Complete Removal of the Lesion as a Guidance in the Management of Patients with Breast Ductal Carcinoma In Situ
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistics
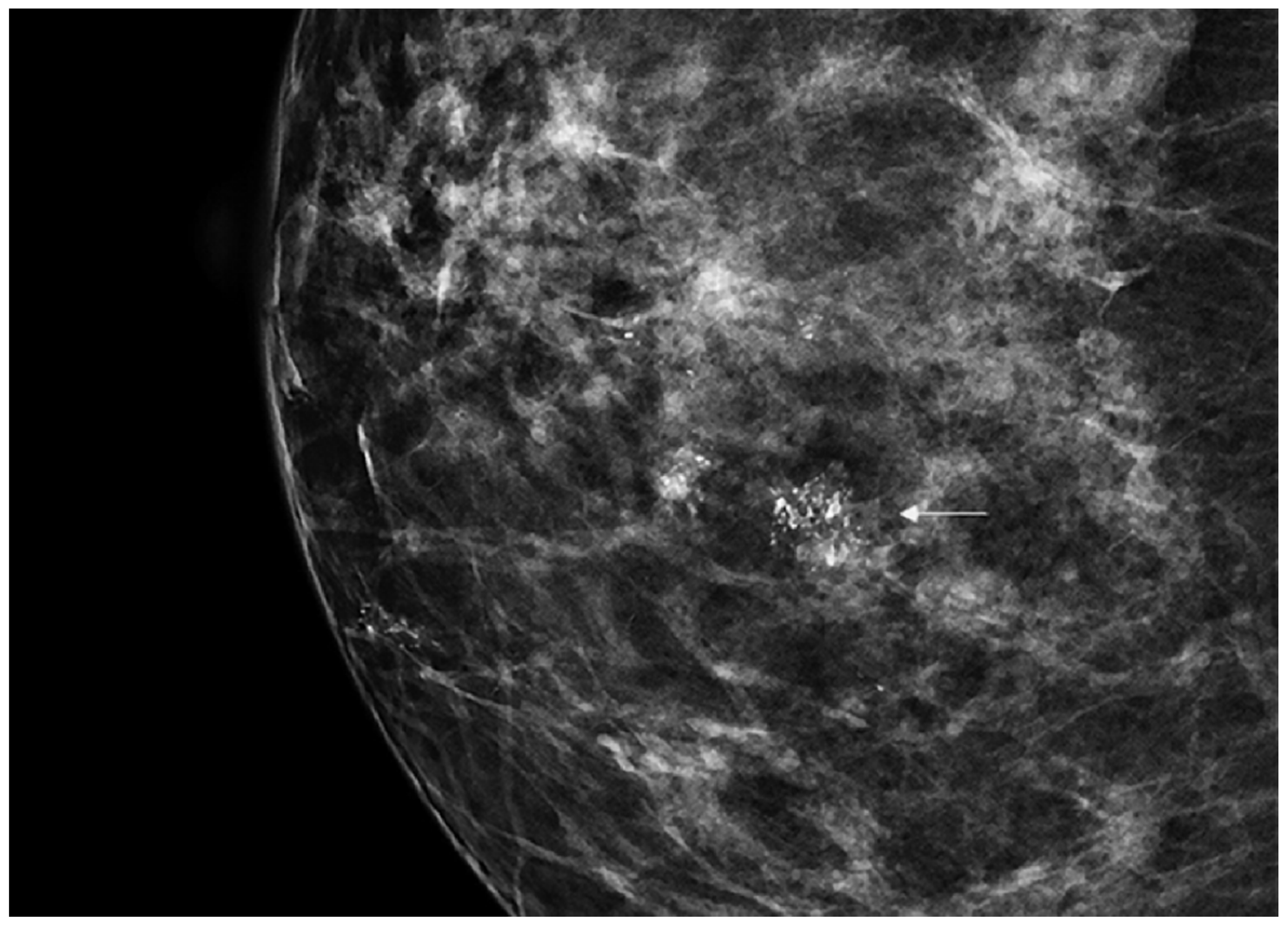
3. Results
3.1. DCIS Subcategories
3.1.1. DIN1C (Low-Grade DCIS)
3.1.2. DIN2 (Intermediate-Grade DCIS)
3.1.3. DIN3 (High-Grade DCIS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Parikh, U.; Chhor, C.M.; Mercado, C.L. Ductal Carcinoma In Situ: The Whole Truth. AJR Am. J. Roentgenol. 2018, 210, 246–255. [Google Scholar] [CrossRef]
- Worni, M.; Akushevich, I.; Greenup, R.; Sarma, D.; Ryser, M.D.; Myers, E.R.; Hwang, E.S. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J. Natl. Cancer Inst. 2015, 107, djv263. [Google Scholar] [CrossRef] [Green Version]
- Narod, S.A.; Iqbal, J.; Giannakeas, V.; Sopik, V.; Sun, P. Breast Cancer Mortality after a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015, 1, 888–896. [Google Scholar] [CrossRef]
- Buerger, H.; Otterbach, F.; Simon, R.; Schäfer, K.L.; Poremba, C.; Diallo, R.; Brinkschmidt, C.; Dockhorn-Dworniczak, B.; Boecker, W. Different genetic pathways in the evolution of invasive breast cancer are associated with distinct morphological subtypes. J. Pathol. 1999, 189, 521–526. [Google Scholar] [CrossRef]
- Simpson, P.T.; Reis-Filho, J.S.; Gale, T.; Lakhani, S.R. Molecular evolution of breast cancer. J. Pathol. 2005, 205, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Tot, T. DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch. 2005, 447, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sollie, T.; Tot, T.; Pinder, S.E.; Amini, R.M.; Blomqvist, C.; Fjällskog, M.L.; Christensson, G.; Abdsaleh, S.; Wärnberg, F. Breast cancer with neoductgenesis: Histopathological criteria and its correlation with mammographic and tumour features. Int. J. Breast Cancer 2014, 2014, 581706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzeroni, M.; DeCensi, A. De-Escalating Treatment of Low-Risk Breast Ductal Carcinoma In Situ. J. Clin. Oncol. 2020, 38, 1252–1254. [Google Scholar] [CrossRef]
- Kanbayashi, C.; Thompson, A.M.; Hwang, E.S.; Partridge, A.H.; Rea, D.W.; Wesseling, J.; Shien, T.; Mizutani, T.; Shibata, T.; Iwata, H. The international collaboration of active surveillance trials for low-risk DCIS (LORIS, LORD, COMET, LORETTA). J. Clin. Oncol. 2019, 37 (Suppl. 15), TPS603. [Google Scholar] [CrossRef]
- Francis, A.; Thomas, J.; Fallowfield, L.; Wallis, M.; Bartlett, J.M.; Brookes, C.; Roberts, T.; Pirrie, S.; Gaunt, C.; Young, J.; et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur. J. Cancer 2015, 51, 2296–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.S.; Hyslop, T.; Lynch, T.; Frank, E.; Pinto, D.; Basila, D.; Collyar, D.; Bennett, A.; Kaplan, C.; Rosenberg, S.; et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: A phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open 2019, 9, e026797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshof, L.E.; Tryfonidis, K.; Slaets, L.; Van Leeuwen-Stok, A.E.; Skinner, V.P.; Dif, N.; Pijnappel, R.M.; Bijker, N.; Rutgers, E.J.; Wesseling, J. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ-The LORD study. Eur. J. Cancer 2015, 51, 1497–1510. [Google Scholar] [CrossRef] [Green Version]
- Kanbayashi, C.; Iwata, H. Current approach and future perspective for ductal carcinoma in situ of the breast. Jpn. J. Clin. Oncol. 2017, 47, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, M.E.; Turner, R.M.; Ciatto, S.; Marinovich, M.L.; French, J.R.; Macaskill, P.; Houssami, N. Ductal carcinoma in situ at core-needle biopsy: Meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 2011, 260, 119–128. [Google Scholar] [CrossRef]
- Fahrbach, K.; Sledge, I.; Cella, C.; Linz, H.; Ross, S.D. A comparison of the accuracy of two minimally invasive breast biopsy methods: A systematic literature review and meta-analysis. Arch. Gynecol. Obstet 2006, 274, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Monti, S.; Mastropasqua, M.G. DCIS and LCIS are confusing and outdated terms. They should be abandoned in favor of ductal intraepithelial neoplasia (DIN) and lobular intraepithelial neoplasia (LIN). Breast 2013, 8, 47–61. [Google Scholar] [CrossRef]
- Mastropasqua, M.G.; Viale, G. Clinical and pathological assessment of high-risk ductal and lobular breast lesions: What surgeons must know. Eur. J. Surg. Oncol. 2017, 43, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.C.; Bose, S.; Chen, Y.Y.; Connolly, J.L.; De Baca, M.E.; Fitzgibbons, P.L.; Hayes, D.F.; Kleer, C.; O’Malley, F.P.; Page, D.L.; et al. Members of the Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch. Pathol. Lab. Med. 2009, 133, 15–25. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013; ISBN 155903016X. [Google Scholar]
- Soumian, S.; Verghese, E.T.; Booth, M.; Sharma, N.; Chaudhri, S.; Bradley, S.; Umranikar, S.; Millican-Slater, R.A.; Hanby, A.M.; Francis, A. Concordance between vacuum assisted biopsy and postoperative histology: Implications for the proposed Low Risk DCIS Trial (LORIS). Eur. J. Surg. Oncol. 2013, 39, 1337–1340. [Google Scholar] [CrossRef]
- Pilewskie, M.; Stempel, M.; Rosenfeld, H.; Eaton, A.; Van Zee, K.J.; Morrow, M. Do LORIS Trial Eligibility Criteria Identify a Ductal Carcinoma In Situ Patient Population at Low Risk of Upgrade to Invasive Carcinoma? Ann. Surg. Oncol. 2016, 23, 3487–3493. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.J.; Ryser, M.D.; Partridge, A.H.; Thompson, A.M.; Thomas, J.S.; Wesseling, J.; Hwang, E.S. Surgical Upstaging Rates for Vacuum Assisted Biopsy Proven DCIS: Implications for Active Surveillance Trials. Ann. Surg. Oncol. 2017, 24, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.C.; Chen, S.C.; Ueng, S.H.; Yu, C.C. Ductal Carcinoma In Situ Underestimation of Microcalcifications Only by Stereotactic Vacuum-Assisted Breast Biopsy: A New Predictor of Specimens without Microcalcifications. J. Clin. Med. 2020, 9, 2999. [Google Scholar] [CrossRef] [PubMed]



| Study | LORIS [11] | COMET [12] | LORD [13] | LORETTA [14] |
|---|---|---|---|---|
| Country | UK | USA | EU | Japan |
| Year of activation | 2014 | 2017 | 2017 | 2017 |
| Accrual target (number of patients) | 932 | 1200 | 1240 | 340 |
| Minimum age at diagnosis (years) | 48 | 40 | 45 | 40 |
| Comedonecrosis | Excluded | Allowed | Excluded | Excluded |
| Hormone receptor status | Any | HR-positive only | Any | HR-positive only |
| Size of the lesion | Any | Any | Any | <2.5 cm |
| Type of guide for biopsy | Stereotactic (vacuum-assisted) | Stereotactic (vacuum-assisted) | Stereotactic (vacuum-assisted) | Stereotactic and ultrasound (vacuum-assisted) |
| Endocrine therapy | Optional | Optional | Not allowed | Mandatory |
| Clincico-Pathologic Features | DIN1C | DIN2 | DIN3 | Overall |
|---|---|---|---|---|
| Patient number | 408 | 1262 | 503 | 2173 |
| Age at VABB, mean (years) | 50 (40–82) | 54 (43–87) | 49 (44–85) | 54 (40–87) |
| Mean diameter of the lesion (mm) | 22 (5–75) | 20 (7–60) | 25 (4–80) | 20 (5–80) |
| BIRADS (Breast Imaging-Reporting and Data System) [20] | ||||
| BIRADS 3 | 10 (2.4%) | 2 (0.2%) | 0 (0%) | 12 (0.5%) |
| BIRADS 4a | 308 (75.6%) | 952 (75.5%) | 10 (1.9%) | 1270 (58.4%) |
| BIRADS 4b | 60 (14.7%) | 248 (19.6%) | 102 (20.2%) | 410 (18.9%) |
| BIRADS 4c | 27 (6.6%) | 50 (3.9%) | 345 (68.7%) | 422 (19.4%) |
| BIRADS 5 | 3 (0.7%) | 10 (0.8%) | 46 (9.2%) | 59 (2.7%) |
| Absence of residual disease post-VABB | 159 (39%) | 420 (33.3%) | 206 (41%) | 785 (36.1%) |
| Family history | 230 (56.3%) | 754 (59.7%) | 330 (65.6%) | 1314 (60.5%) |
| Residual Disease Status of Diagnostic Categories | Comments | p-Value for Testing Differences between the Two Proportions (Absence and Presence of Residual Disease) | ||||
|---|---|---|---|---|---|---|
| Absence of Residual Disease Post-Biopsy (DIN1C) | Percentage of upgrading rate from DCIS to invasive disease = 5.7% | p < 0.05 | ||||
| Final Surgical Evaluation | ||||||
| VABB Result | Negative | DIN1C | IN | Total | ||
| DIN1C | 19 | 131 | 9 | 159 | ||
| Presence of Residual Disease Post-Biopsy (DIN1C) | Percentage of upgrading rate from DCIS to invasive disease = 12% | |||||
| Final Surgical Evaluation | ||||||
| VABB Result | Negative | DIN1C | IN | Total | ||
| DIN1C | 43 | 176 | 30 | 249 | ||
| Absence of Residual Disease Post-Biopsy (DIN2) | Percentage of upgrading rate from DCIS to invasive disease = 7.8% | p < 0.05 | ||||
| Final Surgical Evaluation | ||||||
| VABB Result | Negative | DIN2 | IN | Total | ||
| DIN2 | 49 | 338 | 33 | 420 | ||
| Presence of residual disease post biopsy (DIN2) | Percentage of upgrading rate from DCIS to invasive disease = 18.7% | |||||
| Final surgical evaluation | ||||||
| VABB Result | Negative | DIN2 | IN | Total | ||
| DIN2 | 34 | 650 | 158 | 842 | ||
| Absence of Residual Disease Post-Biopsy (DIN3) | Percentage of upgrading rate from DCIS to invasive disease = 11.2% | p < 0. 05 | ||||
| Final Surgical Evaluation | ||||||
| VABB Result | Negative | DIN3 | IN | Total | ||
| DIN3 | 14 | 169 | 23 | 206 | ||
| Presence of Residual Disease Post-Biopsy (DIN3) | Percentage of upgrading rate from DCIS to invasive disease = 25.9% | |||||
| Final Surgical Evaluation | ||||||
| VABB Result | Negative | DIN3 | IN | Total | ||
| DIN3 | 31 | 189 | 77 | 297 | ||
| Absence of Residual Disease Post-Biopsy (Overall) | Percentage of upgrading rate from DCIS to invasive disease = 8.2% | p < 0.05 | ||||
| Final Surgical Evaluation | ||||||
| VABB Result | Negative | DIN | IN | Total | ||
| Overall | 72 | 638 | 65 | 785 | ||
| Presence of Residual Disease Post-Biopsy (Overall) | Percentage of upgrading rate from DCIS to invasive disease = 19% | |||||
| Final Surgical Evaluation | ||||||
| VABB Result | Negative | DIN | IN | Total | ||
| Overall | 108 | 1015 | 265 | 1388 | ||
| References | No. Patients | Years | Biopsy Type | Upstaging Rate to Invasive Cancer |
|---|---|---|---|---|
| Brennan et al. (meta-analysis of 52 studies) [15] | 7350 | 1996–2011 | Variable | 26% overall 21% non-high-grade 32% high-grade |
| Soumian et al. [21] | 225 | 2001–2010 | VABB | 18% overall 10% low-grade 23% high-grade |
| Pilewskie et al. [22] | 296 | 2009–2012 | Variable | 8% low-grade 22% intermediate-grade |
| Grimm et al. [23] | 307 | 2008–2015 | VABB | 17% overall 7% low-grade 7% intermediate-grade 23% high-grade |
| Current study | 2173 | 2000–2018 | VABB | 15.2% overall 9.6% low-grade 15.1% intermediate-grade 19.3% high-grade |
| Current study (post-biopsy removal of the lesion) | 2173 | 2000–2018 | VABB | 8.2% overall 5.6% low-grade 7.8% intermediate-grade 11.1% high-grade |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicosia, L.; di Giulio, G.; Bozzini, A.C.; Fanizza, M.; Ballati, F.; Rotili, A.; Lazzeroni, M.; Latronico, A.; Abbate, F.; Renne, G.; et al. Complete Removal of the Lesion as a Guidance in the Management of Patients with Breast Ductal Carcinoma In Situ. Cancers 2021, 13, 868. https://doi.org/10.3390/cancers13040868
Nicosia L, di Giulio G, Bozzini AC, Fanizza M, Ballati F, Rotili A, Lazzeroni M, Latronico A, Abbate F, Renne G, et al. Complete Removal of the Lesion as a Guidance in the Management of Patients with Breast Ductal Carcinoma In Situ. Cancers. 2021; 13(4):868. https://doi.org/10.3390/cancers13040868
Chicago/Turabian StyleNicosia, Luca, Giuseppe di Giulio, Anna Carla Bozzini, Marianna Fanizza, Francesco Ballati, Anna Rotili, Matteo Lazzeroni, Antuono Latronico, Francesca Abbate, Giuseppe Renne, and et al. 2021. "Complete Removal of the Lesion as a Guidance in the Management of Patients with Breast Ductal Carcinoma In Situ" Cancers 13, no. 4: 868. https://doi.org/10.3390/cancers13040868









