageas, K.S.; Capanu, M.; Houghton, S.; et al. In situ vaccination with defined factors overcomes T cell exhaustion in distant tumors. J. Clin. Investig. 2019, 129, 3435–3447. [Google Scholar] [CrossRef]
- Suek, N.; Campesato, L.F.; Merghoub, T.; Khalil, D.N. Targeted APC Activation in Cancer Immunotherapy to Enhance the Abscopal Effect. Front. Immunol. 2019, 10, 604. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregoire, H.; Roncali, L.; Rousseau, A.; Cherel, M.; Delneste, Y.; Jeannin, P.; Hindre, F.; Garcion, E. Targeting Tumor Associated Macrophages to Overcome Conventional Treatment Resistance in Glioblastoma. Front. Pharmacol. 2020, 11, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, M.C.; Zhang, C.; Harter, P.N.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.P.; Wels, W.S. CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy. Front. Immunol. 2019, 10, 2683. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Kang, W.Y.; Yoon, Y.; Jin, J.Y.; Song, H.J.; Her, J.H.; Kang, S.M.; Hwang, Y.K.; Kang, K.J.; Joo, K.M.; et al. Natural killer (NK) cells inhibit systemic metastasis of glioblastoma cells and have therapeutic effects against glioblastomas in the brain. BMC Cancer 2015, 15, 1011. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Karathanasis, E.; Ghaghada, K.B. Crossing the barrier: Treatment of brain tumors using nanochain particles. WIREs Nanomed. Nanobiotechnol. 2016, 8, 678–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, Y.; Panchal, R.; Slatkin, D.N.; Smilowitz, H.M. Iodine nanoparticles enhance radiotherapy of intracerebral human glioma in mice and increase efficacy of chemotherapy. Sci. Rep. 2019, 9, 4505. [Google Scholar] [CrossRef] [Green Version]
- Samec, N.; Zottel, A.; Videtic Paska, A.; Jovcevska, I. Nanomedicine and Immunotherapy: A Step Further towards Precision Medicine for Glioblastoma. Molecules 2020, 25, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Preat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Walter, K.A.; Cahan, M.A.; Gur, A.; Tyler, B.; Hilton, J.; Colvin, O.M.; Burger, P.C.; Domb, A.; Brem, H. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 1994, 54, 2207–2212. [Google Scholar] [PubMed]
- Perria, C.; Capuzzo, T.; Cavagnaro, G.; Datti, R.; Francaviglia, N.; Rivano, C.; Tercero, V.E. Fast attempts at the photodynamic treatment of human gliomas. J. Neurosurg. Sci. 1980, 24, 119–129. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three-Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [Green Version]
- De Groof, T.W.M.; Mashayekhi, V.; Fan, T.S.; Bergkamp, N.D.; Sastre Torano, J.; van Senten, J.R.; Heukers, R.; Smit, M.J.; Oliveira, S. Nanobody-Targeted Photodynamic Therapy Selectively Kills Viral GPCR-Expressing Glioblastoma Cells. Mol. Pharm. 2019, 16, 3145–3156. [Google Scholar] [CrossRef] [Green Version]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2019, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, A.; Sofi, R.; Rahman, R.; Smith, S.J.; Teschemacher, A.G.; Kasparov, S. Using Light for Therapy of Glioblastoma Multiforme (GBM). Brain Sci. 2020, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef]
- Stepp, H.; Beck, T.; Pongratz, T.; Meinel, T.; Kreth, F.W.; Tonn, J.; Stummer, W. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef]
- Eljamel, S. Photodynamic applications in brain tumors: A comprehensive review of the literature. Photodiagnosis Photodyn Ther. 2010, 7, 76–85. [Google Scholar] [CrossRef]
- Dupont, C.; Vermandel, M.; Leroy, H.A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 2019, 84, E414–E419. [Google Scholar] [CrossRef] [Green Version]
- Yekula, A.; Yekula, A.; Muralidharan, K.; Kang, K.; Carter, B.S.; Balaj, L. Extracellular Vesicles in Glioblastoma Tumor Microenvironment. Front. Immunol. 2019, 10, 3137. [Google Scholar] [CrossRef]
- Osti, D.; Del Bene, M.; Rappa, G.; Santos, M.; Matafora, V.; Richichi, C.; Faletti, S.; Beznoussenko, G.V.; Mironov, A.; Bachi, A.; et al. Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients. Clin. Cancer Res. 2019, 25, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Simon, T.; Jackson, E.; Giamas, G. Breaking through the glioblastoma micro-environment via extracellular vesicles. Oncogene 2020, 39, 4477–4490. [Google Scholar] [CrossRef]
- Berenguer, J.; Lagerweij, T.; Zhao, X.W.; Dusoswa, S.; van der Stoop, P.; Westerman, B.; de Gooijer, M.C.; Zoetemelk, M.; Zomer, A.; Crommentuijn, M.H.W.; et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J. Extracell. Vesicles 2018, 7, 1446660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, T.; Pinioti, S.; Schellenberger, P.; Rajeeve, V.; Wendler, F.; Cutillas, P.R.; King, A.; Stebbing, J.; Giamas, G. Shedding of bevacizumab in tumour cells-derived extracellular vesicles as a new therapeutic escape mechanism in glioblastoma. Mol. Cancer 2018, 17, 132. [Google Scholar] [CrossRef]
- Buruiana, A.; Florian, S.I.; Florian, A.I.; Timis, T.L.; Mihu, C.M.; Miclaus, M.; Osan, S.; Hrapsa, I.; Cataniciu, R.C.; Farcas, M.; et al. The Roles of miRNA in Glioblastoma Tumor Cell Communication: Diplomatic and Aggressive Negotiations. Int. J. Mol. Sci. 2020, 21, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piwecka, M.; Rolle, K.; Belter, A.; Barciszewska, A.M.; Zywicki, M.; Michalak, M.; Nowak, S.; Naskret-Barciszewska, M.Z.; Barciszewski, J. Comprehensive analysis of microRNA expression profile in malignant glioma tissues. Mol. Oncol. 2015, 9, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, Y.; Moore, L.; Mei, M.; You, Y.; Xu, P.; Wang, B.; Wang, G.; Jia, Z.; Pu, P.; et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab. Investig. 2010, 90, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Belter, A.; Rolle, K.; Piwecka, M.; Fedoruk-Wyszomirska, A.; Naskret-Barciszewska, M.Z.; Barciszewski, J. Inhibition of miR-21 in glioma cells using catalytic nucleic acids. Sci. Rep. 2016, 6, 24516. [Google Scholar] [CrossRef] [Green Version]
- Ananta, J.S.; Paulmurugan, R.; Massoud, T.F. Nanoparticle-Delivered Antisense MicroRNA-21 Enhances the Effects of Temozolomide on Glioblastoma Cells. Mol. Pharm. 2015, 12, 4509–4517. [Google Scholar] [CrossRef]
- Teplyuk, N.M.; Uhlmann, E.J.; Gabriely, G.; Volfovsky, N.; Wang, Y.; Teng, J.; Karmali, P.; Marcusson, E.; Peter, M.; Mohan, A.; et al. Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: First steps toward the clinic. EMBO Mol. Med. 2016, 8, 268–287. [Google Scholar] [CrossRef]
- Sharif, S.; Ghahremani, M.H.; Soleimani, M. Delivery of Exogenous miR-124 to Glioblastoma Multiform Cells by Wharton’s Jelly Mesenchymal Stem Cells Decreases Cell Proliferation and Migration, and Confers Chemosensitivity. Stem. Cell Rev. Rep. 2018, 14, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Sana, J.; Busek, P.; Fadrus, P.; Besse, A.; Radova, L.; Vecera, M.; Reguli, S.; Stollinova Sromova, L.; Hilser, M.; Lipina, R.; et al. Identification of microRNAs differentially expressed in glioblastoma stem-like cells and their association with patient survival. Sci. Rep. 2018, 8, 2836. [Google Scholar] [CrossRef] [Green Version]
- Rubenstein, J.L.; Kim, J.; Ozawa, T.; Zhang, M.; Westphal, M.; Deen, D.F.; Shuman, M.A. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2000, 2, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutouri, C.; Kirkpatrick, N.D.; Chung, E.; Mpekris, F.; Baish, J.W.; Munn, L.L.; Fukumura, D.; Stylianopoulos, T.; Jain, R.K. Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc. Natl. Acad. Sci. USA 2019, 116, 2662–2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montana, V.; Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. 2011, 31, 4858–4867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.N.; Zamler, D.; Baker, G.J.; Kadiyala, P.; Erdreich-Epstein, A.; DeCarvalho, A.C.; Mikkelsen, T.; Castro, M.G.; Lowenstein, P.R. CXCR4 increases in-vivo glioma perivascular invasion, and reduces radiation induced apoptosis: A genetic knockdown study. Oncotarget 2016, 7, 83701–83719. [Google Scholar] [CrossRef] [Green Version]
- Griveau, A.; Seano, G.; Shelton, S.J.; Kupp, R.; Jahangiri, A.; Obernier, K.; Krishnan, S.; Lindberg, O.R.; Yuen, T.J.; Tien, A.C.; et al. A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell 2018, 33, 874–889. [Google Scholar] [CrossRef] [Green Version]
- Rabe, M.; Dumont, S.; Alvarez-Arenas, A.; Janati, H.; Belmonte-Beitia, J.; Calvo, G.F.; Thibault-Carpentier, C.; Sery, Q.; Chauvin, C.; Joalland, N.; et al. Identification of a transient state during the acquisition of temozolomide resistance in glioblastoma. Cell Death Dis. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Struve, N.; Binder, Z.A.; Stead, L.F.; Brend, T.; Bagley, S.J.; Faulkner, C.; Ott, L.; Muller-Goebel, J.; Weik, A.S.; Hoffer, K.; et al. EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene 2020, 39, 3041–3055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Pascual, A.; Hale, J.S.; Kordowski, A.; Pugh, J.; Silver, D.J.; Bayik, D.; Roversi, G.; Alban, T.J.; Rao, S.; Chen, R.; et al. ADAMDEC1 Maintains a Growth Factor Signaling Loop in Cancer Stem Cells. Cancer Discov. 2019, 9, 1574–1589. [Google Scholar] [CrossRef] [Green Version]
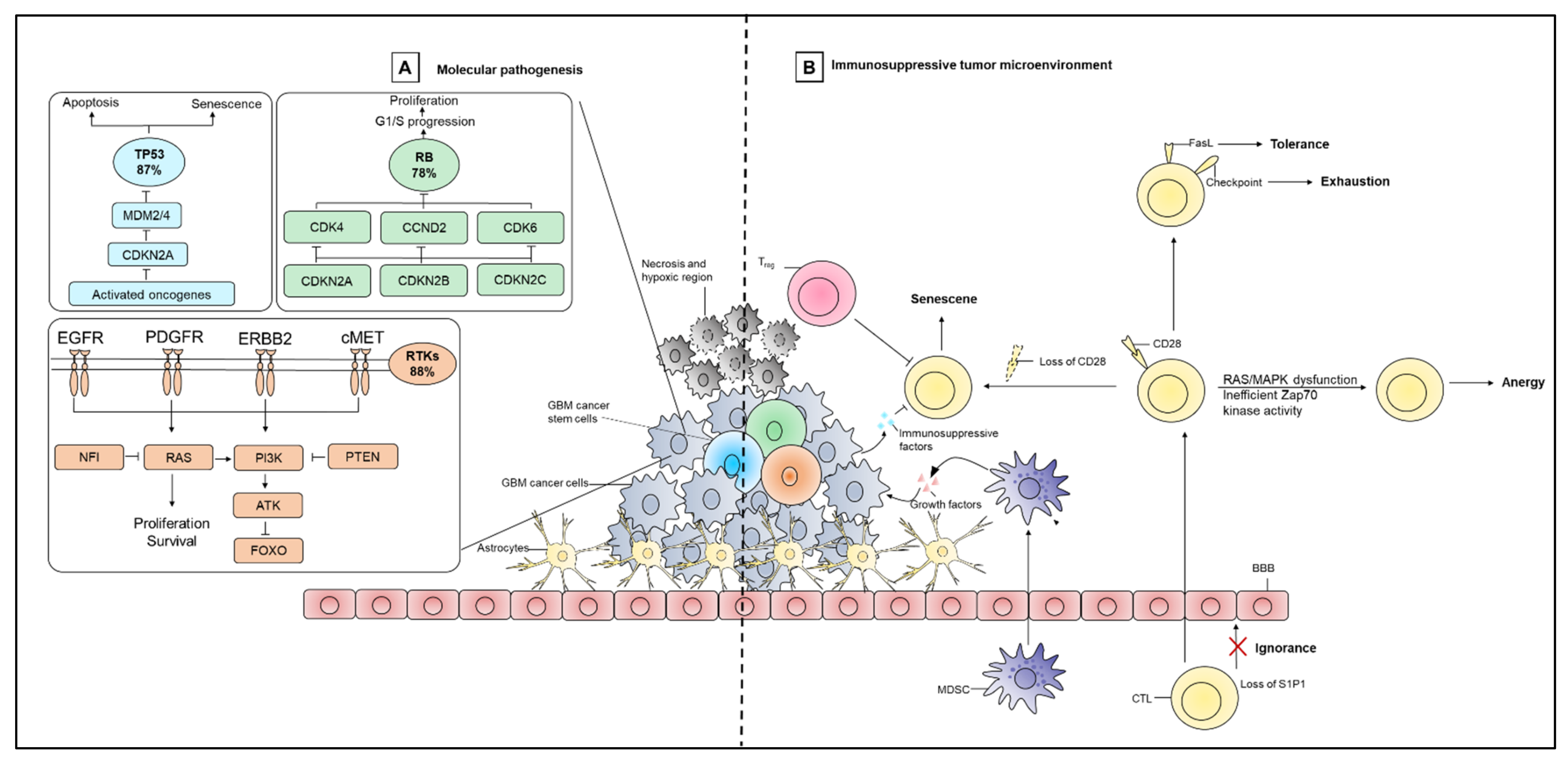
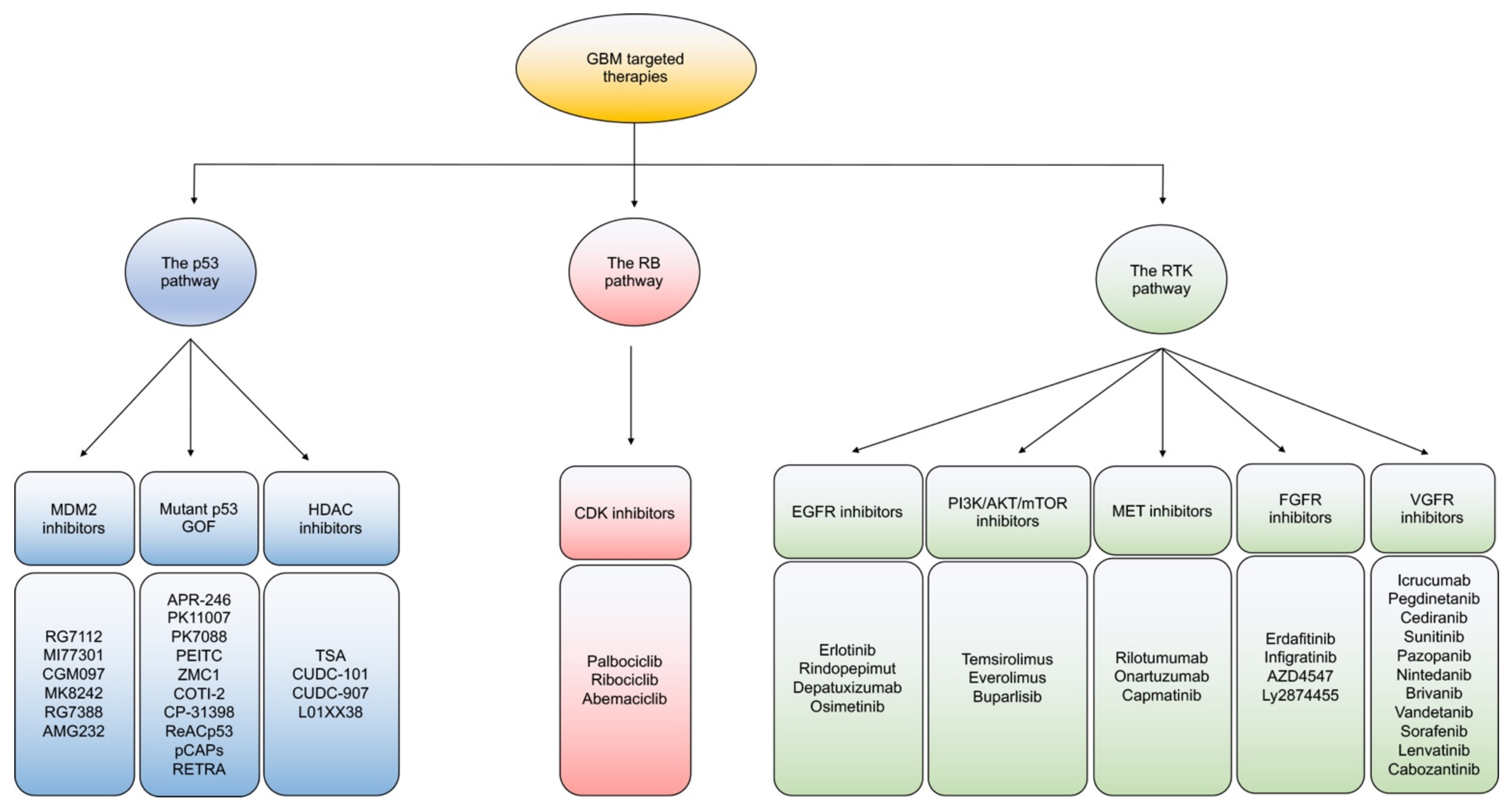
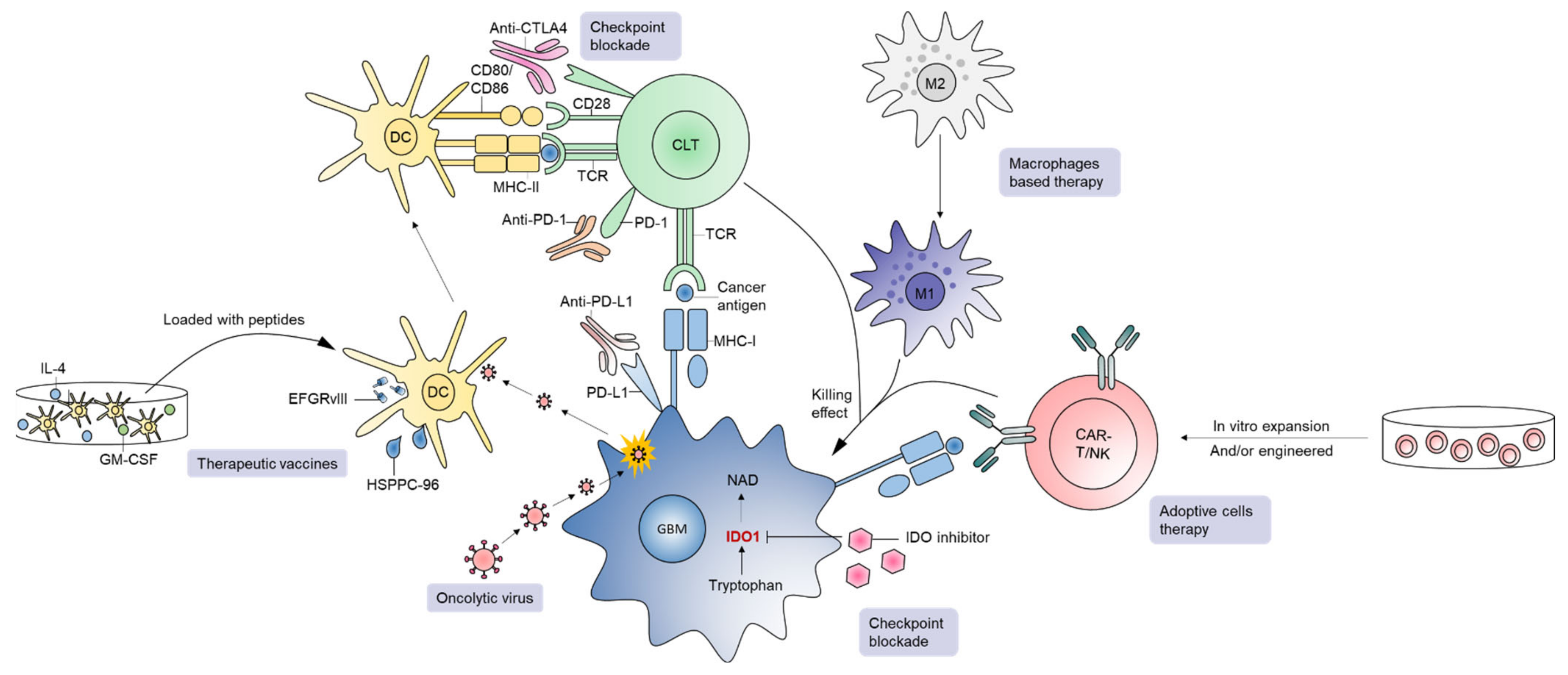
| GBM Subtype | Expression of Signature Genes | Predominant Marker | Distinct Neural Cell Types |
|---|---|---|---|
| Proneural |
| Proneural markers: SOX, DCX, DLL3, ASCL1, TCF4 and Oligodendrocytic development markers: PDGFRA, NKX2-2 and OLIG2 | Oligodendrocyte |
| Mesenchymal |
| Mesenchymal and astrocytic markers CD44, MERTK | Astroglial |
| Classical |
| Neural precursor and stem cell markers NES, NOTCH3, JAG1, LFNG, SMO, GAS1, GLI2 | Murine astrocytes |
| Neural |
| Neuron markers such as NEFL, GABRA1, SYT1 and SLC12A5. | Neuron, oligodendrocytes and astrocytes |
| Targeted Pathway | Targeted Therapy | Drug Name | In Combination | Condition | Phase | N | NCT (Accessed On) |
|---|---|---|---|---|---|---|---|
| The p53 pathway | MDM2 inhibitors | AMG232 | Radiation | Newly Diagnosed and recurrent GBM | I | 86 | NCT03107780 (04/09/2020) |
| RG7388 (Idasanutlin) | Radiation | Newly Diagnosed GBM Without MGMT Promoter Methylation | I/IIa | 350 | NCT03158389 (04/09/2020) | ||
| HDAC inhibitors | SAHA (Vorinostat) | Radiation Pembrolizumab TMZ | Newly Diagnosed GBM | I | 32 | NCT03426891 (04/09/2020) | |
| Isotretinoin TMZ | Recurrent GBM | I/II | 135 | NCT00555399 (04/09/2020) | |||
| Radiation TMZ | Newly Diagnosed GBM | I/II | 125 | NCT00731731 (04/09/2020) | |||
| Bevacizumab | Recurrent GBM | II | 48 | NCT01738646 (04/09/2020) | |||
| CUDC-907 (Fimepinostat) | Surgery | Recurrent GBM | Early I | 30 | NCT03893487 (04/09/2020) | ||
| The Rb pathway | CDK inhibitor | PD-332991 (Palbociclib) | Radiation | Newly Diagnosed GBM Without MGMT Promoter Methylation | I/IIa | 350 | NCT03158389 (04/09/2020) |
| LEE011 (Ribociclib) | Everolimus | Recurrent GBM | Early I | 24 | NCT03834740 (04/09/2020) | ||
| Preoperative GBM | Early I | 48 | NCT02933736 (04/09/2020) | ||||
| LY2835219 (Abemaciclib) | Bevacizumab | Recurrent GBM | Early I | 10 | NCT04074785 (04/09/2020) | ||
| Recurrent GBM | II | 42 | NCT02981940 (04/09/2020) | ||||
| LY3214996 | Recurrent GBM | Early I | 50 | NCT04391595 (04/09/2020) | |||
| TMZ | GBM | II | 280 | NCT02977780 (04/09/2020) | |||
| The RTK pathway | EGFR inhibitors | OSI-774 (Erlotinib) | Relapsed/Refractory GBM | I/II | 11 | NCT00301418 (04/09/2020) | |
| Bevacizumab TMZ | Newly Diagnosed GBM | II | 115 | NCT00720356 (04/09/2020) | |||
| Sorafenib | Progressive or recurrent GBM | II | 56 | NCT00445588 (04/09/2020) | |||
| Cetuximab | Mannitol Radiation | Relapsed/Refractory GBM | II | 37 | NCT02800486 (04/09/2020) | ||
| Mannitol | Newly Diagnosed GBM | I/II | 33 | NCT02861898 (04/09/2020) | |||
| AZD9291 (Osimertinib) | Fludeoxyglucose F-18 PET | Recurrent GBM | II | 12 | NCT03732352 (04/09/2020) | ||
| Nimotuzumab | Radiation TMZ | Newly Diagnosed GBM | II | 39 | NCT03388372 (04/09/2020) | ||
| Newly Diagnosed GBM | III | 150 | NCT00753246 (04/09/2020) | ||||
| CDX-110 (Rindopepimut) | TMZ | Newly Diagnosed, Surgically Resected, EGFRvIII-positive GBM | III | 745 | NCT01480479 (04/09/2020) | ||
| Radiation TMZ | Newly Diagnosed GBM | II | 82 | NCT00458601 (04/09/2020) | |||
| ABT-414 (Depatuxizumab) | TMZ | Recurrent GBM | II | 266 | NCT02343406 (04/09/2020) | ||
| Radiation TMZ | GBM | I | 202 | NCT01800695 (04/09/2020) | |||
| Radiation TMZ | Newly Diagnosed GBM With EGFR Amplification | III | 691 | NCT02573324 (04/09/2020) | |||
| Radiation TMZ | Newly diagnosed or recurrent GBM | I/II | 53 | NCT02590263 (04/09/2020) | |||
| PI3K/AKT/mTOR inhibitors | CCI-779 (Temsirolimus) | Sorafenib Tosylate | Recurrent GBM | I/II | 115 | NCT00329719 (04/09/2020) | |
| Sorafenib Tosylate | Recurrent GBM | I/II | 92 | NCT00335764 (04/09/2020) | |||
| Perifosine | Recurrent GBM | I | 10 | NCT02238496 (04/09/2020) | |||
| TMZ | Recurrent GBM | II | 266 | NCT02343406 (04/09/2020) | |||
| RAD001 (Everolimus) | Ribociclib | Recurrent GBM | Early I | 24 | NCT03834740 (04/09/2020) | ||
| Radiation TMZ | Newly diagnosed GBM | I/II | 122 | NCT00553150 (04/09/2020) | |||
| Radiation TMZ | Newly diagnosed GBM | I/II | 279 | NCT01062399 (04/09/2020) | |||
| Sorafenib | Recurrent GBM | I/II | 118 | NCT01434602 (04/09/2020) | |||
| BKM120 (Buparlisib) | Bevacizumab | Relapsed/Refractory GBM | I/II | 88 | NCT01349660 (04/09/2020) | ||
| Radiation TMZ | Newly diagnosed GBM | I | 38 | NCT01473901 (04/09/2020) | |||
| Lomustine or Carboplatin | Recurrent GBM | Ib/II | 35 | NCT01934361 (04/09/2020) | |||
| Recurrent GBM | II | 65 | NCT01339052 (04/09/2020) | ||||
| MET inhibitors | AMG 102 (Rilotumumab) | Bevacizumab | Recurrent GBM | II | 36 | NCT01113398 (04/09/2020) | |
| (RO5490258) Onartuzumab | Bevacizumab | Recurrent GBM | II | 135 | NCT01632228 (04/09/2020) | ||
| INC280 (Capmatinib) | Bevacizumab | GBM | I | 65 | NCT02386826 (04/09/2020) | ||
| FGFR inhibitors | Infigratinib | Recurrent GBM | Early I | 20 | NCT04424966 (04/09/2020) | ||
| AZD4547 | GBM with FGFR-TACC gene fusion | I/II | 14 | NCT02824133 (01/02/2021) | |||
| VEFGR inhibitors | Cediranib | Bevacizumab Olaparib | Recurrent GBM | II | 70 | NCT02974621 (04/09/2020) | |
| Sunitinib | Lomustine | Recurrent GBM | II/III | 100 | NCT03025893 (04/09/2020) | ||
| Pazopanib | Topotecan | Recurrent GBM | II | 35 | NCT01931098 (04/09/2020) | ||
| N/A | Newly diagnosed GBM | I/II | 51 | NCT02331498 (04/09/2020) | |||
| Vandetanib | Sirolimus | Recurrent GBM | I | 33 | NCT00821080 (04/09/2020) | ||
| Sorafenib | Everolimus | Recurrent GBM | I/II | 118 | NCT01434602 (04/09/2020) | ||
| Lenvatinib | Pembrolizumab | GBM | II | 600 | NCT03797326 (04/09/2020) | ||
| Cabozantinib | N/A | Recurrent GBM | II | 10 | NCT02885324 (04/09/2020) | ||
| Other pathways | TGF-β inhibitors | BCA101 | Pembrolizumab | GBM | I | 292 | NCT04429542 (04/09/2020) |
| LY2157299 (Galunisertib) | Lomustine | Recurrent GBM | II | 180 | NCT01582269 (04/09/2020) | ||
| Proteasome inhibitors | Bortezomib | TMZ | Recurrent GBM with Unmethylated MGMT Promoter to TMZ | 1B/II | 63 | NCT03643549 (04/09/2020) | |
| Bevacizumab TMZ | Recurrent GBM | I | 12 | NCT01435395 (04/09/2020) | |||
| Ixazomib | N/A | GBM | Early phase I | 3 | NCT02630030 (04/09/2020) | ||
| Marizomib | Radiation TMZ | Newly diagnosed GBM | III | 750 | NCT03345095 (04/09/2020) | ||
| Bevacizumab | GBM | I/II | 121 | NCT02330562 (04/09/2020) | |||
| Radiation TMZ Optune | Newly diagnosed GBM | IB | 66 | NCT02903069 (04/09/2020) | |||
| ABI-009 (Nab-Rapamycin) | Newly diagnosed GBM | II | 56 | NCT03463265 (04/09/2020) | |||
| ATM inhibitor | AZD1390 | Radiation | Newly diagnosed and recurrent GBM | I | 132 | NCT03423628 (01/02/2021) | |
| PARP inhibitor | Veliparib | Radiation TMZ | GBM | I/II | 66 | NCT01514201 (01/02/2021) | |
| Radiation TMZ | GBM | II | 115 | NCT03581292 (01/02/2021) | |||
| TMZ | Newly Diagnosed GBM With MGMT Promoter Hypermethylation | II/III | 440 | NCT02152982 (01/02/2021) | |||
| Olaparib | Bevacizumab Cediranib | Recurrent GBM | II | 70 | NCT02974621 (01/02/2021) | ||
| Radiation Pamiparib TMZ | Newly diagnosed and recurrent GBM | I | 30 | NCT04614909 (01/02/2021) | |||
| N/A | GBM | II | 145 | NCT03212274 (01/02/2021) | |||
| Pamiparib | Radiation TMZ | Newly diagnosed and recurrent GBM | I/II | 116 | NCT03150862 (01/02/2021) | ||
| TMZ | Recurrent GBM | I/II | 100 | NCT03914742 (01/02/2021) | |||
| TMZ | Recurrent GBM | I | 78 | NCT03749187 (01/02/2021) |
| Immunotherapies | Drug Name | In Combination | Condition | Phase | N | NCT (Accessed on) |
|---|---|---|---|---|---|---|
| Anti-PD-1 | Spartalizumab | MBG453 | Recurrent GBM | I | 15 | NCT03961971 (11/09/2020) |
| BLZ945 | Advanced/metastatic/recurrent IDH wild-type GBM | I/II | 200 | NCT02829723 (11/09/2020) | ||
| INCMGA00012 | INCAGN01876, stereotactic radiosurgery | Recurrent GBM | II | 32 | NCT04225039 (11/09/2020) | |
| Bevacizumab, radiation | Recurrent GBM | II | 55 | NCT03532295 (11/09/2020) | ||
| Nivolumab | BMS-986016 | Recurrent GBM | I | 63 | NCT02658981 (11/09/2020) | |
| Dendritic vaccine | GBM | I | 6 | NCT02529072 (11/09/2020) | ||
| Bevacizumab | Recurrent GBM | II | 90 | NCT03452579 (11/09/2020) | ||
| Ipilimumab, Radiation | Newly Diagnosed, MGMT Unmethylated Glioblastoma | II | 24 | NCT03367715 (11/09/2020) | ||
| TMZ | Newly Diagnosed Elderly Patients With GBM | II | 102 | NCT04195139 (11/09/2020) | ||
| Ipilimumab, NovoTTF200A (Optune) | Recurrent GBM | II | 60 | NCT03430791 (11/09/2020) | ||
| Ad-RTS-hIL-12, Veledimex | Recurrent or Progressive GBM | I | 21 | NCT03636477 (11/09/2020) | ||
| Ipilimumab | Recurrent GBM | I | 6 | NCT03233152 (11/09/2020) | ||
| Ipilimumab, TMZ | Newly Diagnosed GBM | I | 32 | NCT02311920 (11/09/2020) | ||
| Bevacizumab, Re-irradiation | Recurrent MGMT Methylated GBM | II | 94 | NCT03743662 (11/09/2020) | ||
| Ipilimumab | Recurrent GBM with elevated mutational | II | 37 | NCT04145115 (11/09/2020) | ||
| BMS-986205, TMZ, Radiation | Newly diagnosed GBM | I | 30 | NCT04047706 (11/09/2020) | ||
| IL13Ralpha2-CRT T cells, Ipilimumab | Recurrent GBM | I | 60 | NCT04003649 (11/09/2020) | ||
| Ipilimumab | Newly Diagnosed, MGMT Unmethylated GBM | II/III | 485 | NCT04396860 (11/09/2020) | ||
| TMZ, radiation | Newly Diagnosed, MGMT Methylated GBM | III | 693 | NCT02667587 (11/09/2020) | ||
| TMZ | Newly Diagnosed, MGMT Unmethylated GBM | III | 550 | NCT02617589 (11/09/2020) | ||
| Bevacizumab | Recurrent GBM | II | 40 | NCT03890952 (11/09/2020) | ||
| NeoVax, Ipilimumab | Newly Diagnosed, MGMT Unmethylated GBM | I | 3 | NCT03422094 (11/09/2020) | ||
| Therapeutic vaccine EO2401 | Progressive Glioblastoma | Ib/IIa | 32 | NCT04116658 (11/09/2020) | ||
| N/A | IDH-Mutant GBM with and without hypermutator phenotype | II | 95 | NCT03718767 (11/09/2020) | ||
| Ipilimumab | Recurrent GBM | I | 45 | NCT04323046 (11/09/2020) | ||
| Pembrolizumab | SurVaxM, Sargramostim, Montanide ISA51 | Recurrent GBM | II | 51 | NCT04013672 (11/09/2020) | |
| Radiation, TMZ, NeoAntigen vaccine | MGMT Unmethylated, Newly Diagnosed GBM | I | 56 | NCT02287428 (11/09/2020) | ||
| Bevacizumab, radiation | Recurrent GBM | II | 60 | NCT03661723 (11/09/2020) | ||
| HSPPC-96, TMZ | Newly Diagnosed GBM | II | 108 | NCT03018288 (11/09/2020) | ||
| N/A | Newly Diagnosed GBM | II | 56 | NCT03899857 (11/09/2020) | ||
| Laser Interstitial Thermotherapy | Recurrent GBM | I/II | 34 | NCT03277638 (11/09/2020) | ||
| TTF | Newly Diagnosed GBM | II | 29 | NCT03405792 (11/09/2020) | ||
| TTAC-0001 | Recurrent GBM | I | 9 | NCT03722342 (11/09/2020) | ||
| EGFRvIII-CAR T Cells | Newly diagnosed, MGMT unmethylated GBM | I | 7 | NCT03726515 (11/09/2020) | ||
| Vorinostat, TMZ, radiation | Newly Diagnosed GBM | I | 32 | NCT03426891 (11/09/2020) | ||
| Ferumoxytol | Newly Diagnosed GBM | II | 45 | NCT03347617 (11/09/2020) | ||
| IMA950/Poly-ICLC | Recurrent GBM | I/II | 24 | NCT03665545 (11/09/2020) | ||
| Dendritic Cell Tumor Cell Lysate Vaccine | Recurrent or progressive GBM | I | 40 | NCT04201873 (11/09/2020) | ||
| N/A | Recurrent GBM | II | 20 | NCT02337686 (11/09/2020) | ||
| Oncolytic Polio/Rhinovirus Recombinant (PVSRIPO) | Recurrent GBM | I | 10 | NCT04479241 (11/09/2020) | ||
| Radiation, TMZ | Newly Diagnosed GBM | II | 90 | NCT03197506 (11/09/2020) | ||
| N/A | Recurrent or progressive GBM | I | 35 | NCT02852655 (11/09/2020) | ||
| Adenovirus (DNX-2401) | Recurrent GBM | II | 49 | NCT02798406 (11/09/2020) | ||
| Lenvatinib | GBM | II | 600 | NCT03797326 (11/09/2020) | ||
| Cyclophosphamide, fludarabine, aldesleukin, TIL | Progressive GBM | II | 332 | NCT01174121 (11/09/2020) | ||
| Cyclophosphamide, fludarabine, aldesleukin, TCR | GBM | II | 270 | NCT03412877 (11/09/2020) | ||
| MRI-guided Laser Ablation | Recurrent GBM | I/II | 58 | NCT02311582 (11/09/2020) | ||
| N/A | Recurrent GBM With a Hypermutator Phenotype | Pilot | 44 | NCT02658279 (11/09/2020) | ||
| Radiation, Bevacizumab | Recurrent GBM | I | 32 | NCT02313272 (11/09/2020) | ||
| Radiation, TMZ | GBM | I/II | 50 | NCT02530502 (11/09/2020) | ||
| Cemiplimab | Ad-RTS-hIL-12, Veledimex | Recurrent or progressive GBM | II | 36 | NCT04006119 (11/09/2020) | |
| INO-5401 and INO-9012 | Newly Diagnosed GBM | I/II | 52 | NCT03491683 (11/09/2020) | ||
| Anti-PD-L1 | Atezolizumab | Radiation, TMZ | Newly Diagnosed GBM | I/II | 80 | NCT03174197 (11/09/2020) |
| Ipatasertib | GBM | I/II | 51 | NCT03673787 (11/09/2020) | ||
| Radiation | MGMT unmethylated GBM | I/II | 350 | NCT03158389 (11/09/2020) | ||
| D2C7-IT | Recurrent GBM | I | 18 | NCT04160494 (11/09/2020) | ||
| Avelumab | MRI-guided LITT therapy | Recurrent GBM | I | 30 | NCT03341806 (11/09/2020) | |
| Hypofractionated radiation therapy | IDH mutant GBM | II | 43 | NCT02968940 (11/09/2020) | ||
| VXM01 | Progressive GBM | I/II | 30 | NCT03750071 (11/09/2020) | ||
| TMZ | Newly Diagnosed GBM | II | 30 | NCT03047473 (11/09/2020) | ||
| Axitinib | Recurrent GBM | II | 52 | NCT03291314 (11/09/2020) | ||
| Durvalumab | Bevacizumab, radiation | GBM | II | 159 | NCT02336165 (11/09/2020) | |
| Hypofractionated stereotactic radiation therapy | Recurrent GBM | I/II | 112 | NCT02866747 (11/09/2020) | ||
| Tremelimumab | Recurrent GBM | II | 36 | NCT02794883 (11/09/2020) | ||
| Anti-CLTA4 | Tremelimumab | Durvalumab | Recurrent GBM | II | 36 | NCT02794883 (11/09/2020) |
| Ipilimumab | Nivolumab, Radiation | Newly Diagnosed, MGMT Unmethylated Glioblastoma | II | 24 | NCT03367715 (11/09/2020) | |
| Nivolumab, NovoTTF200A (Optune) | Recurrent GBM | II | 60 | NCT03430791 (11/09/2020) | ||
| Nivolumab | Recurrent GBM | I | 6 | NCT03233152 (11/09/2020) | ||
| Nivolumab | Recurrent GBM with elevated mutational | II | 37 | NCT04145115 (11/09/2020) | ||
| Nivolumab, TMZ | Newly Diagnosed GBM | I | 32 | NCT02311920 (11/09/2020) | ||
| IL13Ralpha2-CRT T cells, Nivolumab | Recurrent GBM | I | 60 | NCT04003649 (11/09/2020) | ||
| Nivolumab | Newly Diagnosed, MGMT Unmethylated GBM | II/III | 485 | NCT04396860 (11/09/2020) | ||
| NeoVax, Nivolumab | Newly Diagnosed, MGMT Unmethylated GBM | I | 3 | NCT03422094 (11/09/2020) | ||
| Nivolumab | Recurrent GBM | I | 45 | NCT04323046 (11/09/2020) | ||
| Anti-IDO1 | BMS-986205 (Linrodostat) | Nivolumab, TMZ, Radiation | Newly diagnosed GBM | I | 30 | NCT04047706 (11/09/2020) |
| Indoximod | TMZ, radiation, cyclophosphamide, etoposide | Newly diagnosed GBM | I | 81 | NCT02502708 (11/09/2020) | |
| TMZ, bevacizumab, stereotactic radiation | GBM | I/II | 160 | NCT02052648 (11/09/2020) | ||
| TMZ, radiation, cyclophosphamide, etoposide, lomustine | Progressive GBM | II | 140 | NCT04049669 (11/09/2020) | ||
| Oncolytic viruses | TG6002 | 5-flucytosine | Recurrent GBM | I/II | 78 | NCT03294486 (11/09/2020) |
| DNX-2440 | N/A | Recurrent GBM | I | 24 | NCT03714334 (11/09/2020) | |
| DNX-2401 | IFNg | Recurrent GBM | I | 37 | NCT02197169 (11/09/2020) | |
| Pembrolizumab | Recurrent GBM | II | 49 | NCT02798406 (11/09/2020) | ||
| TMZ | Recurrent GBM | I | 31 | NCT01956734 (11/09/2020) | ||
| N/A | Recurrent GBM | I | 36 | NCT03896568 (11/09/2020) | ||
| C134 | N/A | Recurrent GBM | I | 24 | NCT03657576 (11/09/2020) | |
| M032 | N/A | Recurrent GBM | I | 36 | NCT02062827 (11/09/2020) | |
| G207 | Radiation | Recurrent GBM | II | 30 | NCT04482933 (11/09/2020) | |
| N/A | Recurrent GBM | I | 15 | NCT03911388 (11/09/2020) | ||
| N/A | Recurrent or progressive GBM | I | 12 | NCT02457845 (11/09/2020) | ||
| Therapeutic vaccines | Percellvac | N/A | Newly diagnosed GBM | I | 10 | NCT02709616 (11/09/2020) |
| Percellvac2 | N/A | Newly diagnosed GBM | I | 10 | NCT02808364 (11/09/2020) | |
| GNOS-PV01 | Plasmid encoded IL-12 | Newly diagnosed, unmethylated GBM | I | 6 | NCT04015700 (11/09/2020) | |
| VBI-1901 | N/A | Recurrent GBM | I/II | 38 | NCT03382977 (11/09/2020) | |
| MTA-based Personalized Vaccine | TTF, Poly-ICLC | Newly diagnosed GBM | I | 20 | NCT03223103 (11/09/2020) | |
| Autologous DCV | TMZ | GBM | II | 28 | NCT04523688 (11/09/2020) | |
| UCPVax | N/A | GBM | I/II | 28 | NCT04280848 (11/09/2020) | |
| NeoAntigen vaccine | Radiation, TMZ, Pembrolizumab | MGMT Unmethylated, Newly Diagnosed GBM | I | 56 | NCT02287428 (11/09/2020) | |
| Dendritic Cell Tumor Cell Lysate Vaccine | Pembrolizumab, Poly ICLC | Recurrent or progressive GBM | I | 40 | NCT04201873 (11/09/2020) | |
| TMZ, radiation, bevacizumab | Newly diagnosed or recurrent GBM | I | 39 | NCT02010606 (11/09/2020) | ||
| NeoVax | Nivolumab, Ipilimumab | Newly Diagnosed, MGMT Unmethylated GBM | I | 3 | NCT03422094 (11/09/2020) | |
| DC/tumor cell fusion vaccine | IL-12, TMZ | Newly diagnosed GBM | I/II | 10 | NCT04388033 (11/09/2020) | |
| Malignant Glioma Tumor Lysate-Pulsed Autologous DCV | N/A | Recurrent GBM | I | 20 | NCT03360708 (11/09/2020) | |
| TMZ | Newly diagnosed GBM | I | 21 | NCT01957956 (11/09/2020) | ||
| 0.2% resiquimod, polyICLC | GBM | 2 | 60 | NCT01204684 (11/09/2020) | ||
| Autologous, tumor lysate-loaded, mature DCs | TMZ, radiation | Newly diagnosed GBM | II | 136 | NCT03395587 (11/09/2020) | |
| Autologous DCV | TMZ, radiation | Newly diagnosed GBM | I/II | 20 | NCT02649582 (11/09/2020) | |
| Autologous DCs loaded with autogeneic glioma stem-like cells (A2B5+) | Surgery, chemotherapy, and radiotherapy. | GBM | II | 100 | NCT01567202 (11/09/2020) | |
| EO2401 | N/A | Progressive or first recurrent GBM | I/II | 32 | NCT04116658 (11/09/2020) | |
| SurVaxM | TMZ, Montanide ISA 51 VG, Sargramostim | Newly diagnosed GBM | II | 64 | NCT02455557 (11/09/2020) | |
| CMV RNA-Pulsed Dendritic Cells | Tetanus-Diphtheria Toxoid Vaccine | Newly diagnosed GBM | II | 120 | NCT02465268 (11/09/2020) | |
| Tetanus-Diphtheria Toxoid Vaccine | Recurrent GBM | I | 11 | NCT03615404 (11/09/2020) | ||
| HSPPC-96 | Pembrolizumab, TMZ | Newly diagnosed GBM | II | 108 | NCT03018288 (11/09/2020) | |
| Autologous DCV | metronomic cyclophosphamide | Recurrent GBM | I/II | 25 | NCT03879512 (11/09/2020) | |
| CMV-specific dendritic cell vaccine | TMZ, Tetanus-Diphtheria Toxoid, GM-CSF | Newly diagnosed Unmethylated GBM | II | 48 | NCT03927222 (11/09/2020) | |
| DEN-STEM | TMZ | IDH wild-type, MGMT methylated GBM | II/III | 60 | NCT03548571 (11/09/2020) | |
| ADCV01 | N/A | GBM | II | 24 | NCT04115761 (11/09/2020) | |
| VXM01 | Avelumab | Progressive GBM | I/II | 30 | NCT03750071 (11/09/2020) | |
| IMA950 | Pembrolizumab/Poly-ICLC | Recurrent GBM | I/II | 24 | NCT03665545 (11/09/2020) | |
| ADCTA-SSI-G1 | Bevacizumab | Recurrent GBM | III | 118 | NCT04277221 (11/09/2020) | |
| AV-GBM-1 | N/A | Newly diagnosed GBM | II | 55 | NCT03400917 (11/09/2020) | |
| Human CMV pp65-LAMP mRNA-pulsed autologous DCs | Variliumab | Newly diagnosed GBM | II | 112 | NCT03688178 (11/09/2020) | |
| Adoptive cell therapy | PD1-TIL | N/A | GBM | I | 40 | NCT03347097 (11/09/2020) |
| TIL | Cyclophosphamide, fludarabine, aldesleukin, pembrolizumab | Progressive GBM | II | 332 | NCT01174121 (11/09/2020) | |
| Autologous T-Cells Express TCRs Reactive Against Mutated Neoantigens | Cyclophosphamide, fludarabine, aldesleukin, pembrolizumab | GBM | II | 270 | NCT03412877 (11/09/2020) | |
| EGFRvIII CAR-T | Pembrolizumab | Newly diagnosed, MGMT unmethylated GBM | I | 7 | NCT03726515 (11/09/2020) | |
| B7-H3 CAR-T | TMZ | Recurrent GBM | I | 12 | NCT04385173 (11/09/2020) | |
| CD147 CAR-T | N/A | Recurrent GBM | I | 31 | NCT04045847 (11/09/2020) | |
| Chlorotoxin (EQ)-CD28-CD3zeta-CD19t-expressing CAR-T | N/A | Recurrent GBM | I | 36 | NCT04214392 (11/09/2020) | |
| IL13Ralpha2 CAR-T | Nivolumab, Ipilimumab | Recurrent GBM | I | 60 | NCT04003649 (11/09/2020) | |
| NKG2D-based CAR-T | N/A | Recurrent GBM | I | 10 | NCT04270461 (11/09/2020) | |
| IL13Ralpha2-specific hinge-optimized 41BB-co-stimulatory CAR Truncated CD19-expressing Autologous T-Lymphocytes | N/A | Recurrent GBM | I | 92 | NCT02208362 (11/09/2020) | |
| Macrophage based therapy | BLZ945 | Spartalizumab | Advanced/metastatic/recurrent IDH wild-type GBM | I/II | 200 | NCT02829723 (11/09/2020) |
| APX005M | N/A | GBM | I | 45 | NCT03389802 (11/09/2020) | |
| NK cell therapy | NK-92/5.28.z Cells | N/A | Recurrent HER2-positive GBM | I | 30 | NCT03383978 (11/09/2020) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.-M.; Guz-Montgomery, K.; Lowe, D.B.; Saha, D. Pathogenetic Features and Current Management of Glioblastoma. Cancers 2021, 13, 856. https://doi.org/10.3390/cancers13040856
Nguyen H-M, Guz-Montgomery K, Lowe DB, Saha D. Pathogenetic Features and Current Management of Glioblastoma. Cancers. 2021; 13(4):856. https://doi.org/10.3390/cancers13040856
Chicago/Turabian StyleNguyen, Hong-My, Kirsten Guz-Montgomery, Devin B. Lowe, and Dipongkor Saha. 2021. "Pathogenetic Features and Current Management of Glioblastoma" Cancers 13, no. 4: 856. https://doi.org/10.3390/cancers13040856
APA StyleNguyen, H.-M., Guz-Montgomery, K., Lowe, D. B., & Saha, D. (2021). Pathogenetic Features and Current Management of Glioblastoma. Cancers, 13(4), 856. https://doi.org/10.3390/cancers13040856







