Photodynamic Therapy as a Potent Radiosensitizer in Head and Neck Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Antibodies and Reagents
2.3. Photodynamic Therapy
2.4. Ionizing Radiation Treatment (XRT)
2.5. Clonogenic Cell Survival Assay
2.6. DEVDase Activity Assay
2.7. Detection of Splice Variants of X-Box Binding Protein-1 (XBP1) mRNA by RT-PCR Analysis
2.8. Establishment of p62-Knockdown WSU12 Cell Line
2.9. Statistical Analysis
2.10. Immunoblot Analysis
3. Results
3.1. HPV-Negative HNSCC Cell Lines Show Increased Basal Autophagy and Fail to Undergo Apoptosis after XRT, Whereas HPV-Positive HNSCC Cell Lines Are Prone to XRT-Induced Apoptosis
3.2. PDT Directed at ER/Mitochondria Promotes Efficacy of XRT in HPV-Negative HNSCC Cells
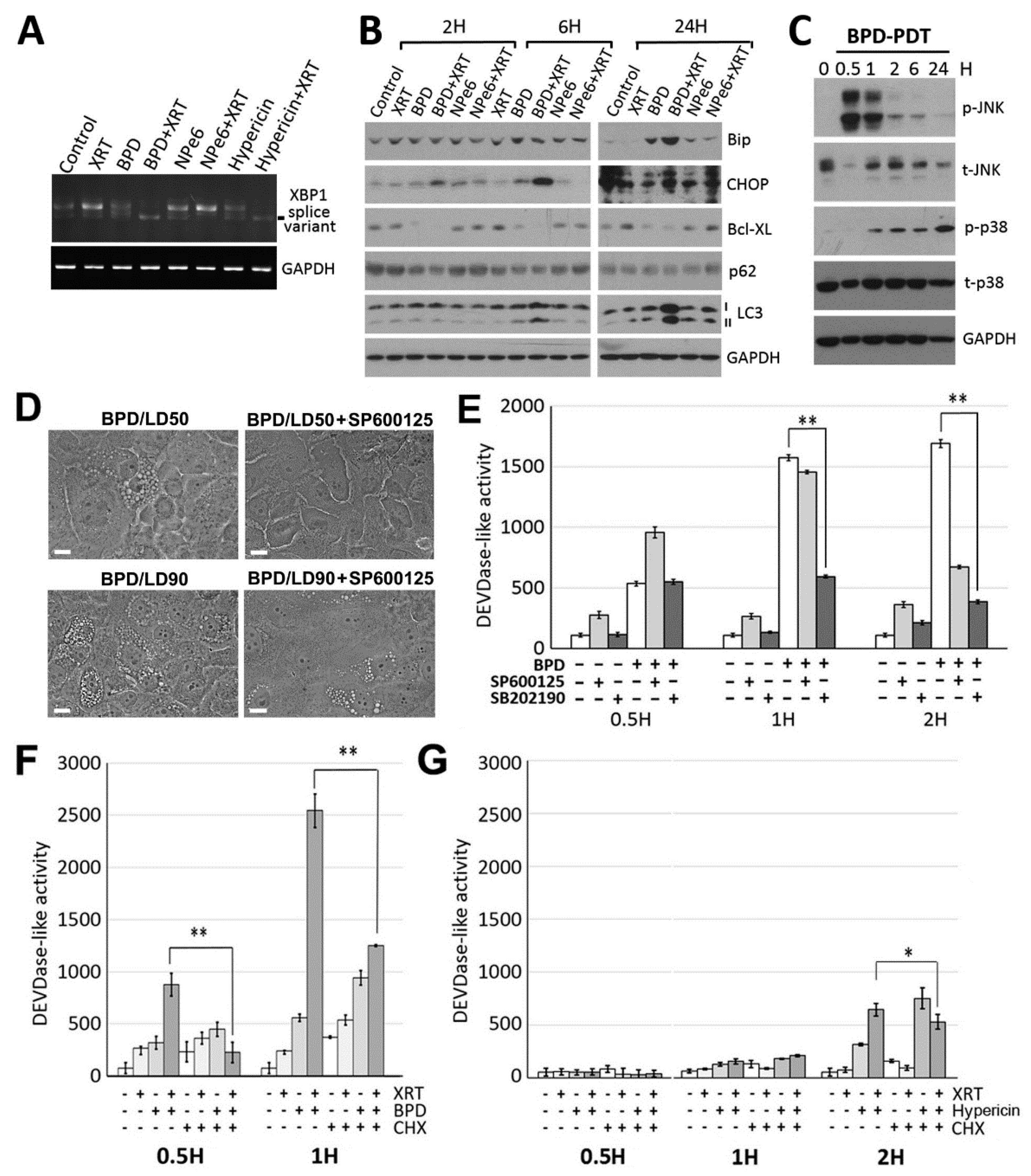
3.3. ER/mitochondria Photodamage Induces Paraptosis via JNK Activation Followed by Apoptosis Induction
3.4. BPD-PDT Sensitizes WSU12 Cells to XRT via Apoptosis Induction; Implication of the Involvement of ER Stress/Paraptotic Signaling Pathways
3.5. A Critical Role of the Autophagic Adaptor p62 in the Signal Relay for BPD-PDT-Mediated Apoptosis and Radiosensitization in Nonviral HNSCC Cells
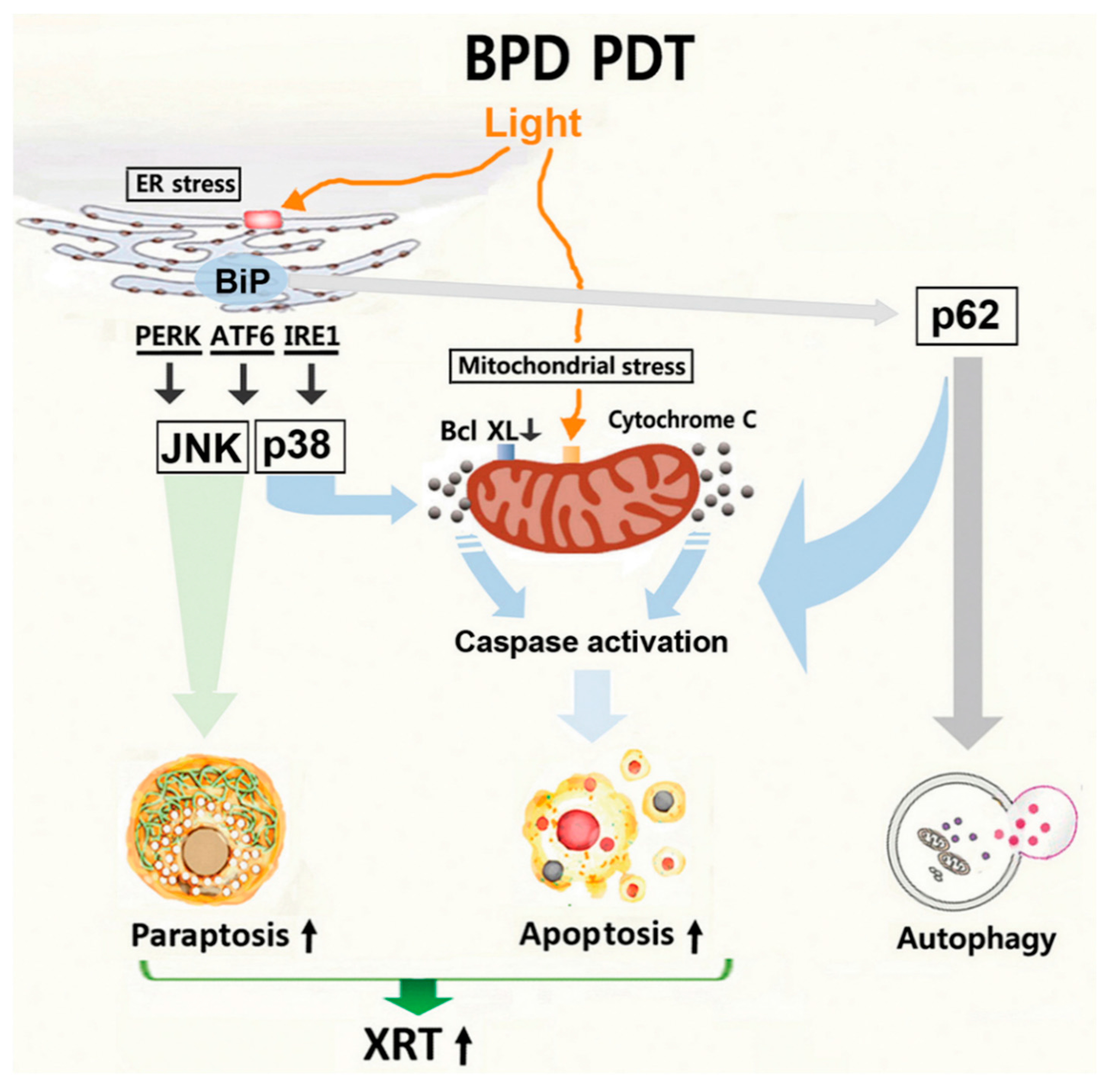
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braakhuis, B.J.; Brakenhoff, R.H.; Leemans, C.R. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann. Oncol. 2012, 23, x173–x177. [Google Scholar] [CrossRef] [PubMed]
- Attner, P.; Du, J.; Nasman, A.; Hammarstedt, L.; Ramqvist, T.; Lindholm, J.; Marklund, L.; Dalianis, T.; Munck-Wikland, E. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int. J. Cancer 2010, 126, 2879–2884. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, L.; Lindquist, D.; Dahlstrand, H.; Romanitan, M.; Dahlgren, L.O.; Joneberg, J.; Creson, N.; Lindholm, J.; Ye, W.; Dalianis, T.; et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int. J. Cancer 2006, 119, 2620–2623. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Hong, A.M.; Dobbins, T.A.; Lee, C.S.; Jones, D.; Harnett, G.B.; Armstrong, B.K.; Clark, J.R.; Milross, C.G.; Kim, J.; O’Brien, C.J.; et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br. J. Cancer 2010, 103, 1510–1517. [Google Scholar] [CrossRef]
- Sethi, S.; Ali-Fehmi, R.; Franceschi, S.; Struijk, L.; van Doorn, L.J.; Quint, W.; Albashiti, B.; Ibrahim, M.; Kato, I. Characteristics and survival of head and neck cancer by HPV status: A cancer registry-based study. Int. J. Cancer 2012, 131, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.K.; Patel, M.R.; Yin, X.Y.; Sundaram, S.; Fritchie, K.; Zhao, N.; Liu, Y.; Freemerman, A.J.; Wilkerson, M.D.; Walter, V.; et al. High XRCC1 protein expression is associated with poorer survival in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2011, 17, 6542–6552. [Google Scholar] [CrossRef]
- Lill, C.; Kornek, G.; Bachtiary, B.; Selzer, E.; Schopper, C.; Mittlboeck, M.; Burian, M.; Wrba, F.; Thurnher, D. Survival of patients with HPV-positive oropharyngeal cancer after radiochemotherapy is significantly enhanced. Wien. Klin. Wochenschr. 2011, 123, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Mellin, H.; Friesland, S.; Lewensohn, R.; Dalianis, T.; Munck-Wikland, E. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int. J. Cancer 2000, 89, 300–304. [Google Scholar] [CrossRef]
- Sedaghat, A.R.; Zhang, Z.; Begum, S.; Palermo, R.; Best, S.; Ulmer, K.M.; Levine, M.; Zinreich, E.; Messing, B.P.; Gold, D.; et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope 2009, 119, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Worden, F.P.; Kumar, B.; Lee, J.S.; Wolf, G.T.; Cordell, K.G.; Taylor, J.M.; Urba, S.G.; Eisbruch, A.; Teknos, T.N.; Chepeha, D.B.; et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: Response and survival positively associated with HPV16 copy number. J. Clin. Oncol. 2008, 26, 3138–3146. [Google Scholar] [CrossRef] [PubMed]
- Ow, T.J.; Pitts, C.E.; Kabarriti, R.; Garg, M.K. Effective Biomarkers and Radiation Treatment in Head and Neck Cancer. Arch. Pathol. Lab. Med. 2015, 139, 1379–1388. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Baine, M.J.; Souchek, J.J.; Menning, M.; Kaur, S.; Yan, Y.; Ouellette, M.M.; Jain, M.; Lin, C.; Batra, S.K. Biological determinants of radioresistance and their remediation in pancreatic cancer. Biochim Biophys Acta. Rev. Cancer 2017, 1868, 69–92. [Google Scholar] [CrossRef]
- Monel, B.; Compton, A.A.; Bruel, T.; Amraoui, S.; Burlaud-Gaillard, J.; Roy, N.; Guivel-Benhassine, F.; Porrot, F.; Genin, P.; Meertens, L.; et al. Zika virus induces massive cytoplasmic vacuolization and paraptosis-like death in infected cells. EMBO. J. 2017, 36, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Codogno, P.; Meijer, A.J. Autophagy and signaling: Their role in cell survival and cell death. Cell Death. Differ. 2005, 12, 1509–1518. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Photodynamic therapy and cell death pathways. Methods. Mol. Biol 2010, 635, 35–46. [Google Scholar] [PubMed]
- Del Duca, E.; Manfredini, M.; Petrini, N.; Farnetani, F.; Chester, J.; Bennardo, L.; Schipani, G.; Tamburi, F.; Sannino, M.; Cannarozzo, G.; et al. Daylight Photodynamic Therapy with 5-aminolevulinic acid 5% gel for the treatment of mild-to-moderate inflammatory acne. G. Ital. Dermatol. Venereol. 2019. [Google Scholar] [CrossRef]
- Nam, J.S.; Kang, M.G.; Kang, J.; Park, S.Y.; Lee, S.J.; Kim, H.T.; Seo, J.K.; Kwon, O.H.; Lim, M.H.; Rhee, H.W.; et al. Endoplasmic Reticulum-Localized Iridium(III) Complexes as Efficient Photodynamic Therapy Agents via Protein Modifications. J. Am. Chem. Soc. 2016, 138, 10968–10977. [Google Scholar] [CrossRef]
- Jung, Y.S.; Najy, A.J.; Huang, W.; Sethi, S.; Snyder, M.; Sakr, W.; Dyson, G.; Huttemann, M.; Lee, I.; Ali-Fehmi, R.; et al. HPV-associated differential regulation of tumor metabolism in oropharyngeal head and neck cancer. Oncotarget 2017, 8, 51530–51541. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F. Autophagy modulation for cancer therapy. Cancer Biol. Ther. 2011, 11, 169–176. [Google Scholar] [CrossRef]
- Carew, J.S.; Kelly, K.R.; Nawrocki, S.T. Autophagy as a target for cancer therapy: New developments. Cancer Manag. Res. 2012, 4, 357–365. [Google Scholar] [PubMed]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death. Dis. 2013, 4, e838. [Google Scholar] [CrossRef]
- Sperandio, S.; Poksay, K.; de Belle, I.; Lafuente, M.J.; Liu, B.; Nasir, J.; Bredesen, D.E. Paraptosis: Mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death. Differ. 2004, 11, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Wang, X.; Wang, P.; Zhang, K.; Liu, Q. Role of p38MAPK in apoptosis and autophagy responses to photodynamic therapy with Chlorin e6. Photodiagnosis. Photodyn. Ther. 2015, 12, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Duan, C.Y.; Chen, S.K.; Zhang, C.Y.; He, T.; Li, H.; Liu, Y.P.; Dai, R.Y. The suppressive role of p38 MAPK in cellular vacuole formation. J. Cell Biochem. 2013, 114, 1789–1799. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends. Cell Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Maurel, M.; Chevet, E.; Tavernier, J.; Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends. Biochem. Sci. 2014, 39, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2alpha/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Luo, Y.; Li, G.; Kessel, D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999, 59, 3429–3432. [Google Scholar] [PubMed]
- Cha-Molstad, H.; Sung, K.S.; Hwang, J.; Kim, K.A.; Yu, J.E.; Yoo, Y.D.; Jang, J.M.; Han, D.H.; Molstad, M.; Kim, J.G.; et al. Amino-terminal arginylation targets endoplasmic reticulum chaperone BiP for autophagy through p62 binding. Nat. Cell Biol. 2015, 17, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Cho, W.J.; Rakowski, J.; Kim, H.E.; Kim, H.C. Effects of HPV Status on Responsiveness to Ionizing Radiation vs Photodynamic Therapy in Head and Neck Cancer Cell lines. Photochem. Photobiol. 2020, 96, 652–657. [Google Scholar] [CrossRef]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Lee, D.H.; Dilly, A.K.; Lee, Y.S.; Choudry, H.A.; Kwon, Y.T.; Bartlett, D.L.; Lee, Y.J. Crosstalk Between Apoptosis and Autophagy Is Regulated by the Arginylated BiP/Beclin-1/p62 Complex. Mol. Cancer Res. 2018, 16, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Ullman, E.; Pan, J.A.; Zong, W.X. Squamous cell carcinoma antigen 1 promotes caspase-8-mediated apoptosis in response to endoplasmic reticulum stress while inhibiting necrosis induced by lysosomal injury. Mol. Cell Biol. 2011, 31, 2902–2919. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Pitti, R.; Lawrence, D.; Pham, V.C.; Lill, J.R.; Ashkenazi, A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 2009, 137, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.; Todryk, S.; Hardwick, N.; Ford, M.; Jacobson, M.; Vile, R.G. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 1998, 4, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Biel, M.A. Photodynamic therapy of head and neck cancers. Methods. Mol. Biol. 2010, 635, 281–293. [Google Scholar] [PubMed]
- Rigual, N.R.; Thankappan, K.; Cooper, M.; Sullivan, M.A.; Dougherty, T.; Popat, S.R.; Loree, T.R.; Biel, M.A.; Henderson, B. Photodynamic therapy for head and neck dysplasia and cancer. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Mimikos, C.; Shafirstein, G.; Arshad, H. Current state and future of photodynamic therapy for the treatment of head and neck squamous cell carcinoma. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 126–129. [Google Scholar] [CrossRef] [PubMed]
- van Doeveren, T.E.M.; Karakullukcu, M.B.; van Veen, R.L.P.; Lopez-Yurda, M.; Schreuder, W.H.; Tan, I.B. Adjuvant photodynamic therapy in head and neck cancer after tumor-positive resection margins. Laryngoscope 2018, 128, 657–663. [Google Scholar] [CrossRef]



| Photosensitizing Agent | Light Dose (mJ/cm2) | WSU12 | UP154 | ||
|---|---|---|---|---|---|
| Paraptosis | Apoptosis | Paraptosis | Apoptosis | ||
| BPD | 45 | + | − | − | − |
| 90 | +++ | + | − | − | |
| 135 | ++ | + | − | − | |
| 180 | + | ++ | − | − | |
| Hypericin | 15 | + | - | − | − |
| 30 | +++ | - | − | − | |
| 45 | ++ | + | − | − | |
| 60 | + | + | − | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.J.; Kessel, D.; Rakowski, J.; Loughery, B.; Najy, A.J.; Pham, T.; Kim, S.; Kwon, Y.T.; Kato, I.; Kim, H.E.; et al. Photodynamic Therapy as a Potent Radiosensitizer in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 1193. https://doi.org/10.3390/cancers13061193
Cho WJ, Kessel D, Rakowski J, Loughery B, Najy AJ, Pham T, Kim S, Kwon YT, Kato I, Kim HE, et al. Photodynamic Therapy as a Potent Radiosensitizer in Head and Neck Squamous Cell Carcinoma. Cancers. 2021; 13(6):1193. https://doi.org/10.3390/cancers13061193
Chicago/Turabian StyleCho, Won Jin, David Kessel, Joseph Rakowski, Brian Loughery, Abdo J. Najy, Tri Pham, Seongho Kim, Yong Tae Kwon, Ikuko Kato, Harold E. Kim, and et al. 2021. "Photodynamic Therapy as a Potent Radiosensitizer in Head and Neck Squamous Cell Carcinoma" Cancers 13, no. 6: 1193. https://doi.org/10.3390/cancers13061193
APA StyleCho, W. J., Kessel, D., Rakowski, J., Loughery, B., Najy, A. J., Pham, T., Kim, S., Kwon, Y. T., Kato, I., Kim, H. E., & Kim, H.-R. C. (2021). Photodynamic Therapy as a Potent Radiosensitizer in Head and Neck Squamous Cell Carcinoma. Cancers, 13(6), 1193. https://doi.org/10.3390/cancers13061193








