Carfilzomib Improves Bone Metabolism in Patients with Advanced Relapsed/Refractory Multiple Myeloma: Results of the CarMMa Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.2. Eligibility Criteria and Treatment Schedule
2.3. Evaluation of SREs and Bone Metabolism
2.4. Statistical Analysis
3. Results
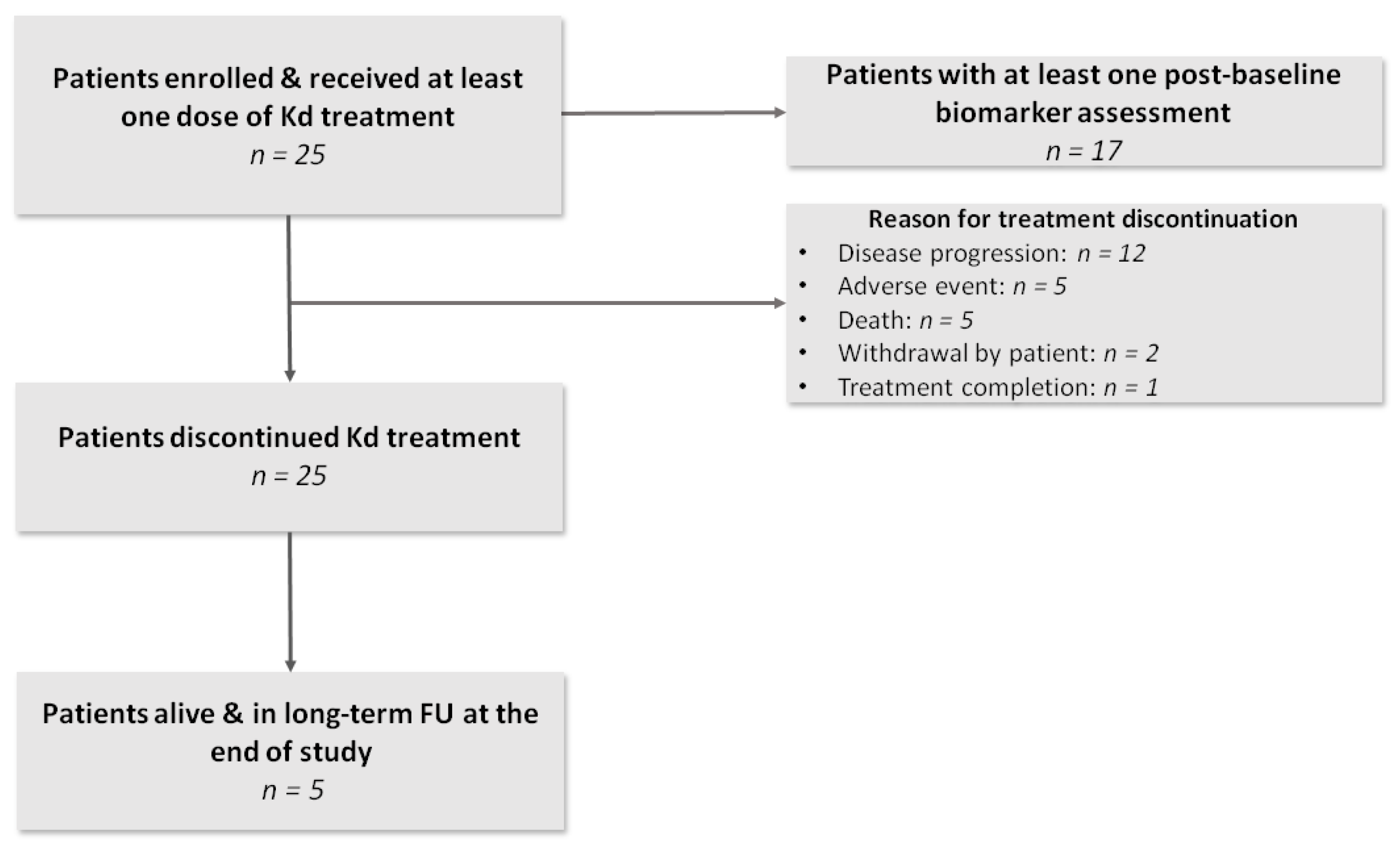
3.1. Patient and Disease Characteristics
3.2. Incidence of SREs during Treatment with Kd
3.3. Effects of Kd on Bone Metabolism
3.3.1. Indices of Bone Remodeling in RRMM Patients at Baseline Compared to Controls
3.3.2. Bone Resorption and Bone Formation
3.3.3. Osteoclast Regulators and Osteoblast Inhibitors
3.3.4. Subgroup Analyses
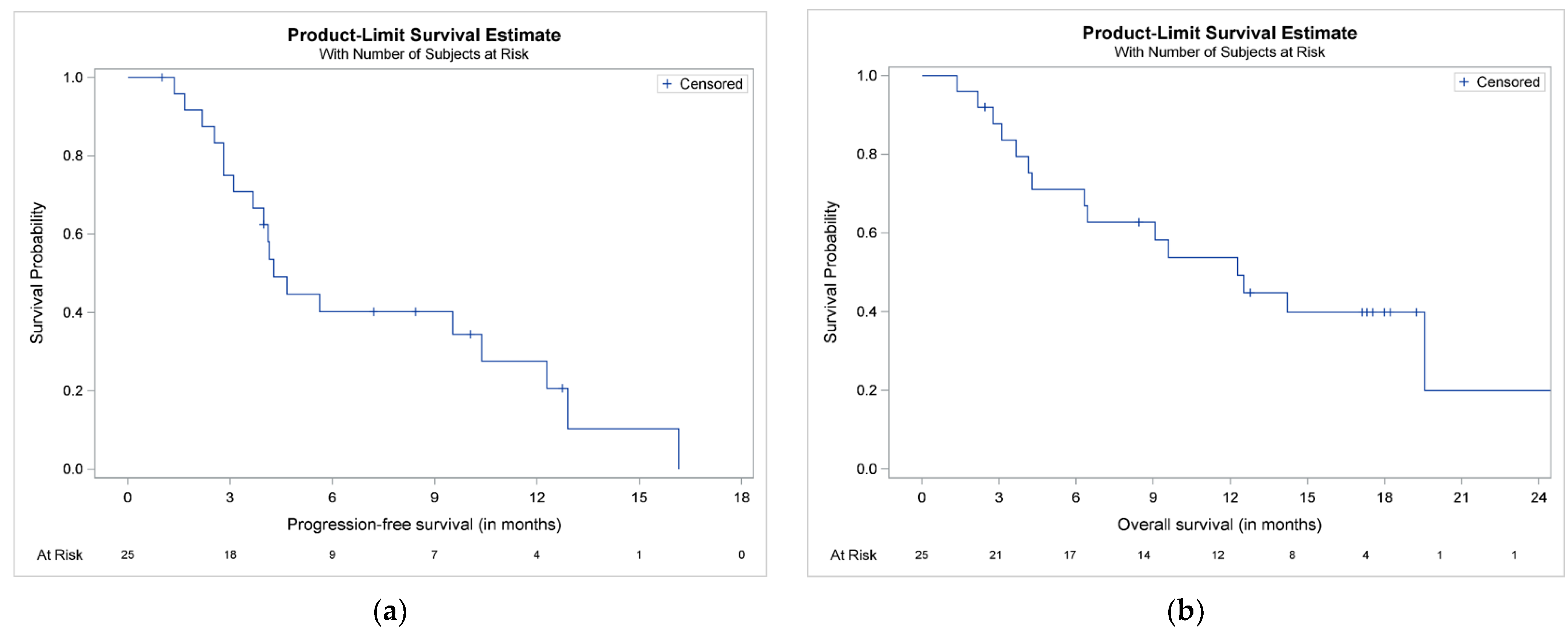
3.4. TtNT, PFS and OS
3.5. Safety Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef] [Green Version]
- Mateos, M.V.; Fink, L.; Koneswaran, N.; Intorcia, M.; Giannopoulou, C.; Niepel, D.; Cavo, M. Bone complications in patients with multiple myeloma in five European countries: A retrospective patient chart review. BMC Cancer 2020, 20, 170. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, O.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Zamagni, E.; Lentzsch, S.; Drake, M.T.; Garcia-Sanz, R.; Abildgaard, N.; Ntanasis-Stathopoulos, I.; Schjesvold, F.; de la Rubia, J.; Kyriakou, C.; et al. Treatment of multiple myeloma-related bone disease: Recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. 2021. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Terpos, E.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Eleutherakis-Papaiakovou, E.; Papatheodorou, A.; Kanellias, N.; Migkou, M.; Fotiou, D.; Dialoupi, I.; et al. Consolidation with carfilzomib, lenalidomide, and dexamethasone (KRd) following ASCT results in high rates of minimal residual disease negativity and improves bone metabolism, in the absence of bisphosphonates, among newly diagnosed patients with multiple myeloma. Blood Cancer J. 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Christoulas, D.; Papatheodorou, A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Fotiou, D.; Ziogas, D.C.; Migkou, M.; et al. Consolidation therapy with the combination of bortezomib and lenalidomide (VR) without dexamethasone in multiple myeloma patients after transplant: Effects on survival and bone outcomes in the absence of bisphosphonates. Am. J. Hematol. 2019, 94, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Kastritis, E.; Roussou, M.; Migkou, M.; Eleutherakis-Papaiakovou, E.; Gavriatopoulou, M.; Gkotzamanidou, M.; Kanellias, N.; Manios, E.; et al. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia 2014, 28, 928–934. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Mohty, B.; Savani, B.; Moreau, P.; Terpos, E. The effects of bortezomib on bone disease in patients with multiple myeloma. Cancer 2014, 120, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Accardi, F.; Toscani, D.; Bolzoni, M.; Dalla Palma, B.; Aversa, F.; Giuliani, N. Mechanism of Action of Bortezomib and the New Proteasome Inhibitors on Myeloma Cells and the Bone Microenvironment: Impact on Myeloma-Induced Alterations of Bone Remodeling. Biomed. Res. Int. 2015, 2015, 172458. [Google Scholar] [CrossRef] [Green Version]
- Zangari, M.; Suva, L.J. The effects of proteasome inhibitors on bone remodeling in multiple myeloma. Bone 2016, 86, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Kastritis, E.; Roussou, M.; Heath, D.; Christoulas, D.; Anagnostopoulos, N.; Eleftherakis-Papaiakovou, E.; Tsionos, K.; Croucher, P.; Dimopoulos, M.A. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia 2008, 22, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Terpos, E.; Richardson, P.G.; Shpilberg, O.; Khuageva, N.K.; Schlag, R.; Dimopoulos, M.A.; Kropff, M.; Spicka, I.; Petrucci, M.T.; et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur. J. Haematol. 2011, 86, 372–384. [Google Scholar] [CrossRef]
- Terpos, E.; Heath, D.J.; Rahemtulla, A.; Zervas, K.; Chantry, A.; Anagnostopoulos, A.; Pouli, A.; Katodritou, E.; Verrou, E.; Vervessou, E.C.; et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br. J. Haematol. 2006, 135, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Sezer, O.; Croucher, P.; Dimopoulos, M.A. Myeloma bone disease and proteasome inhibition therapies. Blood 2007, 110, 1098–1104. [Google Scholar] [CrossRef]
- Terpos, E.; Dimopoulos, M.A.; Sezer, O. The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia 2007, 21, 1875–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangari, M.; Terpos, E.; Zhan, F.; Tricot, G. Impact of bortezomib on bone health in myeloma: A review of current evidence. Cancer Treat. Rev. 2012, 38, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hajek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.-V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. HemaSphere 2021, 5, e528. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Terpos, E.; Dimopoulos, M.A. Real World Treatment of Patients with Relapsed/Refractory Myeloma. Clin. Lymphoma Myeloma Leuk. 2021. [Google Scholar] [CrossRef]
- Zangari, M.; Aujay, M.; Zhan, F.; Hetherington, K.L.; Berno, T.; Vij, R.; Jagannath, S.; Siegel, D.; Keith Stewart, A.; Wang, L.; et al. Alkaline phosphatase variation during carfilzomib treatment is associated with best response in multiple myeloma patients. Eur. J. Haematol. 2011, 86, 484–487. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Blade, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Burch, J.; Rice, S.; Yang, H.; Neilson, A.; Stirk, L.; Francis, R.; Holloway, P.; Selby, P.; Craig, D. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: The secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol. Assess. 2014, 18, 1–180. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Reginster, J.Y.; Crans, G.G.; Diez-Perez, A.; Pinette, K.V.; Delmas, P.D. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J. Bone Miner. Res. 2004, 19, 394–401. [Google Scholar] [CrossRef]
- Kim, C.; Bhatta, S.; Cyprien, L.; Fonseca, R.; Hernandez, R.K. Incidence of skeletal-related events among multiple myeloma patients in the United States at oncology clinics: Observations from real-world data. J. Bone Oncol. 2019, 14, 100215. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; Garcia-Sanz, R.; Durie, B.; Legiec, W.; Krejci, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, E.T.; Li, C.; Zhu, L.; Zhang, B.; Glennane, T.; Zhang, L. What is the relationship between bone turnover markers and skeletal-related events in patients with bone metastases from solid tumors and in patients with multiple myeloma? A systematic review and meta-regression analysis. Bone Rep. 2020, 12, 100272. [Google Scholar] [CrossRef]
- Terpos, E.; Berenson, J.; Cook, R.J.; Lipton, A.; Coleman, R.E. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia 2010, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Dimopoulos, M.A.; Sezer, O.; Roodman, D.; Abildgaard, N.; Vescio, R.; Tosi, P.; Garcia-Sanz, R.; Davies, F.; Chanan-Khan, A.; et al. The use of biochemical markers of bone remodeling in multiple myeloma: A report of the International Myeloma Working Group. Leukemia 2010, 24, 1700–1712. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Katodritou, E.; Symeonidis, A.; Zagouri, F.; Gerofotis, A.; Christopoulou, G.; Gavriatopoulou, M.; Christoulas, D.; Ntanasis-Stathopoulos, I.; Kourakli, A.; et al. Effect of induction therapy with lenalidomide, doxorubicin and dexamethasone on bone remodeling and angiogenesis in newly diagnosed multiple myeloma. Int. J. Cancer 2019, 145, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kortuem, K.M.; Stewart, A.K. Carfilzomib. Blood 2013, 121, 893–897. [Google Scholar] [CrossRef]
- Suvannasankha, A.; Abonour, R.; Farag, S.; Silbermann, R.W.; Wongsaengsak, S.; Cangany, M.H.; Rush-Taylor, A.; Tann, M.; Althouse, S.K.; Perkins, S.M.; et al. Phase 2 Study of Carfilzomib and Bone Metabolism in Patients with Relapsed Multiple Myeloma. Blood 2017, 130, 1826. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurchla, M.A.; Garcia-Gomez, A.; Hornick, M.C.; Ocio, E.M.; Li, A.; Blanco, J.F.; Collins, L.; Kirk, C.J.; Piwnica-Worms, D.; Vij, R.; et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia 2013, 27, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Eda, H.; Santo, L.; Cirstea, D.D.; Yee, A.J.; Scullen, T.A.; Nemani, N.; Mishima, Y.; Waterman, P.R.; Arastu-Kapur, S.; Evans, E.; et al. A novel Bruton’s tyrosine kinase inhibitor CC-292 in combination with the proteasome inhibitor carfilzomib impacts the bone microenvironment in a multiple myeloma model with resultant antimyeloma activity. Leukemia 2014, 28, 1892–1901. [Google Scholar] [CrossRef]
- Takito, J.; Inoue, S.; Nakamura, M. The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. Int. J. Mol. Sci. 2018, 19, 984. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Szydlo, R.; Apperley, J.F.; Hatjiharissi, E.; Politou, M.; Meletis, J.; Viniou, N.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: Proposal for a novel prognostic index. Blood 2003, 102, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Blair, H.C.; Shapiro, I.M.; Wang, B. The Proteasome Inhibitor Carfilzomib Suppresses Parathyroid Hormone-induced Osteoclastogenesis through a RANKL-mediated Signaling Pathway. J. Biol. Chem. 2015, 290, 16918–16928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpos, E.; Kastritis, E.; Christoulas, D.; Gkotzamanidou, M.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Papatheodorou, A.; Dimopoulos, M.A. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann. Oncol. 2012, 23, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.; Mukherjee, S.; Vaghela, N.; Hideshima, T.; Fulciniti, M.; Pozzi, S.; Santo, L.; Cirstea, D.; Patel, K.; Sohani, A.R.; et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc. Natl. Acad. Sci. USA 2010, 107, 5124–5129. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Politou, M.; Szydlo, R.; Goldman, J.M.; Apperley, J.F.; Rahemtulla, A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br. J. Haematol. 2003, 123, 106–109. [Google Scholar] [CrossRef]
- Roussou, M.; Tasidou, A.; Dimopoulos, M.A.; Kastritis, E.; Migkou, M.; Christoulas, D.; Gavriatopoulou, M.; Zagouri, F.; Matsouka, C.; Anagnostou, D.; et al. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 2009, 23, 2177–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet, S.; Pozzi, S.; Patel, K.; Vaghela, N.; Fulciniti, M.T.; Veiby, P.; Hideshima, T.; Santo, L.; Cirstea, D.; Scadden, D.T.; et al. A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia 2011, 25, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Fotiou, D.; Terpos, E. CCL3 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Christoulas, D.; Bagratuni, T.; Bakogeorgos, M.; Gavriatopoulou, M.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Kastritis, E.; Dimopoulos, M.A. Semaphorin 4D correlates with increased bone resorption, hypercalcemia, and disease stage in newly diagnosed patients with multiple myeloma. Blood Cancer J. 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Chen, Y.; Usmani, S.Z.; Ye, S.; Qiang, W.; Papanikolaou, X.; Heuck, C.J.; Yaccoby, S.; Williams, B.O.; Van Rhee, F.; et al. Characterization of the molecular mechanism of the bone-anabolic activity of carfilzomib in multiple myeloma. PLoS ONE 2013, 8, e74191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; De Veirman, K.; Fan, R.; Jian, Q.; Zhang, Y.; Lei, L.; Evans, H.; Wang, Y.; Lei, L.; Wang, B.; et al. ER stress arm XBP1s plays a pivotal role in proteasome inhibition-induced bone formation. Stem Cell Res. Ther. 2020, 11, 516. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhuang, W.; Wang, Q.; Ge, X.; Zhang, X.; Chen, P.; Fu, J.; Li, B. Carfilzomib promotes the osteogenic differentiation potential of mesenchymal stem cells derived from myeloma patients by inhibiting notch1 activity in vitro. Leuk. Res. 2014, 38, 970–976. [Google Scholar] [CrossRef]
- Garrett, I.R.; Chen, D.; Gutierrez, G.; Zhao, M.; Escobedo, A.; Rossini, G.; Harris, S.E.; Gallwitz, W.; Kim, K.B.; Hu, S.; et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J. Clin. Invest. 2003, 111, 1771–1782. [Google Scholar] [CrossRef] [Green Version]
- Zangari, M.; Yaccoby, S.; Pappas, L.; Cavallo, F.; Kumar, N.S.; Ranganathan, S.; Suva, L.J.; Gruenwald, J.M.; Kern, S.; Zhan, F.; et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica 2011, 96, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Gavriatopoulou, M.; Dimopoulos, M.A.; Christoulas, D.; Migkou, M.; Iakovaki, M.; Gkotzamanidou, M.; Terpos, E. Dickkopf-1: A suitable target for the management of myeloma bone disease. Expert Opin. Ther. Targets 2009, 13, 839–848. [Google Scholar] [CrossRef]
- Politou, M.C.; Heath, D.J.; Rahemtulla, A.; Szydlo, R.; Anagnostopoulos, A.; Dimopoulos, M.A.; Croucher, P.I.; Terpos, E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int. J. Cancer 2006, 119, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, G.; Oranger, A.; Mori, G.; Specchia, G.; Rinaldi, E.; Curci, P.; Zallone, A.; Rizzi, R.; Grano, M.; Colucci, S. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann. N. Y. Acad. Sci. 2011, 1237, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.; Brunetti, G.; Oranger, A.; Mori, G.; Sardone, F.; Specchia, G.; Rinaldi, E.; Curci, P.; Liso, V.; Passeri, G.; et al. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011, 1, e27. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Katodritou, E.; Bratengeier, C.; Gkotzamanidou, M.; Michalis, E.; Delimpasi, S.; Pouli, A.; Meletis, J.; Kastritis, E.; et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: Reduction post-bortezomib monotherapy. Int. J. Cancer 2012, 131, 1466–1471. [Google Scholar] [CrossRef]
- Eda, H.; Santo, L.; Wein, M.N.; Hu, D.Z.; Cirstea, D.D.; Nemani, N.; Tai, Y.T.; Raines, S.E.; Kuhstoss, S.A.; Munshi, N.C.; et al. Regulation of Sclerostin Expression in Multiple Myeloma by Dkk-1: A Potential Therapeutic Strategy for Myeloma Bone Disease. J. Bone Miner. Res. 2016, 31, 1225–1234. [Google Scholar] [CrossRef] [Green Version]
- McDonald, M.M.; Reagan, M.R.; Youlten, S.E.; Mohanty, S.T.; Seckinger, A.; Terry, R.L.; Pettitt, J.A.; Simic, M.K.; Cheng, T.L.; Morse, A.; et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 2017, 129, 3452–3464. [Google Scholar] [CrossRef] [Green Version]
- Kleber, M.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A.; Terpos, E. Monoclonal antibodies against RANKL and sclerostin for myeloma-related bone disease: Can they change the standard of care? Expert Rev. Hematol. 2019, 12, 651–663. [Google Scholar] [CrossRef]
| Variables | Overall (n = 25) | SRE during the Study Interval (n = 7) | No SRE during the Study Interval (n = 18) | p-Value a |
|---|---|---|---|---|
| Age at enrollment (years), | 67.5 (53.2–76.8) | 67.5 (56.1–76.8) | 67.7 (53.2–76.2) | 0.739 |
| Age at diagnosis (years) | 64.0 (41.1–73.9) | 66.4 (45.5–73.3) | 63.2 (41.1–73.9) | 0.785 |
| Time from diagnosis (years) | 4.3 (0.4–19.4) | 2.0 (0.4–10.6) | 4.4 (0.9–19.4) | 0.138 |
| Male sex | 12 (48.0%) | 4 (57.1%) | 8 (44.4%) | 0.673 |
| Greek ethnicity | 25 (100%) | 7 (100%) | 18 (100%) | |
| Women, postmenopausal | 13 (152.0%) | 3 (42.9%) | 10 (55.6%) | 0.236 |
| BMI (kg/m2) | 26.4 (17.7–34.3) | 29.8 (23.1–34.3) | 25.6 (17.7–33.3) | 0.127 |
| ECOG PS at Kd initiation | ||||
| 0 | 13 (52.0%) | 3 (42.9%) | 10 (55.6%) | 0.252 |
| 1 | 7 (28.0%) | 1 (14.3%) | 6 (33.3%) | |
| 2 or higher | 5 (20.0%) | 3 (42.9%) | 2 (11.1%) | |
| ISS at diagnosis | ||||
| I | 8 (32.0%) | 0 (0%) | 8 (44.4%) | 0.092 |
| II | 9 (36.0%) | 3 (42.9%) | 6 (33.3%) | |
| III | 8 (32.0%) | 4 (57.1%) | 4 (22.2%) | |
| R-ISS at diagnosis | ||||
| I | 7 (28.0%) | 0 (0%) | 7 (38.9%) | 0.159 |
| II | 12 (48.0%) | 5 (71.4%) | 7 (38.9%) | |
| III | 6 (24.0%) | 2 (28.6%) | 4 (22.2%) | |
| ISS at Kd initiation | ||||
| I | 9 (36.0%) | 2 (28.6%) | 7 (38.9%) | 0.295 |
| II | 8 (32.0%) | 1 (14.3%) | 7 (38.9%) | |
| III | 8 (32.0%) | 4 (57.1%) | 4 (22.2%) | |
| R-ISS at Kd initiation | ||||
| I | 6 (24.0%) | 1 (14.3%) | 5 (27.8%) | 0.188 |
| II | 12 (48.0%) | 2 (28.6%) | 10 (55.6%) | |
| III | 7 (28.0%) | 4 (57.1%) | 3 (16.7%) | |
| Prior ASCT | 14 (56.0%) | 3 (42.9%) | 11 (61.1%) | 0.656 |
| Prior radiotherapy | 7 (28.0%) | 4 (57.1%) | 3 (16.7%) | 0.066 |
| Prior lines of therapy | 3.0 (1.0–8.0) | 3.0 (1.0–5.0) | 3.5 (1.0–8.0) | 0.294 |
| Refractoriness to: | ||||
| PI | 11 (44.0%) | 4 (57.1%) | 7 (38.9%) | 0.656 |
| IMiD | 16 (64.0%) | 5 (71.4%) | 11 (61.1%) | >0.999 |
| PI and IMiD | 10 (40.0%) | 3 (42.9%) | 7 (38.9%) | >0.999 |
| Pomalidomide | 5 (20.0%) | 2 (28.6%) | 3 (16.7%) | 0.597 |
| Daratumumab | 5 (20.0%) | 2 (28.6%) | 3 (16.7%) | 0.597 |
| Last line of therapy | 14 (56.0%) | 6 (85.7%) | 8 (44.4%) | 0.090 |
| Prior use of bisphosphonates (during the last prior therapy) | 19 (76.0%) | 6 (85.7%) | 13 (72.2%) | 0.637 |
| Prior use of proteasome inhibitor | 22 (88.0%) | 7 (100.0%) | 15 (83.3%) | 0.534 |
| No bone disease at diagnosis | 9 (36.0%) | 2 (28.6%) | 7 (38.9%) | >0.999 |
| Lytic bone lesions at Kd initiation | ||||
| None | 4 (16.0%) | 1 (14.3%) | 3 (16.7%) | 0.466 |
| 1–3 | 6 (24.0%) | 1 (14.3%) | 5 (27.8%) | |
| 4–10 | 7 (28.0%) | 1 (14.3%) | 6 (33.3%) | |
| More than 10 | 8 (32.0%) | 4 (57.1%) | 4 (22.2%) | |
| Prior history of SREs | 9 (36.0%) | 3 (42.9%) | 6 (33.3%) | 0.673 |
| Variables | Baseline | 2 Months | 4 Months | 6 Months | 8 Months | 10 Months | 12 Months |
|---|---|---|---|---|---|---|---|
| bALP (μg/L) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 10.9 (9.1, 11.7) | 12.1 (9.1, 15.4) | 11.6 (9.1, 14.1) | 13.6 (8.1, 14.8) | 16.0 (6.2, 17.4) | 15.0 (7.0, 18.1) | 17.1 (14.5, 19.7) |
| Median percent change from baseline (Q1, Q3) | 12.1 (−9.4, 29.5) | 3.5 (−19.7, 37.8) | 16.1 (−36.3, 30.6) | 37.7 (−45.1, 67.0) | 27.8 (−38.4, 58.0) | 56.6 (23.7, 89.5) | |
| p-value for absolute change a | 0.487 | 0.597 | 0.825 | 0.963 | 0.696 | NA | |
| OC (ng/mL) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 9.2 (5.5, 11.3) | 10.5 (8.8, 14.1) | 12.4 (9.9, 19.2) | 13.9 (11.1, 18.9) | 15.9 (7.3, 23.8) | 16.8 (3.8, 19.7) | 17.1 (13.3, 20.8) |
| Median percent change from baseline (Q1, Q3) | 23.4 (19.0, 65.2) | 64.4 (35.5, 242.2) | 89.7 (39.2, 169.3) | 61.2 (33.0, 216.9) | 71.7 (49.6, 167.4) | 65.8 (44.8, 86.7) | |
| p-value for absolute change a | 0.257 | 0.099 | 0.030 | 0.033 | 0.203 | NA | |
| P1NP (pg/mL) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 542.2 (294.8, 746.4) | 384.9 (226.3, 775.3) | 490.2 (411.6, 777.5) | 442.8 (419.7, 789.0) | 884.9 (461.1, 2072.1) | 652.0 (447.6, 2567.2) | 992.5 (701.3, 1283.7) |
| Median percent change from baseline (Q1, Q3) | 7.9 (−30.8, 21.9) | 38.4 (−34.1, 105.9) | 20.6 (−41.5, 33.7) | 42.4 (24.9, 110.4) | 92.8 (11.8, 173.8) | 58.2 (20.2, 96.2) | |
| p-value for absolute change a | 0.918 | 0.437 | 0.469 | 0.059 | 0.061 | NA | |
| CTX (ng/mL) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 0.7 (0.3, 0.9) | 0.4 (0.2, 0.6) | 0.3 (0.2, 0.5) | 0.2 (0.2, 0.4) | 0.1 (0.1, 0.4) | 0.2 (0.1, 0.3) | 0.3 (0.2, 0.4) |
| Median percent change from baseline (Q1, Q3) | −31.3 (−43.0, −15.0) | −48.4 (−63.5, 42.8) | −43.5 (−64.6, −31.4) | −59.9 (−86.1, −48.0) | −63.7 (−74.9, −31.2) | −74.2 (−79.7, −68.6) | |
| p-value for absolute change a | 0.048 | 0.054 | 0.029 | <0.001 | 0.001 | NA | |
| TRACP-5B (U/L) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 5 | 2 |
| Median biomarker value (Q1, Q3) | 3.4 (1.7, 4.0) | 1.9 (1.0, 2.1) | 1.2 (0.8, 2.0) | 1.3 (1.1, 1.9) | 1.0 (0.9, 1.1) | 0.9 (0.9, 0.9) | 1.3 (0.9, 1.8) |
| Median percent change from baseline (Q1, Q3) | −35.3 (−49.7, −9.5) | −48.6 (−66.0, −21.6) | −22.8 (−66.3, −17.9) | −64.0 (−70.2, −52.9) | −72.1 (−73.6, −59.1) | −58.3 (−58.8, −57.7) | |
| p-value for absolute change a | 0.002 | <0.001 | 0.043 | <0.001 | <0.001 | NA | |
| RANKL (pmol/L) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 0.3 (0.2, 0.4) | 0.2 (0.1, 0.2) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | 0.1 (0.0, 0.2) | 0.1 (0.1, 0.2) |
| Median percent change from baseline (Q1, Q3) | −47.5 (−52.9, −1.6) | −53.5 (−77.5, 44.9) | −63.2 (−77.0, 3.8) | −71.7 (−84.7, −55.0) | −73.0 (−92.9, −58.3) | −82.8 (−87.0, −78.6) | |
| p-value for absolute change a | 0.032 | 0.001 | 0.001 | <0.001 | <0.001 | NA | |
| RANKL/OPG ratio | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 0.072 (0.000, 0.123) | 0.036 (0.000, 0.101) | 0.031 (0.000, 0.098) | 0.017 (0.000, 0.077) | 0.010 (0.011, 0.064) | 0.009 (0.000, 0.033) | 0.005 (0.00, 0.021) |
| Median percent change from baseline (Q1, Q3) | −52.2 (−69.2, 10.6) | −60.4 (−86.8, 44.5) | −77.0 (−85.1, 5.3) | −86.9 (−93.5, −48.8) | −84.9 (−94.7, −47.0) | −92.9 (−94.9, −91.0) | |
| p-value for absolute change a | 0.026 | <0.001 | <0.001 | <0.001 | <0.001 | NA | |
| SOST (pmol/L) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 47.6 (38.0, 65.1) | 37.2 (29.4, 41.7) | 33.2 (25.0, 45.8) | 31.8 (25.5, 63.1) | 28.0 (22.3, 53.9) | 36.9 (20.2, 64.7) | 27.8 (20.0, 35.7) |
| Median percent change from baseline (Q1, Q3) | −24.0 (−39.6, 6.6) | −31.0 (−44.5, −5.6) | −27.4 (−32.0, 25.5) | −36.7 (−48.4, −26.6) | −38.9 (−52.0, 0.5) | −50.8 (−55.3, −46.2) | |
| p-value for absolute change a | 0.272 | 0.306 | 0.869 | 0.597 | 0.191 | NA | |
| Dkk1 (pmol/L) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 41.6 (28.2, 63.7) | 36.9 (26.9, 62.5) | 33.7 (18.5, 58.4) | 37.0 (32.0, 49.2) | 29.0 (21.5, 32.7) | 26.1 (9.1, 31.0) | 14.4 (8.4, 20.4) |
| Median percent change from baseline (Q1, Q3) | −24.0 (−27.8, 3.7) | −21.0 (−58.3, −14.8) | −31.5 (−59.3, −23.8) | −61.4 (−68.6, −39.3) | −64.2 (−82.6, −29.2) | −78.0 (−84.0, −72.0) | |
| p-value for absolute change a | 0.856 | 0.393 | 0.399 | 0.037 | 0.005 | NA | |
| Activin-A (pg/mL) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 652.0 (498.6, 903.5) | 462.2 (358.2, 538.3) | 418.7 (334.5, 519.6) | 378.7 (366.9, 504.5) | 392.0 (275.4, 488.5) | 357.5 (280.5, 422.7) | 287.5 (256.8, 318.2) |
| Median percent change from baseline (Q1, Q3) | −22.7 (−39.9, −5.7) | −37.3 (−63.4, −16.2) | −48.5 (−59.0, −21.1) | −40.2 (−66.9, −30.0) | −58.0 (−61.7, −27.4) | −55.3 (−58.2, −52.4) | |
| p-value for absolute change a | 0.015 | 0.007 | 0.008 | 0.008 | <0.001 | NA | |
| CCL3 (ng/mL) | |||||||
| n | 25 | 17 | 11 | 9 | 8 | 7 | 2 |
| Median biomarker value (Q1, Q3) | 77.8 (61.8, 91.6) | 70.5 (44.0, 89.4) | 68.0 (47.0, 72.0) | 62.1 (61.2, 71.1) | 58.1 (37.7, 65.2) | 50.7 (9.1, 57.9) | 34.1 (3.9, 64.3) |
| Median percent change from baseline (Q1, Q3) | −3.9 (−36.4, 8.7) | −17.8 (−24.4, 43.8) | −17.4 (−29.5, −11.9) | −33.0 (−55.2, −11.7) | −44.5 (−87.5, −21.7) | −55.3 (−94.6, −16.1) | |
| p-value for absolute change a | 0.849 | 0.577 | 0.958 | 0.668 | 0.063 | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpos, E.; Ntanasis-Stathopoulos, I.; Katodritou, E.; Kyrtsonis, M.-C.; Douka, V.; Spanoudakis, E.; Papatheodorou, A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Gavriatopoulou, M.; et al. Carfilzomib Improves Bone Metabolism in Patients with Advanced Relapsed/Refractory Multiple Myeloma: Results of the CarMMa Study. Cancers 2021, 13, 1257. https://doi.org/10.3390/cancers13061257
Terpos E, Ntanasis-Stathopoulos I, Katodritou E, Kyrtsonis M-C, Douka V, Spanoudakis E, Papatheodorou A, Eleutherakis-Papaiakovou E, Kanellias N, Gavriatopoulou M, et al. Carfilzomib Improves Bone Metabolism in Patients with Advanced Relapsed/Refractory Multiple Myeloma: Results of the CarMMa Study. Cancers. 2021; 13(6):1257. https://doi.org/10.3390/cancers13061257
Chicago/Turabian StyleTerpos, Evangelos, Ioannis Ntanasis-Stathopoulos, Eirini Katodritou, Marie-Christine Kyrtsonis, Vassiliki Douka, Emmanouil Spanoudakis, Athanasios Papatheodorou, Evangelos Eleutherakis-Papaiakovou, Nikolaos Kanellias, Maria Gavriatopoulou, and et al. 2021. "Carfilzomib Improves Bone Metabolism in Patients with Advanced Relapsed/Refractory Multiple Myeloma: Results of the CarMMa Study" Cancers 13, no. 6: 1257. https://doi.org/10.3390/cancers13061257
APA StyleTerpos, E., Ntanasis-Stathopoulos, I., Katodritou, E., Kyrtsonis, M.-C., Douka, V., Spanoudakis, E., Papatheodorou, A., Eleutherakis-Papaiakovou, E., Kanellias, N., Gavriatopoulou, M., Makras, P., Kastritis, E., & Dimopoulos, M. A. (2021). Carfilzomib Improves Bone Metabolism in Patients with Advanced Relapsed/Refractory Multiple Myeloma: Results of the CarMMa Study. Cancers, 13(6), 1257. https://doi.org/10.3390/cancers13061257











