Therapeutic Implications of the Immunoscore in Patients with Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
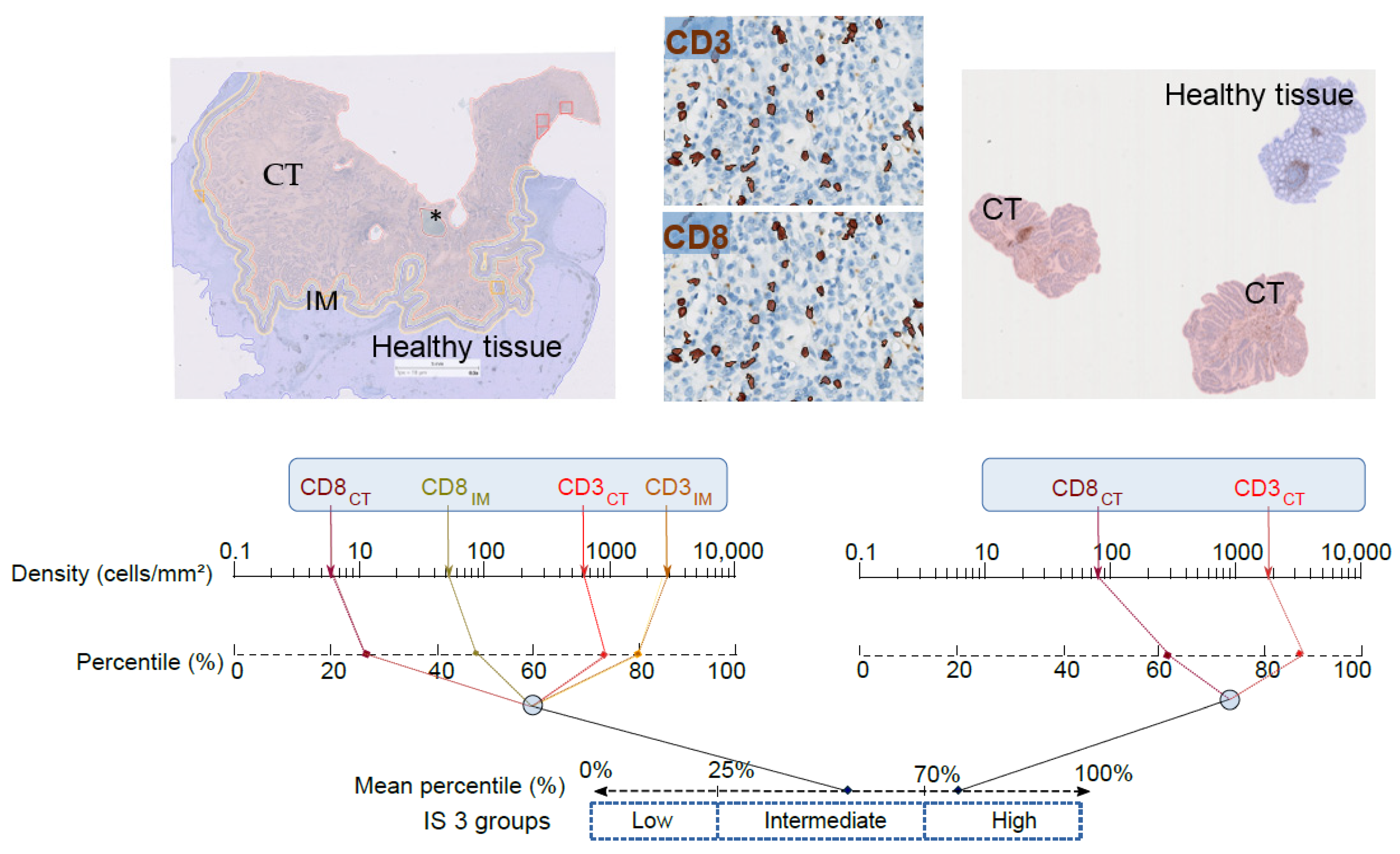
2. Conceptual Bases and Development of the IS
3. IS and Adjuvant Chemotherapies in Colon Cancer (CC)
4. The IS and ISB in Rectal Cancer
5. Immunoscore and Immunotherapy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Araghi, M.; Soerjomataram, I.; Bardot, A.; Ferlay, J.; Cabasag, C.J.; Morrison, D.S.; De, P.; Tervonen, H.; Walsh, P.M.; Bucher, O.; et al. Changes in Colorectal Cancer Incidence in Seven High-Income Countries: A Population-Based Study. Lancet Gastroenterol. Hepatol. 2019, 4, 511–518. [Google Scholar] [CrossRef]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons, Inc: Chichester, UK; Hoboken, NJ, USA, 2017; ISBN 978-1-119-26357-9. [Google Scholar]
- Nagtegaal, I.D.; Quirke, P.; Schmoll, H.-J. Has the New TNM Classification for Colorectal Cancer Improved Care? Nat. Rev. Clin. Oncol. 2011, 9, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The Immune Contexture and Immunoscore in Cancer Prognosis and Therapeutic Efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Jass, J.R. Lymphocytic Infiltration and Survival in Rectal Cancer. J. Clin. Pathol. 1986, 39, 585–589. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Angell, H.K.; Bedognetti, D.; Marincola, F.M. The Continuum of Cancer Immunosurveillance: Prognostic, Predictive, and Mechanistic Signatures. Immunity 2013, 39, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International Validation of the Consensus Immunoscore for the Classification of Colon Cancer: A Prognostic and Accuracy Study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Marliot, F.; Chen, X.; Kirilovsky, A.; Sbarrato, T.; Sissy, C.E.; Batista, L.; den Eynde, M.V.; Haicheur-Adjouri, N.; Anitei, M.-G.; Musina, A.-M.; et al. Analytical Validation of the Immunoscore and Its Associated Prognostic Value in Patients with Colon Cancer. J. Immunother. Cancer 2020, 8, e000272. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Digestive System Tumours, WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1, ISBN 978-92-832-4499-8. [Google Scholar]
- Mlecnik, B.; Bifulco, C.; Bindea, G.; Marliot, F.; Lugli, A.; Lee, J.J.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter International Society for Immunotherapy of Cancer Study of the Consensus Immunoscore for the Prediction of Survival and Response to Chemotherapy in Stage III Colon Cancer. JCO 2020, JCO.19.03205. [Google Scholar] [CrossRef]
- Pagès, F.; André, T.; Taieb, J.; Vernerey, D.; Henriques, J.; Borg, C.; Marliot, F.; Jannet, R.B.; Louvet, C.; Mineur, L.; et al. Prognostic and Predictive Value of the Immunoscore in Stage III Colon Cancer Patients Treated with Oxaliplatin in the Prospective IDEA France PRODIGE-GERCOR Cohort Study. Ann. Oncol. 2020, 31, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rebe, C.; Ghiringhelli, F. 5-Fluorouracil Selectively Kills Tumor-Associated Myeloid-Derived Suppressor Cells Resulting in Enhanced T Cell-Dependent Antitumor Immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef]
- Garg, A.D.; More, S.; Rufo, N.; Mece, O.; Sassano, M.L.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Immunogenic Cell Death Induction by Anticancer Chemotherapeutics. OncoImmunology 2017, 6, e1386829. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-Analysis of Treatment Outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef]
- Jones, H.J.S.; Al-Najami, I.; Cunningham, C. Quality of Life after Rectal-Preserving Treatment of Rectal Cancer. Eur. J. Surg. Oncol. 2020, 46, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Dayde, D.; Tanaka, I.; Jain, R.; Tai, M.; Taguchi, A. Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. IJMS 2017, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- El Sissy, C.; Kirilovsky, A.; den Eynde, M.V.; Muşină, A.-M.; Anitei, M.-G.; Romero, A.; Marliot, F.; Junca, A.; Doyen, J.; Mlecnik, B.; et al. A Diagnostic Biopsy-Adapted Immunoscore Predicts Response to Neoadjuvant Treatment and Selects Patients with Rectal Cancer Eligible for a Watch-and-Wait Strategy. Clin. Cancer Res. 2020, 26, 5198–5207. [Google Scholar] [CrossRef] [PubMed]
- George, T.J.; Allegra, C.J.; Yothers, G. Neoadjuvant Rectal (NAR) Score: A New Surrogate Endpoint in Rectal Cancer Clinical Trials. Curr. Colorectal Cancer Rep. 2015, 11, 275–280. [Google Scholar] [CrossRef]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological Features of Rectal Cancer after Preoperative Radiochemotherapy. Int. J. Colorectal Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef]
- Appelt, A.L.; Pløen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jørgensen, J.C.R.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-Dose Chemoradiotherapy and Watchful Waiting for Distal Rectal Cancer: A Prospective Observational Study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef]
- Buckley, H.; Wilson, C.; Ajithkumar, T. High-Dose-Rate Brachytherapy in the Management of Operable Rectal Cancer: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 111–127. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Pellat, A.; Boussion, H.; Svrcek, M.; Lopez-Trabada, D.; Trouilloud, I.; Afchain, P.; André, T. Immunotherapy and Metastatic Colorectal Cancers with Microsatellite Instability or Mismatch Repair Deficiency. Bull. Cancer 2019, 106, 137–142. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Huebner, L.J.; Finnes, H.D.; Muranyi, A.; Clements, J.; Singh, S.; Hubbard, J.M.; McWilliams, R.R.; Shanmugam, K.; Sinicrope, F.A. Intratumoral CD3+ and CD8+ T-Cell Densities in Patients with DNA Mismatch Repair–Deficient Metastatic Colorectal Cancer Receiving Programmed Cell Death-1 Blockade. JCO Precis. Oncol. 2019, 1–7. [Google Scholar] [CrossRef]
- Kishore, C.; Bhadra, P. Current Advancements and Future Perspectives of Immunotherapy in Colorectal Cancer Research. Eur. J. Pharmacol. 2021, 893, 173819. [Google Scholar] [CrossRef] [PubMed]
- Breakstone, R. Colon Cancer and Immunotherapy—Can We Go beyond Microsatellite Instability? Transl. Gastroenterol. Hepatol. 2021, 6. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sissy, C.; Kirilovsky, A.; Zeitoun, G.; Marliot, F.; Haicheur, N.; Lagorce-Pagès, C.; Galon, J.; Pagès, F. Therapeutic Implications of the Immunoscore in Patients with Colorectal Cancer. Cancers 2021, 13, 1281. https://doi.org/10.3390/cancers13061281
El Sissy C, Kirilovsky A, Zeitoun G, Marliot F, Haicheur N, Lagorce-Pagès C, Galon J, Pagès F. Therapeutic Implications of the Immunoscore in Patients with Colorectal Cancer. Cancers. 2021; 13(6):1281. https://doi.org/10.3390/cancers13061281
Chicago/Turabian StyleEl Sissy, Carine, Amos Kirilovsky, Guy Zeitoun, Florence Marliot, Nacilla Haicheur, Christine Lagorce-Pagès, Jérôme Galon, and Franck Pagès. 2021. "Therapeutic Implications of the Immunoscore in Patients with Colorectal Cancer" Cancers 13, no. 6: 1281. https://doi.org/10.3390/cancers13061281
APA StyleEl Sissy, C., Kirilovsky, A., Zeitoun, G., Marliot, F., Haicheur, N., Lagorce-Pagès, C., Galon, J., & Pagès, F. (2021). Therapeutic Implications of the Immunoscore in Patients with Colorectal Cancer. Cancers, 13(6), 1281. https://doi.org/10.3390/cancers13061281





