Comparative Analysis of Genetic Alterations, HPV-Status, and PD-L1 Expression in Neuroendocrine Carcinomas of the Cervix
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DNA Preparation and Next-Generation Sequencing
2.3. Classification of Oncogenic/Pathogenic Mutations
2.4. Detection of Copy Number Alterations Using the Taqman Assay
2.5. Identification of Human Papillomavirus (HPV) Genotyping by Sanger Sequencing
2.6. Detection of High-Risk HPV Types
2.7. Immunohistochemistry
2.8. Large-Scale Genomic Datasets
2.9. Clinical Association and Actionability Analysis
2.10. Statistical Analysis
3. Results
3.1. Patient Demographics
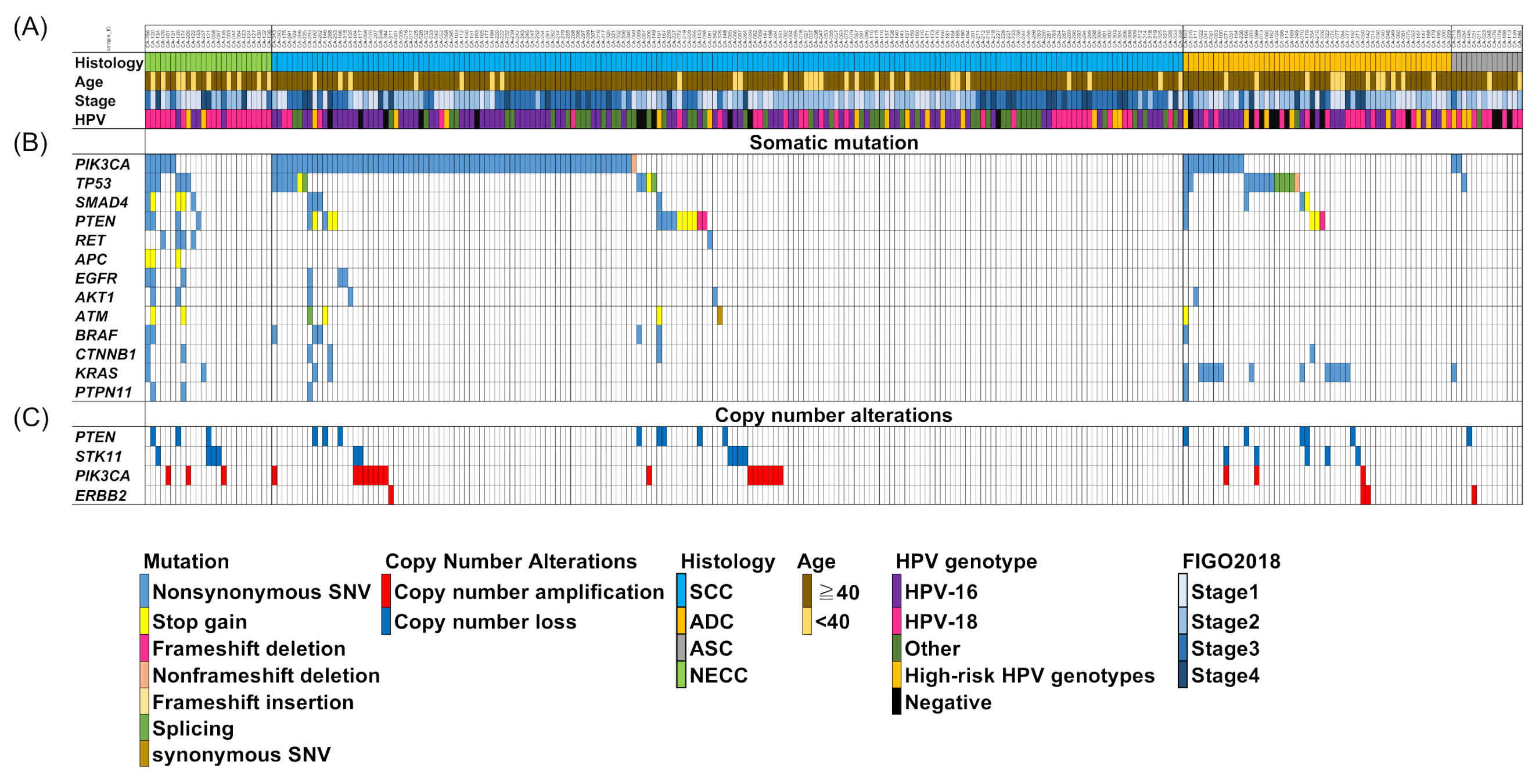
3.2. Different Genetic Alterations between Neuroendocrine Carcinoma of the Cervix (NECC) and Other Histological Types in Patients with Japanese Cervical Cancer
3.3. Comparison of Frequency of Mutation between NECC and Other Histological Types of Cervical Cancer in the Project GENIE Database
3.4. Immunohistichemical Detection of the Expression of RB1 and PD-L1
3.5. Actionable Mutations in NECC
3.6. Association between HPV Genotypes and Genetic Alterations in NECC
3.7. Prognostic Factors of NECC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative Study of Lung and Extrapulmonary Poorly Differentiated Neuroendocrine Carcinomas: A SEER Database Analysis of 162,983 Cases. Cancer 2018, 124, 807–815. [Google Scholar] [CrossRef]
- Nagase, S.; Ohta, T.; Takahashi, F.; Enomoto, T. 2017 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual Report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology: Annual Patients Report for 2015 and Annual Treatment Report for 2010. J. Obstet. Gynaecol. Res. 2019, 45, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvo, G.; Gonzalez Martin, A.; Gonzales, N.R.; Frumovitz, M. Updates and Management Algorithm for Neuroendocrine Tumors of the Uterine Cervix. Int. J. Gynecol. Cancer 2019, 29, 986–995. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Tischoff, I.; Dogan, A.; Hilal, Z.; Schultheis, B.; Kern, P.; Rezniczek, G.A. Neuroendocrine Carcinoma of the Cervix: A Systematic Review of the Literature. BMC Cancer 2018, 18, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Kasamatsu, T.; Tsuda, H.; Fukunaga, M.; Sakamoto, A.; Kaku, T.; Nakanishi, T.; Hasumi, Y.; Iwata, T.; Baba, T.; et al. Prognostic Factors and Optimal Therapy for Stages I-II Neuroendocrine Carcinomas of the Uterine Cervix: A Multi-Center Retrospective Study. Gynecol. Oncol. 2018, 148, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Dancey, J.E.; Bedard, P.L.; Onetto, N.; Hudson, T.J. The Genetic Basis for Cancer Treatment Decisions. Cell 2012, 148, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Lyons, Y.A.; Frumovitz, M.; Soliman, P.T. Response to MEK Inhibitor in Small Cell Neuroendocrine Carcinoma of the Cervix with a KRAS mutation. Gynecol. Oncol. Rep. 2014, 10, 28–29. [Google Scholar] [CrossRef] [Green Version]
- Paraghamian, S.E.; Longoria, T.C.; Eskander, R.N. Metastatic Small Cell Neuroendocrine Carcinoma of the Cervix Treated with the PD-1 Inhibitor, Nivolumab: A Case Report. Gynecol. Oncol. Res. Pract. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, D.; Zheng, G.; Schoolmeester, J.K.; Li, Z.; Pallavajjala, A.; Haley, L.; Conner, M.G.; Vang, R.; Hung, C.F.; Wu, T.C.; et al. Next-Generation Sequencing Reveals Recurrent Somatic Mutations in Small Cell Neuroendocrine Carcinoma of the Uterine Cervix. Am. J. Surg. Pathol. 2018, 42, 750–760. [Google Scholar] [CrossRef]
- Frumovitz, M.; Burzawa, J.K.; Byers, L.A.; Lyons, Y.A.; Ramalingam, P.; Coleman, R.L.; Brown, J. Sequencing of Mutational Hotspots in Cancer-Related Genes in Small Cell Neuroendocrine Cervical Cancer. Gynecol. Oncol. 2016, 141, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Eskander, R.N.; Elvin, J.; Gay, L.; Ross, J.S.; Miller, V.A.; Kurzrock, R. Unique Genomic Landscape of High-Grade Neuroendocrine Cervical Carcinoma: Implications for Rethinking Current Treatment Paradigms. JCO Precis. Oncol. 2020, 4. [Google Scholar] [CrossRef]
- Hillman, R.T.; Cardnell, R.; Fujimoto, J.; Lee, W.C.; Zhang, J.; Byers, L.A.; Ramalingam, P.; Leitao, M.; Swisher, E.; Futreal, P.A.; et al. Comparative Genomics of High Grade Neuroendocrine Carcinoma of the Cervix. PLoS ONE 2020, 15, e0234505. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castle, P.E.; Pierz, A.; Stoler, M.H. A systematic Review and Meta-Analysis on the Attribution of Human Papillomavirus (HPV) in Neuroendocrine Cancers of the Cervix. Gynecol. Oncol. 2018, 148, 422–429. [Google Scholar] [CrossRef]
- Hirose, S.; Murakami, N.; Takahashi, K.; Kuno, I.; Takayanagi, D.; Asami, Y.; Matsuda, M.; Shimada, Y.; Yamano, S.; Sunami, K.; et al. Genomic Alterations in STK11 Can Predict Clinical Outcomes in Cervical Cancer Patients. Gynecol. Oncol. 2020, 156, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2014; 307p. [Google Scholar]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; Ms, M.J.; Shah, S.; Hanks, D.; Wang, J.; et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch. Pathol. Lab. Med. 2019, 143, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Henken, F.E.; Banerjee, N.S.; Snijders, P.J.; Meijer, C.J.; De-Castro Arce, J.; Rösl, F.; Broker, T.R.; Chow, L.T.; Steenbergen, R.D. PIK3CA-Mediated PI3-Kinase Signalling Is Essential for HPV-Induced Transformation in Vitro. Mol. Cancer 2011, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Cancer Genome Atlas Research Network; Albert Einstein College of Medicine; Analytical Biological Services; Barretos Cancer Hospital; Baylor College of Medicine; Beckman Research Institute of City of Hope; Buck Institute for Research on Aging; Canada’s Michael Smith Genome Sciences Centre; Harvard Medical School; Helen, F.; et al. Integrated Genomic and Molecular Characterization of Cervical Cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Fraguas, S.; Barberán, S.; Cebrià, F. EGFR Signaling Regulates Cell Proliferation, Differentiation and Morphogenesis during Planarian Regeneration and Homeostasis. Dev. Biol. 2011, 354, 87–101. [Google Scholar] [CrossRef]
- Wei, H.; Wang, X.W.; Chen, K.M.; Ling, S.R.; Yi, C.J. Analysis of Gene Mutation Associated with Tyrosine Kinase Inhibitor Sensitivity of Epidermal Growth Factor Receptor in Cervical Cancer Patients. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6280–6287. [Google Scholar] [CrossRef]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The Roles of TGFβ in the Tumour Microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt Signaling in Cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Ambros, R.A.; Park, J.S.; Shah, K.V.; Kurman, R.J. Evaluation of Histologic, Morphometric, and Immunohistochemical Criteria in the Differential Diagnosis of Small Cell Carcinomas of the Cervix with Particular Reference to Human Papillomavirus Types 16 and 18. Mod. Pathol. 1991, 4, 586–593. [Google Scholar] [PubMed]
- Stoler, M.H.; Mills, S.E.; Gersell, D.J.; Walker, A.N. Small-Cell Neuroendocrine Carcinoma of the Cervix. A Human Papillomavirus Type 18-Associated Cancer. Am. J. Surg. Pathol. 1991, 15, 28–32. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Alqahtani, A.; Ayesh, H.S.K.; Halawani, H. PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers 2019, 12, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Z.Y.; Yeo, H.Y.; Chen, Y.T.; Chang, K.M.; Chen, T.C.; Chuang, Y.J.; Chang, S.J. PI3K Inhibitor Enhances the Cytotoxic Response to Etoposide and Cisplatin in a Newly Established Neuroendocrine Cervical Carcinoma Cell Line. Oncotarget 2017, 8, 45323–45334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbiah, V.; Gainor, J.F.; Rahal, R.; Brubaker, J.D.; Kim, J.L.; Maynard, M.; Hu, W.; Cao, Q.; Sheets, M.P.; Wilson, D.; et al. Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov. 2018, 8, 836–849. [Google Scholar] [CrossRef] [Green Version]
- Drilon, A.E.; Subbiah, V.; Oxnard, G.R.; Bauer, T.M.; Velcheti, V.; Lakhani, N.J.; Besse, B.; Park, K.; Patel, J.D.; Cabanillas, M.E.; et al. A phase 1 Study of LOXO-292, a Potent and Highly Selective RET Inhibitor, in Patients with RET-Altered Cancers. J. Clin. Oncol. 2018, 36 (Suppl. 15), 102. [Google Scholar] [CrossRef]
- Oser, M.G.; Fonseca, R.; Chakraborty, A.A.; Brough, R.; Spektor, A.; Jennings, R.B.; Flaifel, A.; Novak, J.S.; Gulati, A.; Buss, E.; et al. Cells Lacking the RB1 Tumor Suppressor Gene Are Hyperdependent on Aurora B Kinase for Survival. Cancer Discov. 2019, 9, 230–247. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Du, J.; Parsons, S.H.; Merzoug, F.F.; Webster, Y.; Iversen, P.W.; Chio, L.C.; Van Horn, R.D.; Lin, X.; Blosser, W.; et al. Aurora A Kinase Inhibition Is Synthetic Lethal with Loss of the RB1 Tumor Suppressor Gene. Cancer Discov. 2019, 9, 248–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, C., Jr.; Johnson, C.E.; Balaji, V.N.; Balaji, G.A.; Hammer, R.D.; Kannan, R. Identification and Validation of a PD-L1 Binding Peptide for Determination of PDL1 Expression in Tumors. Sci. Rep. 2017, 7, 13682. [Google Scholar] [CrossRef] [Green Version]
- Emancipator, K.; Huang, L.; Aurora-Garg, D.; Bal, T.; Cohen, E.E.W.; Harrington, K.; Soulières, D.; Le Tourneau, C.; Licitra, L.; Burtness, B.; et al. Comparing Programmed Death Ligand 1 Scores for Predicting Pembrolizumab Efficacy in Head and Neck Cancer. Mod. Pathol. 2020. [Google Scholar] [CrossRef]
- Lin, H.; Wei, S.; Hurt, E.M.; Green, M.D.; Zhao, L.; Vatan, L.; Szeliga, W.; Herbst, R.; Harms, P.W.; Fecher, L.A.; et al. Host Expression of PD-L1 Determines Efficacy of PD-L1 Pathway Blockade-Mediated Tumor Regression. J. Clin. Investig. 2018, 128, 805–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.; Zhang, D.; Xu, C.; Hance, K.W.; Marelli, B.; Qi, J.; Yu, H.; Qin, G.; Sircar, A.; Hernández, V.M.; et al. Enhanced Preclinical Antitumor Activity of M7824, a Bifunctional Fusion Protein Simultaneously Targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marté, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin. Cancer. Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Alejo, M.; Alemany, L.; Clavero, O.; Quiros, B.; Vighi, S.; Seoud, M.; Cheng-Yang, C.; Garland, S.M.; Juanpere, N.; Lloreta, J.; et al. Contribution of Human Papillomavirus in Neuroendocrine Tumors from a Series of 10,575 Invasive Cervical Cancer Cases. Papillomavirus Res. 2018, 5, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Chang, C.J.; Huang, H.J.; Hsueh, S.; Chao, A.; Yang, J.E.; Lin, C.T.; Huang, S.L.; Hong, J.H.; Chou, H.H.; et al. Role of Human Papillomavirus Genotype in Prognosis of Early-Stage Cervical Cancer Undergoing Primary Surgery. J. Clin. Oncol. 2007, 25, 3628–3634. [Google Scholar] [CrossRef]
- Onuki, M.; Matsumoto, K.; Tenjimbayashi, Y.; Tasaka, N.; Akiyama, A.; Sakurai, M.; Minaguchi, T.; Oki, A.; Satoh, T.; Yoshikawa, H. Human Papillomavirus Genotype and Prognosis of Cervical Cancer: Favorable Survival of Patients with HPV16-Positive Tumors. Papillomavirus Res. 2018, 6, 41–45. [Google Scholar] [CrossRef]
- Bratman, S.V.; Bruce, J.P.; O’Sullivan, B.; Pugh, T.J.; Xu, W.; Yip, K.W.; Liu, F.F. Human Papillomavirus Genotype Association with Survival in Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016, 2, 823–826. [Google Scholar] [CrossRef] [Green Version]
- Alexandraki, K.I.; Tsoli, M.; Kyriakopoulos, G.; Angelousi, A.; Nikolopoulos, G.; Kolomodi, D.; Kaltsas, G.A. Current Concepts in the Diagnosis and Management of Neuroendocrine Neoplasms of Unknown Primary Origin. Minerva. Endocrinol. 2019, 44, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A.; Westra, W.H. Human Papillomavirus-Related Small Cell Carcinoma of the Oropharynx. Am. J. Surg. Pathol. 2011, 35, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.W.; Nucci, M.R.; Brodsky, J.; Crum, C.P.; Hirsch, M.S. Biomarker-Assisted Diagnosis of Ovarian, Cervical and Pulmonary Small Cell Carcinomas: The Role of TTF-1, WT-1 and HPV Analysis. Histopathology 2007, 51, 305–312. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | NECC (25) | SCC (180) | ADC (53) | ASC (14) | |
|---|---|---|---|---|---|
| Age (Year) | Median (Range) | 43 (2868) | 55 (25–89) | 51 (30–82) | 47 (37–60) |
| Stage (FIGO2018), n (%) | I | 10 (40.0) | 40 (22.2) | 18 (34.0) | 7 (50.0) |
| II | 7 (28.0) | 58 (32.2) | 25 (47.2) | 6 (42.9) | |
| III | 4 (16.0) | 60 (33.3) | 3 (5.7) | 1 (7.1) | |
| IV | 4 (16.0) | 22 (12.2) | 7 (13.2) | 0 (0.0) | |
| HPV positivity, n(%) | 25 (100) | 169 (93.8) | 44 (83.0) | 11 (78.6) | |
| Treatment, n(%) | Surgery only | 6 (24.0) | 34 (18.9) | 23 (43.4) | 4 (28.6) |
| RH | 6 (24.0) | 33 (13.3) | 21 (39.6) | 4 (28.6) | |
| RH+PAN | 0 (0.0) | 1 (0.6) | 1 (1.9) | 0 (0.0) | |
| TAH+BSO+PLND+OMT | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | |
| Surgery+adj-Treatment | 9 (36.0) | 57 (31.7) | 21 (39.6) | 9 (64.3) | |
| RH | 7 (24.0) | 43 (20.6) | 16 (30.2) | 6 (42.9) | |
| RH+PAN | 1 (4.0) | 10 (5.6) | 3 (5.7) | 3 (21.4) | |
| MRHx+BSO+PLND | 0 (0.0) | 1 (0.6) | 1 (1.9) | 0 (0.0) | |
| TAH+BSO+PLND | 0 (0.0) | 2 (0.6) | 1 (0.0) | 0 (0.0) | |
| TAH+BSO | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NACT+RH+adjCT | 1 (4.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | |
| NACRT+RT | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | |
| RT | 1 (4.0) | 25 (13.9) | 3 (5.7) | 0 (0.0) | |
| CCRT only | 3 (12.0) | 62 (34.4) | 6 (11.3) | 1 (7.1) | |
| RT following CT | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| CT only | 2 (7.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Palliative care only | 2 (7.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayanagi, D.; Hirose, S.; Kuno, I.; Asami, Y.; Murakami, N.; Matsuda, M.; Shimada, Y.; Sunami, K.; Komatsu, M.; Hamamoto, R.; et al. Comparative Analysis of Genetic Alterations, HPV-Status, and PD-L1 Expression in Neuroendocrine Carcinomas of the Cervix. Cancers 2021, 13, 1215. https://doi.org/10.3390/cancers13061215
Takayanagi D, Hirose S, Kuno I, Asami Y, Murakami N, Matsuda M, Shimada Y, Sunami K, Komatsu M, Hamamoto R, et al. Comparative Analysis of Genetic Alterations, HPV-Status, and PD-L1 Expression in Neuroendocrine Carcinomas of the Cervix. Cancers. 2021; 13(6):1215. https://doi.org/10.3390/cancers13061215
Chicago/Turabian StyleTakayanagi, Daisuke, Sou Hirose, Ikumi Kuno, Yuka Asami, Naoya Murakami, Maiko Matsuda, Yoko Shimada, Kuniko Sunami, Masaaki Komatsu, Ryuji Hamamoto, and et al. 2021. "Comparative Analysis of Genetic Alterations, HPV-Status, and PD-L1 Expression in Neuroendocrine Carcinomas of the Cervix" Cancers 13, no. 6: 1215. https://doi.org/10.3390/cancers13061215
APA StyleTakayanagi, D., Hirose, S., Kuno, I., Asami, Y., Murakami, N., Matsuda, M., Shimada, Y., Sunami, K., Komatsu, M., Hamamoto, R., Kato, M. K., Matsumoto, K., Kohno, T., Kato, T., Shiraishi, K., & Yoshida, H. (2021). Comparative Analysis of Genetic Alterations, HPV-Status, and PD-L1 Expression in Neuroendocrine Carcinomas of the Cervix. Cancers, 13(6), 1215. https://doi.org/10.3390/cancers13061215







