Interactome Mapping of eIF3A in a Colon Cancer and an Immortalized Embryonic Cell Line Using Proximity-Dependent Biotin Identification
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
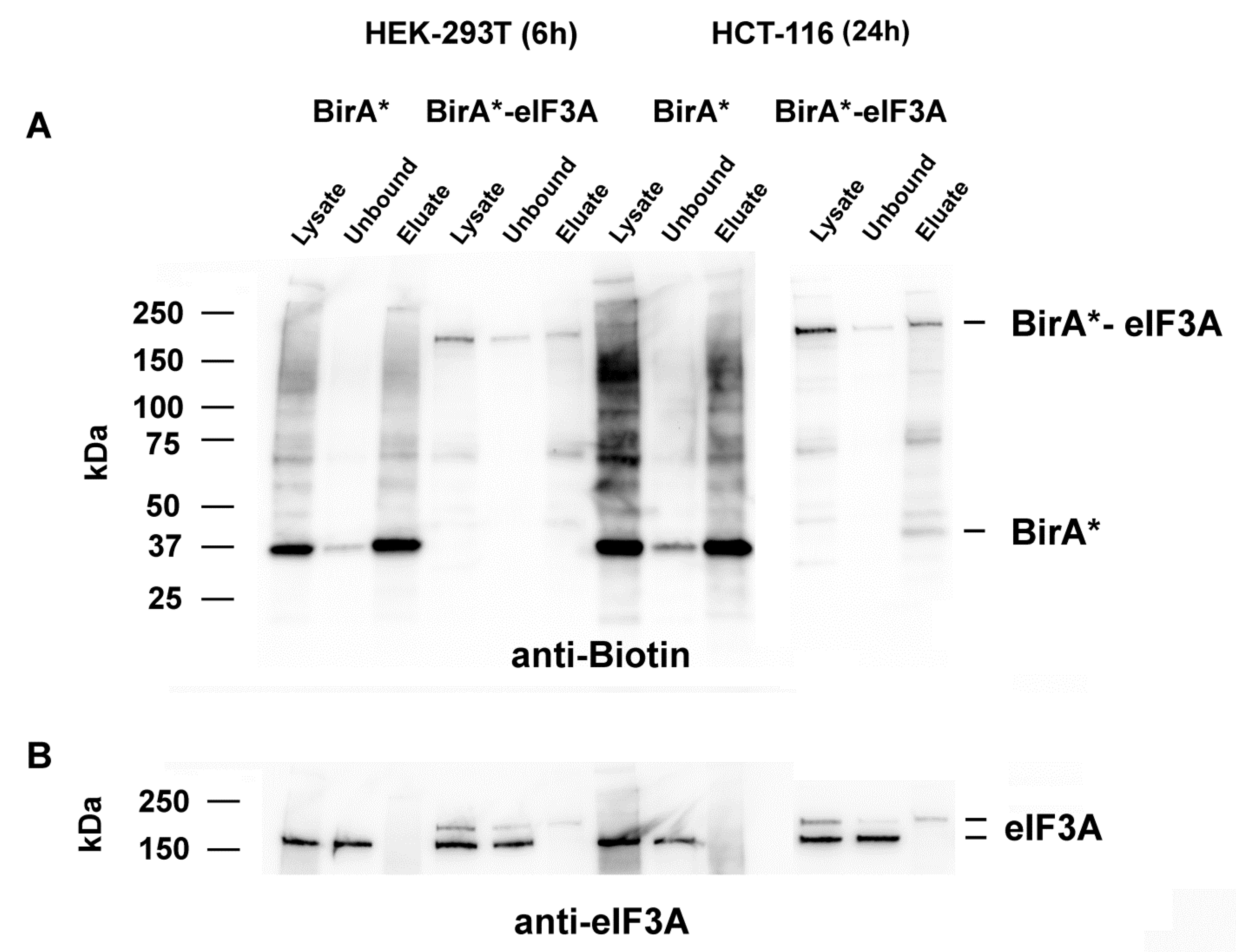
2.1. Identification of Cellular Proteins Biotinylated by BirA*-eIF3A
2.2. eIF3A Interacting Proteins
2.3. Gene Ontology Analysis of eIF3A-Proximal Proteins
2.4. Interaction Network of eIF3A

3. Discussion
4. Materials and Methods
4.1. Plasmids and Cloning
4.2. Cell Lines and Cell Culture
4.3. Proximity-Dependent Protein Labeling Methods—Sample Preparation for Mass Spectrometry
4.4. Mass Spectrometry
4.5. Immunoprecipitation and Immunoblotting
4.6. Immunofluorescence
4.7. Overrepresentation Analysis
4.8. Network Visualization using Cytoscape
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merrick, W.C. Eukaryotic protein synthesis: Still a mystery. J. Biol. Chem. 2010, 285, 21197–21201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, C.E.; Lorsch, J.R. A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 2012, 19, 568–576. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Sorensen, P.H. Translational control of aberrant stress responses as a hallmark of cancer. J. Pathol. 2018, 244, 650–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, G.; Martínez-Salas, E. Structural insights into viral IRES-dependent translation mechanisms. Curr. Opin. Virol. 2015, 12, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Erzberger, J.P.; Stengel, F.; Pellarin, R.; Zhang, S.; Schaefer, T.; Aylett, C.H.S.; Cimermančič, P.; Boehringer, D.; Sali, A.; Aebersold, R.; et al. Molecular architecture of the 40S⋅eIF1⋅eIF3 translation initiation complex. Cell 2014, 158, 1123–1135. [Google Scholar] [CrossRef] [Green Version]
- Cate, J.H.D. Human eIF3: From “blobology” to biological insight. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonetti, A.; Brito Querido, J.; Myasnikov, A.G.; Mancera-Martinez, E.; Renaud, A.; Kuhn, L.; Hashem, Y. eIF3 Peripheral Subunits rearrangement after mRNA binding and start-codon recognition. Mol. Cell 2016, 63, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Querido, J.B.; Sokabe, M.; Kraatz, S.; Gordiyenko, Y.; Skehel, J.M.; Fraser, C.S.; Ramakrishnan, V. Structure of a human 48S translational initiation complex. Science 2020, 369, 1220–1227. [Google Scholar] [CrossRef]
- Sha, Z.; Brill, L.M.; Cabrera, R.; Kleifeld, O.; Scheliga, J.S.; Glickman, M.H.; Chang, E.C.; Wolf, D.A. The eIF3 interactome reveals the translasome, a supercomplex linking protein synthesis and degradation machineries. Mol. Cell 2009, 36, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, C.E.; Beznosková, P.; Vlčkova, V.; Chiu, W.-L.; Zhou, F.; Valášek, L.S.; Hinnebusch, A.G.; Lorsch, J.R. Eukaryotic translation initiation factor 3 plays distinct roles at the mRNA entry and exit channels of the ribosomal preinitiation complex. Elife 2016, 5. [Google Scholar] [CrossRef]
- Cai, Q.; Todorovic, A.; Andaya, A.; Gao, J.; Leary, J.A.; Cate, J.H.D. Distinct regions of human eIF3 are sufficient for binding to the HCV IRES and the 40S ribosomal subunit. J. Mol. Biol. 2010, 403, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Khan, D.; Bhat, P.; Das, S. HCV-like IRESs sequester eIF3: Advantage virus. Trends Microbiol. 2014, 22, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Panthu, B.; Denolly, S.; Faivre-Moskalenko, C.; Ohlmann, T.; Cosset, F.-L.; Jalinot, P. Unlike for cellular mRNAs and other viral internal ribosome entry sites (IRESs), the eIF3 subunit e is not required for the translational activity of the HCV IRES. J. Biol. Chem. 2020, 295, 1843–1856. [Google Scholar] [CrossRef]
- Szamecz, B.; Rutkai, E.; Cuchalová, L.; Munzarová, V.; Herrmannová, A.; Nielsen, K.H.; Burela, L.; Hinnebusch, A.G.; Valásek, L. eIF3a cooperates with sequences 5’ of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRNA. Genes Dev. 2008, 22, 2414–2425. [Google Scholar] [CrossRef] [Green Version]
- Pulos-Holmes, M.C.; Srole, D.N.; Juarez, M.G.; Lee, A.S.-Y.; McSwiggen, D.T.; Ingolia, N.T.; Cate, J.H. Repression of ferritin light chain translation by human eIF3. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Proshkin, S.A.; Shematorova, E.K.; Souslova, E.A.; Proshkina, G.M.; Shpakovski, G.V. A minor isoform of the human RNA polymerase II subunit hRPB11 (POLR2J) interacts with several components of the translation initiation factor eIF3. Biochem. Biokhimiia 2011, 76, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Hershey, J.W.B. The role of eIF3 and its individual subunits in cancer. Biochim. Biophys. Acta 2015, 1849, 792–800. [Google Scholar] [CrossRef]
- Spilka, R.; Ernst, C.; Bergler, H.; Rainer, J.; Flechsig, S.; Vogetseder, A.; Lederer, E.; Benesch, M.; Brunner, A.; Geley, S.; et al. eIF3a is over-expressed in urinary bladder cancer and influences its phenotype independent of translation initiation. Cell. Oncol. Dordr. 2014, 37, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Liu, Y.; Yao, M.Y.; Jin, J. Eukaryotic Translation Initiation Factor 3a (eIF3a) promotes cell proliferation and motility in pancreatic cancer. J. Korean Med. Sci. 2016, 31, 1586–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syntichaki, P.; Troulinaki, K.; Tavernarakis, N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature 2007, 445, 922–926. [Google Scholar] [CrossRef]
- Charmpilas, N.; Daskalaki, I.; Papandreou, M.E.; Tavernarakis, N. Protein synthesis as an integral quality control mechanism during ageing. Ageing Res. Rev. 2015, 23, 75–89. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, B.; Nass, N.; Schatz, C.; Haybaeck, J. Impact of eukaryotic translation initiation factors on breast cancer: Still much to investigate. Cancers 2020, 12, 1984. [Google Scholar] [CrossRef] [PubMed]
- de la Parra, C.; Walters, B.A.; Geter, P.; Schneider, R.J. Translation initiation factors and their relevance in cancer. Curr. Opin. Genet. Dev. 2018, 48, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ramon, Y.; Cajal, S.; Castellvi, J.; Hümmer, S.; Peg, V.; Pelletier, J.; Sonenberg, N. Beyond molecular tumor heterogeneity: Protein synthesis takes control. Oncogene 2018, 37, 2490–2501. [Google Scholar] [CrossRef] [Green Version]
- Robichaud, N.; Sonenberg, N. Translational control and the cancer cell response to stress. Curr. Opin. Cell Biol. 2017, 45, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ilan, L.; Osman, F.; Namer, L.S.; Eliahu, E.; Cohen-Chalamish, S.; Ben-Asouli, Y.; Banai, Y.; Kaempfer, R. PKR activation and eIF2α phosphorylation mediate human globin mRNA splicing at spliceosome assembly. Cell Res. 2017, 27, 688–704. [Google Scholar] [CrossRef] [Green Version]
- Kaempfer, R.; Ilan, L.; Cohen-Chalamish, S.; Turgeman, O.; Namer, L.S.; Osman, F. Control of mRNA Splicing by Intragenic RNA activators of stress signaling: Potential implications for human disease. Front. Genet. 2019, 10, 464. [Google Scholar] [CrossRef]
- Wek, R.C.; Jiang, H.-Y.; Anthony, T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006, 34, 7–11. [Google Scholar] [CrossRef]
- Fullwood, M.J.; Zhou, W.; Shenolikar, S. Targeting phosphorylation of eukaryotic initiation factor-2α to treat human disease. Prog. Mol. Biol. Transl. Sci. 2012, 106, 75–106. [Google Scholar] [CrossRef]
- Yu, W.; Xiang, Y.; Luo, G.; Zhao, X.; Xiao, B.; Cheng, Y.; Feng, C.; Duan, C.; Xia, X.; Wong, V.K.W.; et al. Salubrinal Enhances doxorubicin sensitivity in human cholangiocarcinoma cells through promoting DNA damage. Cancer Biother. Radiopharm. 2018, 33, 258–265. [Google Scholar] [CrossRef]
- Biffo, S.; Manfrini, N.; Ricciardi, S. Crosstalks between translation and metabolism in cancer. Curr. Opin. Genet. Dev. 2018, 48, 75–81. [Google Scholar] [CrossRef]
- De, A.; Jacobson, B.A.; Peterson, M.S.; Jay-Dixon, J.; Kratzke, M.G.; Sadiq, A.A.; Patel, M.R.; Kratzke, R.A. 4EGI-1 represses cap-dependent translation and regulates genome-wide translation in malignant pleural mesothelioma. Investig. New Drugs 2018, 36, 217–229. [Google Scholar] [CrossRef]
- Chu, J.; Zhang, W.; Cencic, R.; Devine, W.G.; Beglov, D.; Henkel, T.; Brown, L.E.; Vajda, S.; Porco, J.A.; Pelletier, J. Amidino-rocaglates: A potent class of EIF4A inhibitors. Cell Chem. Biol. 2019, 26, 1586–1593.e3. [Google Scholar] [CrossRef]
- Iwasaki, S.; Floor, S.N.; Ingolia, N.T. Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature 2016, 534, 558–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, J.; Cargnello, M.; Topisirovic, I.; Pelletier, J. Translation initiation factors: Reprogramming protein synthesis in cancer. Trends Cell Biol. 2016, 26, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Duarte, A.; Lacerda, R.; Menezes, J.; Romão, L. eIF3: A factor for human health and disease. RNA Biol. 2018, 15, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Haybaeck, J.; O’Connor, T.; Spilka, R.; Spizzo, G.; Ensinger, C.; Mikuz, G.; Brunhuber, T.; Vogetseder, A.; Theurl, I.; Salvenmoser, W.; et al. Overexpression of p150, a part of the large subunit of the eukaryotic translation initiation factor 3, in colon cancer. Anticancer Res. 2010, 30, 1047–1055. [Google Scholar] [PubMed]
- Roux, K.J.; Kim, D.I.; Burke, B. BioID: A screen for protein-protein interactions. Curr. Protoc. Protein Sci. 2013, 74, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.-C.; Abe, K.T.; Raught, B. Getting to know the neighborhood: Using proximity-dependent biotinylation to characterize protein complexes and map organelles. Curr. Opin. Chem. Biol. 2019, 48, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.-K.H.; Hartig, R.; Weinert, S.; Haybaeck, J.; Nass, N. G-Protein-coupled estrogen receptor (GPER)-specific agonist G1 induces ER stress leading to cell death in MCF-7 Cells. Biomolecules 2019, 9, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas-Madrid, D.; Nogales-Cadenas, R.; Pascual-Montano, A. GeneCodis3: A non-redundant and modular enrichment analysis tool for functional genomics. Nucleic Acids Res. 2012, 40, W478–W483. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-Y.; Zhang, J.-T.; Zhang, W.; Zhou, H.-H.; Liu, Z.-Q. eIF3a: A new anticancer drug target in the eIF family. Cancer Lett. 2018, 412, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Yin, J.-Y.; Li, X.-P.; Liu, Z.-Q.; Wang, Y.; Chen, J.; Qu, J.; Xu, X.-J.; McLeod, H.L.; He, Y.-J.; et al. The prognostic value of altered eIF3a and its association with p27 in non-small cell lung cancers. PLoS ONE 2014, 9, e96008. [Google Scholar] [CrossRef]
- Miao, B.; Wei, C.; Qiao, Z.; Han, W.; Chai, X.; Lu, J.; Gao, C.; Dong, R.; Gao, D.; Huang, C.; et al. eIF3a mediates HIF1α-dependent glycolytic metabolism in hepatocellular carcinoma cells through translational regulation. Am. J. Cancer Res. 2019, 9, 1079–1090. [Google Scholar] [PubMed]
- Luo, C.-H.; Zhao, M.; Chen, X.-Y.; Shahabi, S.; Qiang, W.; Zeng, L.; Wang, J.; Zhou, H.-H. HE4 and eIF3a Expression Correlates with Surgical Outcome and Overall Survival in Ovarian Cancer Patients with Secondary Cytoreduction. J. Cancer 2018, 9, 2472–2479. [Google Scholar] [CrossRef]
- Spilka, R.; Laimer, K.; Bachmann, F.; Spizzo, G.; Vogetseder, A.; Wieser, M.; Müller, H.; Haybaeck, J.; Obrist, P. Overexpression of eIF3a in squamous cell carcinoma of the oral cavity and its putative relation to chemotherapy response. J. Oncol. 2012, 2012, 901956. [Google Scholar] [CrossRef]
- Xu, X.; Han, L.; Yang, H.; Duan, L.; Zhou, B.; Zhao, Y.; Qu, J.; Ma, R.; Zhou, H.; Liu, Z. The A/G allele of eIF3a rs3740556 predicts platinum-based chemotherapy resistance in lung cancer patients. Lung Cancer Amst. Neth. 2013, 79, 65–72. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Shen, J.; Dong, Z.-Z.; Huang, Q.; Zhong, M.-Z.; Feng, D.-Y.; Zhou, H.-H.; Zhang, J.-T.; Liu, Z.-Q. Effect of eIF3a on response of lung cancer patients to platinum-based chemotherapy by regulating DNA repair. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 4600–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincheira, R.; Chen, Q.; Zhang, J.T. Identification of a 170-kDa protein over-expressed in lung cancers. Br. J. Cancer 2001, 84, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.-R.; Lu, R.-F.; Romano, D.; Pitt, A.; Houslay, M.D.; Milligan, G.; Kolch, W. Eukaryotic translation initiation factor 3, subunit a, regulates the extracellular signal-regulated kinase pathway. Mol. Cell. Biol. 2012, 32, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977, 36, 59–74. [Google Scholar] [CrossRef] [PubMed]
- DuBridge, R.B.; Tang, P.; Hsia, H.C.; Leong, P.M.; Miller, J.H.; Calos, M.P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 1987, 7, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Smart, T.G. HEK293 cell line: A vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 2005, 51, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Zatloukal, B.; Kufferath, I.; Thueringer, A.; Landegren, U.; Zatloukal, K.; Haybaeck, J. Sensitivity and specificity of in situ proximity ligation for protein interaction analysis in a model of steatohepatitis with Mallory-Denk bodies. PLoS ONE 2014, 9, e96690. [Google Scholar] [CrossRef] [PubMed]
- Trinkle-Mulcahy, L. Recent advances in proximity-based labeling methods for interactome mapping. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholler, J.K.; Kanner, S.B. The human p167 gene encodes a unique structural protein that contains centrosomin A homology and associates with a multicomponent complex. DNA Cell Biol. 1997, 16, 515–531. [Google Scholar] [CrossRef]
- Zhang, J.; Song, M.-Q.; Zhu, J.-S.; Zhou, Z.; Xu, Z.-P.; Chen, W.-X.; Chen, N.-W. Identification of differentially-expressed proteins between early submucosal non-invasive and invasive colorectal cancer using 2D-DIGE and mass spectrometry. Int. J. Immunopathol. Pharmacol. 2011, 24, 849–859. [Google Scholar] [CrossRef]
- Theuerkorn, M.; Fischer, G.; Schiene-Fischer, C. Prolyl cis/trans isomerase signalling pathways in cancer. Curr. Opin. Pharmacol. 2011, 11, 281–287. [Google Scholar] [CrossRef]
- Cassaignau, A.M.E.; Cabrita, L.D.; Christodoulou, J. How does the ribosome fold the proteome? Annu. Rev. Biochem. 2020, 89, 389–415. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Christodoulou, J.; Cabrita, L.D.; Orlova, E.V. The ribosome and its role in protein folding: Looking through a magnifying glass. Acta Crystallogr. Sect. Struct. Biol. 2017, 73, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Hutt, D.M.; Loguercio, S.; Roth, D.M.; Su, A.I.; Balch, W.E. Correcting the F508del-CFTR variant by modulating eukaryotic translation initiation factor 3-mediated translation initiation. J. Biol. Chem. 2018, 293, 13477–13495. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Welch, W.J.; Diprospero, N.A.; Diamond, M.I. Phosphorylation of profilin by ROCK1 regulates polyglutamine aggregation. Mol. Cell. Biol. 2008, 28, 5196–5208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Q.; Christianson, T.A.; Koretsky, T.; Carlson, H.; David, L.; Keeble, W.; Faulkner, G.R.; Speckhart, A.; Bagby, G.C. Nucleophosmin interacts with and inhibits the catalytic function of eukaryotic initiation factor 2 kinase PKR. J. Biol. Chem. 2003, 278, 41709–41717. [Google Scholar] [CrossRef] [Green Version]
- Okuwaki, M.; Saito, S.; Hirawake-Mogi, H.; Nagata, K. The interaction between nucleophosmin/NPM1 and the large ribosomal subunit precursors contribute to maintaining the nucleolar structure. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1868, 118879. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-K.; Chou, C.-T.; Chang, H.-T.; Shu, S.-S.; Kuo, C.-C.; Tsai, J.-Y.; Liao, W.-C.; Wang, J.-L.; Lin, K.-L.; Lu, Y.-C.; et al. Effect of thapsigargin on Ca2+ fluxes and viability in human prostate cancer cells. J. Recept. Signal Transduct. Res. 2011, 31, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-L.; Wagner, S.; Herrmannová, A.; Burela, L.; Zhang, F.; Saini, A.K.; Valásek, L.; Hinnebusch, A.G. The C-terminal region of eukaryotic translation initiation factor 3a (eIF3a) promotes mRNA recruitment, scanning, and, together with eIF3j and the eIF3b RNA recognition motif, selection of AUG start codons. Mol. Cell. Biol. 2010, 30, 4415–4434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, J.X.; Pletscher-Frankild, S.; Tsafou, K.; Stolte, C.; O’Donoghue, S.I.; Schneider, R.; Jensen, L.J. COMPARTMENTS: Unification and visualization of protein subcellular localization evidence. Database 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Poulin, F.; Gingras, A.C.; Olsen, H.; Chevalier, S.; Sonenberg, N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J. Biol. Chem. 1998, 273, 14002–14007. [Google Scholar] [CrossRef] [Green Version]
- Ruhs, S.; Nass, N.; Bartling, B.; Brömme, H.-J.; Leuner, B.; Somoza, V.; Friess, U.; Silber, R.-E.; Simm, A. Preconditioning with Maillard reaction products improves antioxidant defence leading to increased stress tolerance in cardiac cells. Exp. Gerontol. 2010, 45, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Dyer, B.W.; Ferrer, F.A.; Klinedinst, D.K.; Rodriguez, R. A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal. Biochem. 2000, 282, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Nass, N.; Brömme, H.-J.; Hartig, R.; Korkmaz, S.; Sel, S.; Hirche, F.; Ward, A.; Simm, A.; Wiemann, S.; Lykkesfeldt, A.E.; et al. Differential response to α-oxoaldehydes in tamoxifen resistant MCF-7 breast cancer cells. PLoS ONE 2014, 9, e101473. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Saez, P.; Chagoyen, M.; Tirado, F.; Carazo, J.M.; Pascual-Montano, A. GENECODIS: A web-based tool for finding significant concurrent annotations in gene lists. Genome Biol. 2007, 8, R3. [Google Scholar] [CrossRef] [Green Version]
- Nogales-Cadenas, R.; Carmona-Saez, P.; Vazquez, M.; Vicente, C.; Yang, X.; Tirado, F.; Carazo, J.M.; Pascual-Montano, A. GeneCodis: Interpreting gene lists through enrichment analysis and integration of diverse biological information. Nucleic Acids Res. 2009, 37, W317–W322. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Term | Annotation | Genes Found | Genes in Term | Hyp._pval_adj. |
| Biological Process | ||||
| translational initiation | GO:0006413 | 18 | 147 | 5.52e × 10−19 |
| translation | GO:0006412 | 21 | 315 | 2.94e × 10−17 |
| SRP-dependent cotranslational protein targeting to membrane | GO:0006614 | 13 | 89 | 1.24e × 10−14 |
| viral transcription | GO:0019083 | 13 | 109 | 1.44e × 10−13 |
| nuclear-transcribed mRNA catabolic process, nonsense-mediated decay | GO:0000184 | 13 | 117 | 2.98e × 10−13 |
| cytoplasmic translational initiation | GO:0002183 | 5 | 17 | 8.87e × 10−7 |
| viral process | GO:0016032 | 15 | 577 | 1.59e × 10−6 |
| formation of cytoplasmic translation initiation complex | GO:0001732 | 4 | 16 | 4.93e × 10−5 |
| neutrophil degranulation | GO:0043312 | 12 | 484 | 5.46e × 10−5 |
| viral translational termination-reinitiation | GO:0075525 | 3 | 5 | 5.56e × 10−5 |
| response to unfolded protein | GO:0006986 | 6 | 74 | 5.76e × 10−5 |
| protein stabilization | GO:0050821 | 8 | 191 | 7.58e × 10−5 |
| protein refolding | GO:0042026 | 4 | 20 | 7.98e × 10−5 |
| gluconeogenesis | GO:0006094 | 5 | 45 | 7.99e × 10−5 |
| negative regulation of apoptotic process | GO:0043066 | 12 | 547 | 1.43e × 10−4 |
| glycolytic process | GO:0006096 | 4 | 36 | 7.05e × 10−4 |
| cellular response to interleukin-7 | GO:0098761 | 3 | 12 | 7.49e × 10−4 |
| cytoplasmic translation | GO:0002181 | 4 | 42 | 1.24e × 10−3 |
| carboxylic acid metabolic process | GO:0019752 | 3 | 18 | 2.30e × 10−3 |
| protein folding | GO:0006457 | 6 | 175 | 3.33e × 10−3 |
| ribosomal small subunit biogenesis | GO:0042274 | 3 | 21 | 3.36e × 10−3 |
| chaperone-mediated protein complex assembly | GO:0051131 | 3 | 21 | 3.36e × 10−3 |
| canonical glycolysis | GO:0061621 | 3 | 27 | 6.09e × 10−3 |
| chaperone-mediated protein folding | GO:0061077 | 3 | 30 | 6.98e × 10−3 |
| establishment of cell polarity | GO:0030010 | 3 | 35 | 9.78e × 10−3 |
| Cellular Component | ||||
| Term | Annotation | Term_Genes_Found | Term_Genes | hyp_pval_adj |
| extracellular exosome | GO:0070062 | 45 | 2170 | 1.70e × 10−20 |
| cytosol | GO:0005829 | 59 | 5175 | 1.33e × 10−16 |
| focal adhesion | GO:0005925 | 20 | 418 | 2.06e × 10−14 |
| ribosome | GO:0005840 | 15 | 237 | 2.16e × 10−12 |
| cytosolic small ribosomal subunit | GO:0022627 | 8 | 47 | 7.04e × 10−10 |
| ribonucleoprotein complex | GO:1990904 | 11 | 160 | 1.93e × 10−9 |
| nucleus | GO:0005634 | 55 | 6594 | 4.74e × 10−9 |
| cytoplasm | GO:0005737 | 52 | 6873 | 1.18e × 10−6 |
| ficolin-1-rich granule lumen | GO:1904813 | 8 | 124 | 1.20e × 10−6 |
| cytosolic large ribosomal subunit | GO:0022625 | 6 | 59 | 3.43e × 10−6 |
| eukaryotic 48S preinitiation complex | GO:0033290 | 4 | 15 | 7.32e × 10−6 |
| eukaryotic translation initiation factor 3 complex | GO:0005852 | 4 | 16 | 8.92e × 10−6 |
| eukaryotic 43S preinitiation complex | GO:0016282 | 4 | 17 | 1.07e × 10−5 |
| mitochondrial nucleoid | GO:0042645 | 5 | 44 | 1.62e × 10−5 |
| cornified envelope | GO:0001533 | 5 | 45 | 1.70e × 10−5 |
| small ribosomal subunit | GO:0015935 | 4 | 29 | 8.39e × 10−5 |
| secretory granule lumen | GO:0034774 | 6 | 115 | 1.05e × 10−4 |
| nucleoplasm | GO:0005654 | 32 | 3719 | 1.27e × 10−4 |
| polysomal ribosome | GO:0042788 | 4 | 34 | 1.29e × 10−4 |
| mitochondrion | GO:0005739 | 19 | 1553 | 1.30e × 10−4 |
| vesicle | GO:0031982 | 7 | 183 | 1.30e × 10−4 |
| melanosome | GO:0042470 | 5 | 99 | 5.70e × 10−4 |
| membrane | GO:0016020 | 49 | 7884 | 1.11e × 10−3 |
| desmosome | GO:0030057 | 3 | 26 | 1.51e × 10−3 |
| protein-containing complex | GO:0032991 | 11 | 745 | 1.94e × 10−3 |
| perinuclear region of cytoplasm | GO:0048471 | 10 | 722 | 5.48e × 10−3 |
| postsynaptic density | GO:0014069 | 6 | 282 | 7.45e × 10−3 |
| nuclear matrix | GO:0016363 | 4 | 109 | 7.67e × 10−3 |
| cell-cell junction | GO:0005911 | 5 | 200 | 9.23e × 10−3 |
| Molecular function | ||||
| Term | Annotation | Term_Genes_Found | Term_Genes | hyp_pval_adj |
| RNA binding | GO:0003723 | 47 | 1531 | 4.51e × 10−28 |
| cadherin binding | GO:0045296 | 16 | 316 | 5.24e × 10−11 |
| structural constituent of ribosome | GO:0003735 | 13 | 203 | 3.62e × 10−10 |
| unfolded protein binding | GO:0051082 | 8 | 115 | 2.51e × 10−6 |
| mRNA binding | GO:0003729 | 9 | 217 | 2.31e × 10−5 |
| rRNA binding | GO:0019843 | 5 | 45 | 6.81e × 10−5 |
| protein folding chaperone | GO:0044183 | 4 | 27 | 2.16e × 10−4 |
| translation initiation factor activity | GO:0003743 | 5 | 60 | 2.17e × 10−4 |
| protein binding | GO:0005515 | 71 | 12556 | 4.09e × 10−4 |
| double-stranded RNA binding | GO:0003725 | 5 | 72 | 4.27e × 10−4 |
| NAD binding | GO:0051287 | 4 | 39 | 6.20e × 10−4 |
| oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | GO:0016616 | 4 | 43 | 8.42e × 10−4 |
| MHC class II protein complex binding | GO:0023026 | 3 | 16 | 9.24e × 10−4 |
| nucleotide binding | GO:0000166 | 20 | 1781 | 1.07e × 10−3 |
| ATPase activity | GO:0016887 | 7 | 237 | 1.43e × 10−3 |
| DNA polymerase binding | GO:0070182 | 3 | 20 | 1.51e × 10−3 |
| ribosome binding | GO:0043022 | 4 | 55 | 1.58e × 10−3 |
| single-stranded DNA binding | GO:0003697 | 5 | 111 | 1.80e × 10−3 |
| mRNA 5’-UTR binding | GO:0048027 | 3 | 26 | 2.16e × 10−3 |
| ATP binding | GO:0005524 | 17 | 1489 | 2.42e × 10−3 |
| protein homodimerization activity | GO:0042803 | 11 | 686 | 2.48e × 10−3 |
| ubiquitin protein ligase binding | GO:0031625 | 7 | 298 | 3.25e × 10−3 |
| catalytic activity | GO:0003824 | 9 | 506 | 3.40e × 10−3 |
| transcription coactivator activity | GO:0003713 | 7 | 306 | 3.55e × 10−3 |
| miRNA binding | GO:0035198 | 3 | 34 | 3.91e × 10−3 |
| GTPase binding | GO:0051020 | 3 | 35 | 4.13e × 10−3 |
| disordered domain specific binding | GO:0097718 | 3 | 38 | 4.97e × 10−3 |
| lyase activity | GO:0016829 | 5 | 167 | 6.45e × 10−3 |
| tau protein binding | GO:0048156 | 3 | 45 | 7.51e × 10−3 |
| chaperone binding | GO:0051087 | 4 | 105 | 8.40e × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, D.-K.; Engler, A.; Stoimenovski, D.; Hartig, R.; Kaehne, T.; Kalinski, T.; Naumann, M.; Haybaeck, J.; Nass, N. Interactome Mapping of eIF3A in a Colon Cancer and an Immortalized Embryonic Cell Line Using Proximity-Dependent Biotin Identification. Cancers 2021, 13, 1293. https://doi.org/10.3390/cancers13061293
Vo D-K, Engler A, Stoimenovski D, Hartig R, Kaehne T, Kalinski T, Naumann M, Haybaeck J, Nass N. Interactome Mapping of eIF3A in a Colon Cancer and an Immortalized Embryonic Cell Line Using Proximity-Dependent Biotin Identification. Cancers. 2021; 13(6):1293. https://doi.org/10.3390/cancers13061293
Chicago/Turabian StyleVo, Diep-Khanh, Alexander Engler, Darko Stoimenovski, Roland Hartig, Thilo Kaehne, Thomas Kalinski, Michael Naumann, Johannes Haybaeck, and Norbert Nass. 2021. "Interactome Mapping of eIF3A in a Colon Cancer and an Immortalized Embryonic Cell Line Using Proximity-Dependent Biotin Identification" Cancers 13, no. 6: 1293. https://doi.org/10.3390/cancers13061293