Quality of Life in Brain Tumor Patients and Their Relatives Heavily Depends on Social Support Factors during the COVID-19 Pandemic
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire Instruments
2.1.1. Demographic and Disease Characteristics
2.1.2. Living Conditions
2.1.3. Personal Behavior
2.1.4. Isolation Questionnaire
2.1.5. QoL Outcome Measures
2.2. Study Population and Recruitment
2.3. Statistics
3. Results
3.1. Patient Characteristics
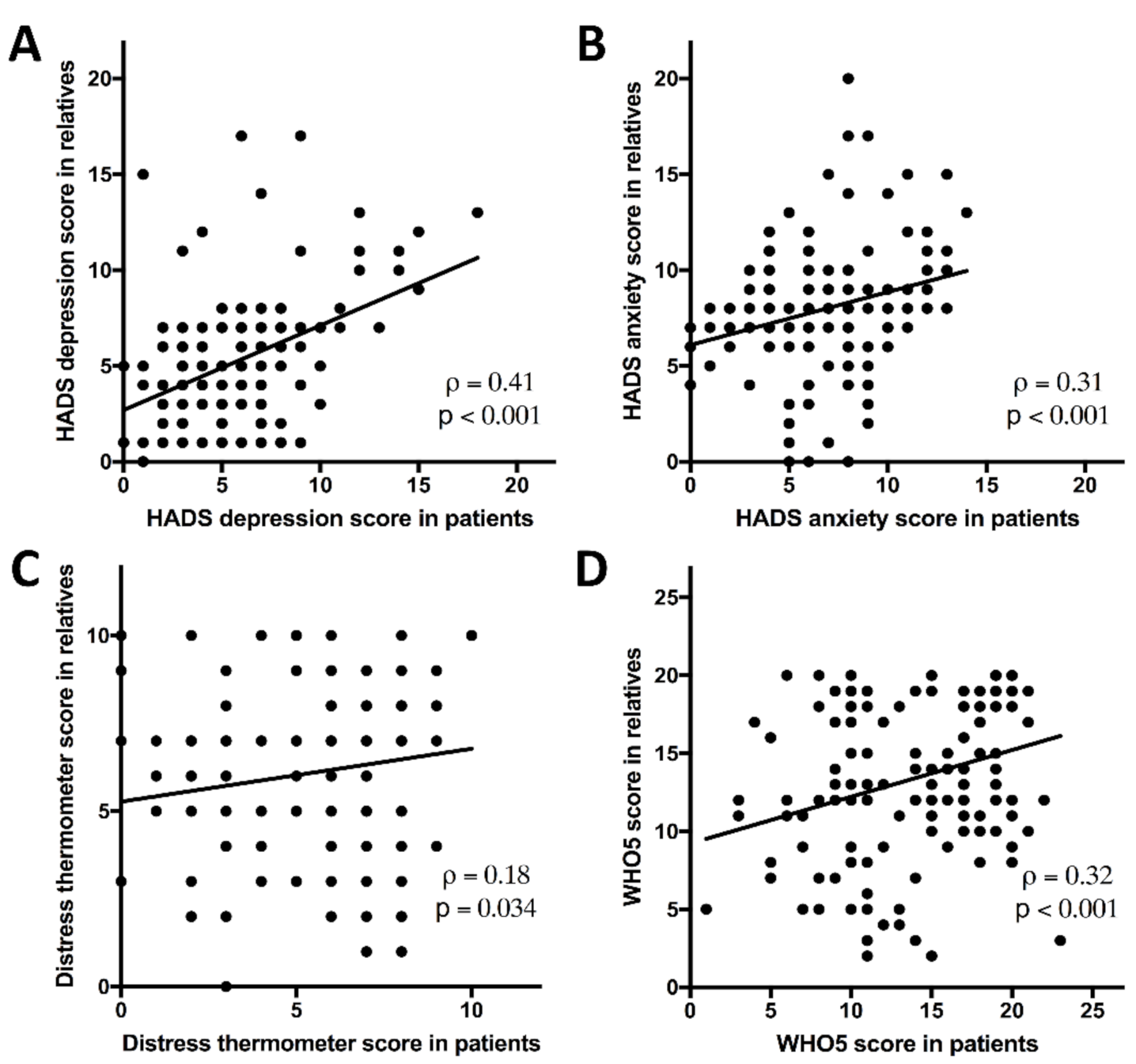
3.2. Intra-Family Correlations
3.3. Changes over Time
3.4. Influencing Factors
4. Discussion
4.1. Mental Health Challenges for Patients during the COVID Pandemic
4.2. Findings on Relatives’ Quality of Life
4.3. Changes over Time
4.4. Social Support Is a Strong Predictor of Quality of Life
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webb, L. COVID-19 lockdown: A perfect storm for older people’s mental health. J. Psychiatr. Ment. Health Nurs. 2020, 12644. [Google Scholar] [CrossRef] [PubMed]
- Torales, J.; O’Higgins, M.; Castaldelli-Maia, J.M.; Ventriglio, A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatry 2020, 66, 317–320. [Google Scholar] [CrossRef]
- Rossi, R.; Socci, V.; Talevi, D.; Mensi, S.; Niolu, C.; Pacitti, F.; Di Marco, A.; Rossi, A.; Siracusano, A.; Di Lorenzo, G. COVID-19 Pandemic and Lockdown Measures Impact on Mental Health Among the General Population in Italy. Front. Psychiatry 2020, 11, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Galea, S.; Merchant, R.M.; Lurie, N. The Mental Health Consequences of COVID-19 and Physical Distancing. JAMA Intern. Med. 2020, 180, 817. [Google Scholar] [CrossRef]
- Bäuerle, A.; Graf, J.; Jansen, C.; Dörrie, N.; Junne, F.; Teufel, M.; Skoda, E.M. Correspondence an e-mental health intervention to support burdened people in times of the COVID-19 pandemic: Cope it. J. Public Health 2020, 42, 647–648. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Okwan-Duodu, D.; Basho, R.; Cui, X. COVID-19 in cancer patients: Risk, clinical features, and management. Cancer Biol. Med. 2020, 17, 519–527. [Google Scholar]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.-C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Z.; Ma, Z.; Mao, Y.; Li, X.; Wilson, A.; Qin, H.; Ou, J.; Peng, K.; Zhou, F.; et al. Epidemiology of mental health problems among patients with cancer during COVID-19 pandemic. Transl. Psychiatry 2020, 10, 263. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, C.; Xiao, J.; Zhao, D.; Tang, H.; Wu, H.; Chen, J. Association between depression and brain tumor: A systematic review and meta-analysis. Oncotarget 2017, 8, 94932–94943. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Zhang, L.; Fan, F.; Chen, J.; Xia, K.; Liu, Z. Screening for distress in patients with primary brain tumor using distress thermometer: A systematic review and meta-analysis. BMC Cancer 2018, 18, 124. [Google Scholar] [CrossRef]
- Stieb, S.; Fischbeck, S.; Wagner, W.; Appels, J.; Wiewrodt, D. High psychosocial burden in relatives of malignant brain tumor patients. Clin. Neurol. Neurosurg. 2018, 170, 1–6. [Google Scholar] [CrossRef]
- Biagioli, V.; Piredda, M.; Annibali, O.; Tirindelli, M.C.; Pignatelli, A.; Marchesi, F.; Mauroni, M.R.; Soave, S.; Del Giudice, E.; Ponticelli, E.; et al. Factors influencing the perception of protective isolation in patients undergoing haematopoietic stem cell transplantation: A multicentre prospective study. Eur. J. Cancer Care 2019, 28, 1–11. [Google Scholar] [CrossRef]
- Hinz, A.; Krauss, O.; Hauss, J.P.; Höckel, M.; Kortmann, R.D.; Stolzenburg, J.U.; Schwarz, R. Anxiety and depression in cancer patients compared with the general population. Eur. J. Cancer Care 2010, 19, 522–529. [Google Scholar] [CrossRef]
- Ownby, K.K. Use of the Distress Thermometer in Clinical Practice. J. Adv. Pract. Oncol. 2019, 10, 175–179. [Google Scholar]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 well-being index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between 1/1, or do they. J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Caron, J.; Cargo, M.; Daniel, M.; Liu, A. Predictors of Quality of Life in Montreal, Canada: A Longitudinal Study. Community Ment. Health J. 2019, 55, 189–201. [Google Scholar] [CrossRef]
- Archer, S.; Pinto, A.; Vuik, S.; Bicknell, C.; Faiz, O.; Byrne, B.; Johnston, M.; Skapinakis, P.; Athanasiou, T.; Vincent, C.; et al. Surgery, Complications, and Quality of Life: A Longitudinal Cohort Study Exploring the Role of Psychosocial Factors. Ann. Surg. 2019, 270, 95–101. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of Life and Satisfaction with Outcome among Prostate-Cancer Survivors. N. Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef]
- Cameron, I.M.; Scott, N.W.; Adler, M.; Reid, I.C. A comparison of three methods of assessing differential item functioning (DIF) in the Hospital Anxiety Depression Scale: Ordinal logistic regression, Rasch analysis and the Mantel chi-square procedure. Qual. Life Res. 2014, 23, 2883–2888. [Google Scholar] [CrossRef]
- Troschel, F.M.; Brandt, R.; Wiewrodt, R.; Stummer, W.; Wiewrodt, D. High-Intensity Physical Exercise in a Glioblastoma Patient under Multimodal Treatment. Med. Sci. Sports Exerc. 2019, 51, 2429–2433. [Google Scholar] [CrossRef]
- Goebel, S.; Von Harscher, M.; Maximilian Mehdorn, H. Comorbid mental disorders and psychosocial distress in patients with brain tumours and their spouses in the early treatment phase. Support. Care Cancer 2011, 19, 1797–1805. [Google Scholar] [CrossRef]
- Goebel, S.; Strenge, H.; Mehdorn, H.M. Acute stress in patients with brain cancer during primary care. Support. Care Cancer 2012, 20, 1425–1434. [Google Scholar] [CrossRef]
- Cordes, M.C.; Scherwath, A.; Ahmad, T.; Cole, A.M.; Ernst, G.; Oppitz, K.; Lanfermann, H.; Bremer, M.; Steinmann, D. Distress, anxiety and depression in patients with brain metastases before and after radiotherapy. BMC Cancer 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pringle, A.M.; Taylor, R.; Whittle, I.R. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br. J. Neurosurg. 1999, 13, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Troschel, F.M.; Ramroth, C.; Lemcke, L.; Clasing, J.; Troschel, A.S.; Dugas, M.; Stummer, W.; Wiewrodt, R.; Brandt, R.; Wiewrodt, D. Feasibility, Safety and Effects of a One-Week, Ski-Based Exercise Intervention in Brain Tumor Patients and Their Relatives: A Pilot Study. J. Clin. Med. 2020, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Voisin, M.R.; Oliver, K.; Farrimond, S.; Chee, T.; Arzbaecher, J.; Kruchko, C.; Maher, M.E.; Tse, C.; Cashman, R.; Daniels, M.; et al. Brain tumors and COVID-19: The patient and caregiver experience*. Neuro-Oncol. Adv. 2020, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Goebel, S.; Mehdorn, H.M. Measurement of psychological distress in patients with intracranial tumours: The NCCN distress thermometer. J. Neurooncol. 2011, 104, 357–364. [Google Scholar] [CrossRef]
- Mainio, A.; Tuunanen, S.; Hakko, H.; Niemelä, A.; Koivukangas, J.; Räsänen, P. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: A follow-up from 1990 to 2003. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Keir, S.T.; Guill, A.B.; Carter, K.E.; Boole, L.C.; Gonzales, L.; Friedman, H.S. Differential levels of stress in caregivers of brain tumor patients—Observations from a pilot study. Support. Care Cancer 2006, 14, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Krug, K.; Miksch, A.; Peters-Klimm, F.; Engeser, P.; Szecsenyi, J. Correlation between patient quality of life in palliative care and burden of their family caregivers: A prospective observational cohort study. BMC Palliat. Care 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedz, C.L.; Green, M.J.; Benzeval, M.; Campbell, D.; Craig, P.; Demou, E.; Leyland, A.; Pearce, A.; Thomson, R.; Whitley, E.; et al. Mental health and health behaviours before and during the initial phase of the COVID-19 lockdown: Longitudinal analyses of the UK Household Longitudinal Study. J. Epidemiol. Community Health 2021, 75, 224–231. [Google Scholar]
- Pierce, M.; Hope, H.; Ford, T.; Hatch, S.; Hotopf, M.; John, A.; Kontopantelis, E.; Webb, R.; Wessely, S.; McManus, S.; et al. Mental health before and during the COVID-19 pandemic: A longitudinal probability sample survey of the UK population. Lancet Psychiatry 2020, 7, 883–892. [Google Scholar] [CrossRef]
- Du, J.; Mayer, G.; Hummel, S.; Oetjen, N.; Gronewold, N.; Zafar, A.; Schultz, J.-H. Identifying post-COVID vulnerable groups: A cross-sectional survey among different professions in China during the final stage of lockdown (Preprint). J. Med. Internet Res. 2020, 22, 1–20. [Google Scholar] [CrossRef]
- Pan, K.Y.; Kok, A.A.L.; Eikelenboom, M.; Horsfall, M.; Jörg, F.; Luteijn, R.A.; Rhebergen, D.; Oppen, P.; van Giltay, E.J.; Penninx, B.W.J.H. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: A longitudinal study of three Dutch case-control cohorts. Lancet Psychiatry 2020, 8, 121–129. [Google Scholar] [CrossRef]
- Hamza, C.A.; Ewing, L.; Heath, N.L.; Goldstein, A.L. When Social Isolation Is Nothing New: A Longitudinal Study Psychological Distress During COVID-19 Among University Students With and Without Preexisting Mental Health Concerns. Can. Psychol. 2020. [Google Scholar] [CrossRef]
- Fancourt, D.; Steptoe, A.; Bu, F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: A longitudinal observational study. Lancet Psychiatry 2020, 8, 141–149. [Google Scholar] [CrossRef]
- Gollwitzer, M.; Platzer, C.; Zwarg, C.; Göritz, A.S. Public acceptance of Covid-19 lockdown scenarios. Int. J. Psychol. 2020, 49. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhou, S.J.; Guo, Z.C.; Zhang, L.G.; Min, H.J.; Li, X.M.; Chen, J.X. The Effect of Social Support on Mental Health in Chinese Adolescents During the Outbreak of COVID-19. J. Adolesc. Health 2020, 67, 514–518. [Google Scholar] [CrossRef]
- Saltzman, L.Y.; Hansel, T.C.; Bordnick, P.S. Loneliness, Isolation, and Social Support Factors in Post-COVID-19 Mental Health. Psychol. Trauma Theory Res. Pract. Policy 2020, 12, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.G.; Dinitto, D.M. The digital divide among low-income homebound older adults: Internet use patterns, ehealth literacy, and attitudes toward computer/internet use. J. Med. Internet Res. 2013, 15, e93. [Google Scholar] [CrossRef] [PubMed]
- Pieh, C.; Budimir, S.; Probst, T. The effect of age, gender, income, work, and physical activity on mental health during coronavirus disease (COVID-19) lockdown in Austria. J. Psychosom. Res. 2020, 136, 110186. [Google Scholar] [CrossRef]
- Balsamo, M.; Carlucci, L. Italians on the Age of COVID-19: The Self-Reported Depressive Symptoms through Web-Based Survey. Front. Psychol. 2020, 11, 569276. [Google Scholar] [CrossRef]
- Naomi, A.S. Access to Nature Has Always Been Important; With COVID-19, It Is Essential. Health Environ. Res. Des. J. 2020, 13, 242–244. [Google Scholar]
- Cheng, J.X.; Zhang, X.; Liu, B.L. Health-related quality of life in patients with high-grade glioma. Neuro Oncol. 2009, 11, 41–50. [Google Scholar] [CrossRef]
- Weller, M.; Preusser, M. How we treat patients with brain tumour during the COVID-19 pandemic. ESMO Open 2020, 4, 19–21. [Google Scholar]
- Liu, R.; Page, M.; Solheim, K.; Fox, S.; Chang, S.M. Quality of life in adults with brain tumors: Current knowledge and future directions. Neuro Oncol. 2009, 11, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Gabel, N.; Altshuler, D.B.; Brezzell, A.; Briceño, E.M.; Boileau, N.R.; Miklja, Z.; Kluin, K.; Ferguson, T.; McMurray, K.; Wang, L.; et al. Health Related Quality of Life in Adult Low and High-Grade Glioma Patients Using the National Institutes of Health Patient Reported Outcomes Measurement Information System (PROMIS) and Neuro-QOL Assessments. Front. Neurol. 2019, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Steginga, S.; Langbecker, D.; Dunn, J.; Walker, D.; Eakin, E. Quality of life among patients with a brain tumor and their carers. J. Psychosom. Res. 2007, 63, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Hickmann, A.K.; Hechtner, M.; Nadji-Ohl, M.; Janko, M.; Reuter, A.K.; Kohlmann, K.; Haug, M.; Grüninger, S.; Deininger, M.; Ganslandt, O.; et al. Evaluating patients for psychosocial distress and supportive care needs based on health-related quality of life in primary brain tumors: A prospective multicenter analysis of patients with gliomas in an outpatient setting. J. Neurooncol. 2017, 131, 135–151. [Google Scholar] [CrossRef] [PubMed]



| All Participants | n | All (n = 100) | Patients (n = 63) | Relatives (n = 37) | p |
| Age, years, mean (SD) | 100 | 48.2 (13.2) | 48.3 (12.2) | 48.1 (14.9) | 0.95 + |
| Center | 100 | 0.77 # | |||
| Münster, n (%) | 74 (74.0) | 46 (73.0) | 28 (75.7) | ||
| Bochum, n (%) | 26 (26.0) | 17 (27.0) | 9 (24.3) | ||
| Sex | 100 | 0.001 # | |||
| Male, n (%) | 44 (44.0) | 36 (57.1) | 8 (21.6) | ||
| Female, n (%) | 56 (56.0) | 27 (42.9) | 29 (78.4) | ||
| Number of questionnaires per participant, median (IQR) | 100 | 9 (2–11) | 10 (2–11) | 8 (2–11) | 0.13 x |
| BMI, kg/m2 (IQR) | 100 | 24.5 (20.7–27.0) | 24.5 (21.3–27.2) | 23.6 (20.5–26.9) | 0.29 x |
| Living facilities with outdoor area | 97 | 0.58 # | |||
| No, n (%) | 19 (19.6) | 13 (21.3) | 6 (16.7) | ||
| Yes, n (%) | 78 (80.4) | 48 (78.7) | 30 (83.3) | ||
| Relationship status | 98 | 0.06 # | |||
| Single, n (%) | 14 (14.3) | 12 (16.7) | 2 (5.6) | ||
| In a relationship, n (%) | 84 (85.7) | 50 (83.3) | 34 (94.4) | ||
| Flatmates | 100 | 0.62 # | |||
| No, n (%) | 13 (13.0) | 9 (14.3) | 4 (10.8) | ||
| Yes, n (%) | 87 (87.0) | 54 (85.7) | 33 (89.2) | ||
| Day job | 96 | 0.067 # | |||
| No, n (%) | 38 (39.6) | 28 (46.7) | 10 (27.8) | ||
| Yes, n (%) | 58 (60.4) | 32 (53.3) | 26 (72.2) | ||
| House vs. Apartment | 97 | 0.11 # | |||
| House, n (%) | 66 (68.0) | 38 (62.3) | 28 (77.8) | ||
| Apartment, n (%) | 31 (32.0) | 23 (37.7) | 9 (23.2) | ||
| Area | 97 | 0.041 # | |||
| <100 m2, n (%) | 37 (38.1) | 28 (45.9) | 9 (25.0) | ||
| >100 m2, n (%) | 60 (61.9) | 33 (54.1) | 27 (75.0) | ||
| Diagnosis of the patient within the family | 100 | 0.90 # | |||
| Meningeoma, n (%) | 7 (7.0) | 5 (7.9) | 2 (5.4) | ||
| Astrocytoma, n (%) | 35 (35.0) | 23 (36.5) | 12 (32.4) | ||
| GBM, n (%) | 31 (31.0) | 20 (31.7) | 11 (29.7) | ||
| Oligodendroglioma, n (%) | 15 (15.0) | 8 (12.7) | 7 (18.9) | ||
| Others *, n (%) | 12 (12.0) | 7 (11.3) | 5 (13.5) | ||
| WHO brain tumor grading § | 100 | 0.84 # | |||
| Low grade (WHO I + II), n (%) | 50 (50.0) | 32 (50.8) | 18 (48.7) | ||
| High grade (WHO III + IV), n (%) | 50 (50.0) | 31 (49.2) | 19 (51.4) | ||
| Ongoing therapy | 100 | 0.94 # | |||
| No, n (%) | 68 (68.0) | 43 (68.3) | 25 (67.6) | ||
| Yes, n (%) | 32 (33.0) | 20 (31.7) | 12 (32.4) | ||
| All Questionnaires | n | All (n = 729) | Patients (n = 484) | Relatives (n = 245) | p |
| Physical exercise frequency | 729 | <0.001 # | |||
| Occasionally (<1/week), n (%) | 179 (24.6) | 138 (28.5) | 41 (16.7) | ||
| Often (≥1/week), n (%) | 550 (75.5) | 346 (71.5) | 204 (83.3) | ||
| Social contacts/week | 729 | 0.74 # | |||
| 0–3, n (%) | 227 (31.1) | 157 (32.4) | 70 (28.6) | ||
| 4–6, n (%) | 186 (25.5) | 120 (24.8) | 66 (26.9) | ||
| 7–10, n (%) | 136 (18.7) | 88 (18.2) | 48 (19.6) | ||
| 10+, n (%) | 180 (24.7) | 119 (24.6) | 61 (24.9) | ||
| HADS-Depression, median (IQR) | 729 | 6 (3–9) | 7 (3–10) | 6 (3–7) | <0.001 x |
| HADS Anxiety, median (IQR) | 729 | 8 (5–10) | 8 (5–10) | 8 (6–10) | 0.99 x |
| Distress Thermometer, median (IQR) | 729 | 6 (4–8) | 6 (3–8) | 6 (4–8) | 0.19 x |
| WHO5, median (IQR) | 729 | 48 (32–72) | 48 (28–72) | 52 (36–72) | 0.013 x |
| Univariable Analyses | HADS Depression (Scale 0–21, Per Point) | HADS Anxiety (Scale 0–21, Per Point) | Distress Thermometer (Scale 0–10, Per Point) | WHO5 (Scale 0–100%, Per 4%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | n | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Sex | 729 | ||||||||
| Female | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Male | 1.53 (1.18–1.98) | 0.001 | 0.8 (0.62–1.04) | 0.099 | 1.01 (0.78–1.31) | 0.92 | 0.95 (0.73–1.23) | 0.69 | |
| Role | 729 | ||||||||
| Relatives | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Patients | 1.58 (1.21–2.06) | 0.001 | 1 (0.77–1.3) | 0.99 | 0.84 (0.64–1.09) | 0.19 | 0.72 (0.55–0.93) | 0.014 | |
| Center | 729 | ||||||||
| Münster | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Bochum | 0.81 (0.61–1.08) | 0.15 | 0.81 (0.61–1.08) | 0.16 | 1.01 (0.75–1.35) | 0.96 | 1 (0.75–1.33) | 1.00 | |
| Age | 729 | 1.05 (1.04–1.06) | <0.001 | 1.03 (1.02–1.04) | <0.001 | 1.01 (1–1.02) | 0.12 | 0.97 (0.96–0.98) | <0.001 |
| BMI | 729 | 1.05 (1.02–1.08) | 0.001 | 0.99 (0.96–1.01) | 0.33 | 0.98 (0.95–1.01) | 0.13 | 1.01 (0.99–1.04) | 0.36 |
| Physical exercise frequency | 729 | ||||||||
| Occasionally (<1/week) | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Often (≥1/week) | 0.6 (0.45–0.8) | 0.001 | 1.05 (0.78–1.42) | 0.74 | 1.11 (0.83–1.48) | 0.49 | 1.77 (1.32–2.38) | <0.001 | |
| Living facilities with outdoor area | 726 | ||||||||
| No | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Yes | 0.57 (0.41–0.8) | 0.001 | 0.9 (0.66–1.22) | 0.49 | 0.98 (0.71–1.35) | 0.88 | 1.96 (1.43–2.69) | <0.001 | |
| Relationship status | 724 | ||||||||
| Single | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| In a relationship | 0.76 (0.54–1.06) | 0.11 | 1.04 (0.74–1.46) | 0.82 | 0.98 (0.7–1.36) | 0.88 | 1.55 (1.11–2.17) | 0.011 | |
| Flatmates | 729 | ||||||||
| No | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Yes | 0.82 (0.57–1.16) | 0.25 | 1.11 (0.78–1.59) | 0.56 | 1.17 (0.83–1.65) | 0.37 | 0.77 (0.54–1.09) | 0.14 | |
| Social contacts/week | 729 | ||||||||
| 0–3 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| 4–6 | 0.70 (0.49–0.98) | 0.039 | 0.89 (0.64–1.25) | 0.51 | 0.75 (0.53–1.05) | 0.092 | 1.01 (0.72–1.41) | 0.97 | |
| 7–10 | 0.52 (0.36–0.78) | 0.001 | 0.72 (0.5–1.05) | 0.091 | 0.72 (0.5–1.05) | 0.091 | 1.14 (0.79–1.66) | 0.49 | |
| 10+ | 0.26 (0.18–0.36) | <0.001 | 0.53 (0.38–0.75) | <0.001 | 0.38 (0.27–0.54) | <0.001 | 2.04 (1.44–2.9) | <0.001 | |
| Day job | 725 | ||||||||
| No | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Yes | 0.55 (0.42–0.71) | <0.001 | 0.58 (0.45–0.76) | <0.001 | 0.87 (0.67–1.12) | 0.27 | 1.97 (1.52–2.55) | <0.001 | |
| House vs. Apartment | 726 | ||||||||
| House | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Apartment | 0.74 (0.56–0.97) | 0.027 | 0.66 (0.50–0.86) | 0.002 | 0.83 (0.63–1.09) | 0.17 | 1 (0.76–1.31) | 1.00 | |
| Area | 726 | ||||||||
| <100 m2 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| >100 m2 | 1.13 (0.98–1.29) | 0.086 | 1.13 (0.99–1.29) | 0.072 | 1.11 (0.97–1.26) | 0.13 | 0.93 (0.82–1.06) | 0.29 | |
| WHO grade | 729 | ||||||||
| low grade (I + II) | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| high grade (III + IV) | 0.9 (0.7–1.16) | 0.43 | 0.94 (0.73–1.22) | 0.67 | 1.02 (0.79–1.33) | 0.85 | 1.33 (1.03–1.71) | 0.029 | |
| Ongoing therapy | 729 | ||||||||
| No | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| Yes | 1.02 (0.78–1.34) | 0.87 | 1.5 (1.14–1.97) | 0.004 | 1.79 (1.36–2.35) | <0.001 | 0.74 (0.57–0.96) | 0.025 | |
| Multivariable Analyses | HADS Depression (Scale 0–25, Per Point) | HADS Anxiety (Scale 0–25, Per Point) | Distress Thermometer (Scale 0–10, Per Point) | WHO5 (Scale 0–100%, Per 4%) | ||||
|---|---|---|---|---|---|---|---|---|
| n = 723 | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Role | ||||||||
| Relatives | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | ||||
| Patients | 1.51 (1.13–2) | 0.005 | 1.05 (0.8–1.39) | 0.719 | 0.82 (0.61–1.09) | 0.169 | 0.87 (0.66–1.15) | 0.343 |
| Age | 1.05 (1.04–1.07) | <0.001 | 1.03 (1.02–1.04) | <0.001 | 1.01 (0.99–1.02) | 0.308 | 0.97 (0.96–0.98) | <0.001 |
| Physical exercise frequency | ||||||||
| Occasionally (<1/week) | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | ||||
| Often (≥1/week) | 0.73 (0.53–0.99) | 0.046 | 1.11 (0.81–1.52) | 0.52 | 1.12 (0.82–1.53) | 0.466 | 1.63 (1.19–2.23) | 0.002 |
| Living facilities with outdoor area | ||||||||
| No | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | ||||
| Yes | 0.17 (0.11–0.26) | <0.001 | 0.43 (0.28–0.65) | <0.001 | 0.72 (0.47–1.09) | 0.119 | 3.3 (2.16–5.04) | <0.001 |
| Social contacts/week | ||||||||
| 0–3 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | ||||
| 4–6 | 0.68 (0.48–0.95) | 0.026 | 0.83 (0.59–1.16) | 0.267 | 0.67 (0.48–0.95) | 0.026 | 1.04 (0.74–1.46) | 0.829 |
| 7–10 | 0.38 (0.25–0.56) | <0.001 | 0.64 (0.43–0.93) | 0.02 | 0.65 (0.44–0.95) | 0.026 | 1.39 (0.95–2.03) | 0.088 |
| 10+ | 0.3 (0.21–0.43) | <0.001 | 0.61 (0.43–0.87) | 0.006 | 0.35 (0.24–0.51) | <0.001 | 1.73 (1.2–2.49) | 0.003 |
| Day job | ||||||||
| No | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | ||||
| Yes | 0.65 (0.5–0.86) | 0.002 | 0.69 (0.52–0.92) | 0.01 | 0.88 (0.67–1.16) | 0.369 | 1.8 (1.36–2.38) | <0.001 |
| Living facilities | ||||||||
| House | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | ||||
| Apartment | 0.33 (0.23–0.47) | <0.001 | 0.55 (0.39–0.78) | 0.001 | 0.86 (0.6–1.22) | 0.393 | 1.52 (1.07–2.17) | 0.021 |
| Ongoing therapy | ||||||||
| No | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | Ref. 1.00 | ||||
| Yes | 1.17 (0.88–1.56) | 0.282 | 1.74 (1.3–2.32) | <0.001 | 1.95 (1.46–2.6) | <0.001 | 0.58 (0.43–0.77) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troschel, F.M.; Ahndorf, F.; Wille, L.-M.; Brandt, R.; Jost, J.; Rekowski, S.; Eich, H.T.; Stummer, W.; Wiewrodt, R.; Jetschke, K.; et al. Quality of Life in Brain Tumor Patients and Their Relatives Heavily Depends on Social Support Factors during the COVID-19 Pandemic. Cancers 2021, 13, 1276. https://doi.org/10.3390/cancers13061276
Troschel FM, Ahndorf F, Wille L-M, Brandt R, Jost J, Rekowski S, Eich HT, Stummer W, Wiewrodt R, Jetschke K, et al. Quality of Life in Brain Tumor Patients and Their Relatives Heavily Depends on Social Support Factors during the COVID-19 Pandemic. Cancers. 2021; 13(6):1276. https://doi.org/10.3390/cancers13061276
Chicago/Turabian StyleTroschel, Fabian M., Franziska Ahndorf, Lisa-Marie Wille, Ralf Brandt, Johanna Jost, Sylvia Rekowski, Hans Theodor Eich, Walter Stummer, Rainer Wiewrodt, Kathleen Jetschke, and et al. 2021. "Quality of Life in Brain Tumor Patients and Their Relatives Heavily Depends on Social Support Factors during the COVID-19 Pandemic" Cancers 13, no. 6: 1276. https://doi.org/10.3390/cancers13061276
APA StyleTroschel, F. M., Ahndorf, F., Wille, L.-M., Brandt, R., Jost, J., Rekowski, S., Eich, H. T., Stummer, W., Wiewrodt, R., Jetschke, K., & Wiewrodt, D. (2021). Quality of Life in Brain Tumor Patients and Their Relatives Heavily Depends on Social Support Factors during the COVID-19 Pandemic. Cancers, 13(6), 1276. https://doi.org/10.3390/cancers13061276







