Comparing the Safety and Efficacy of Microwave Ablation Using ThermosphereTM Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis
Abstract
Simple Summary
Abstract
1. Introduction
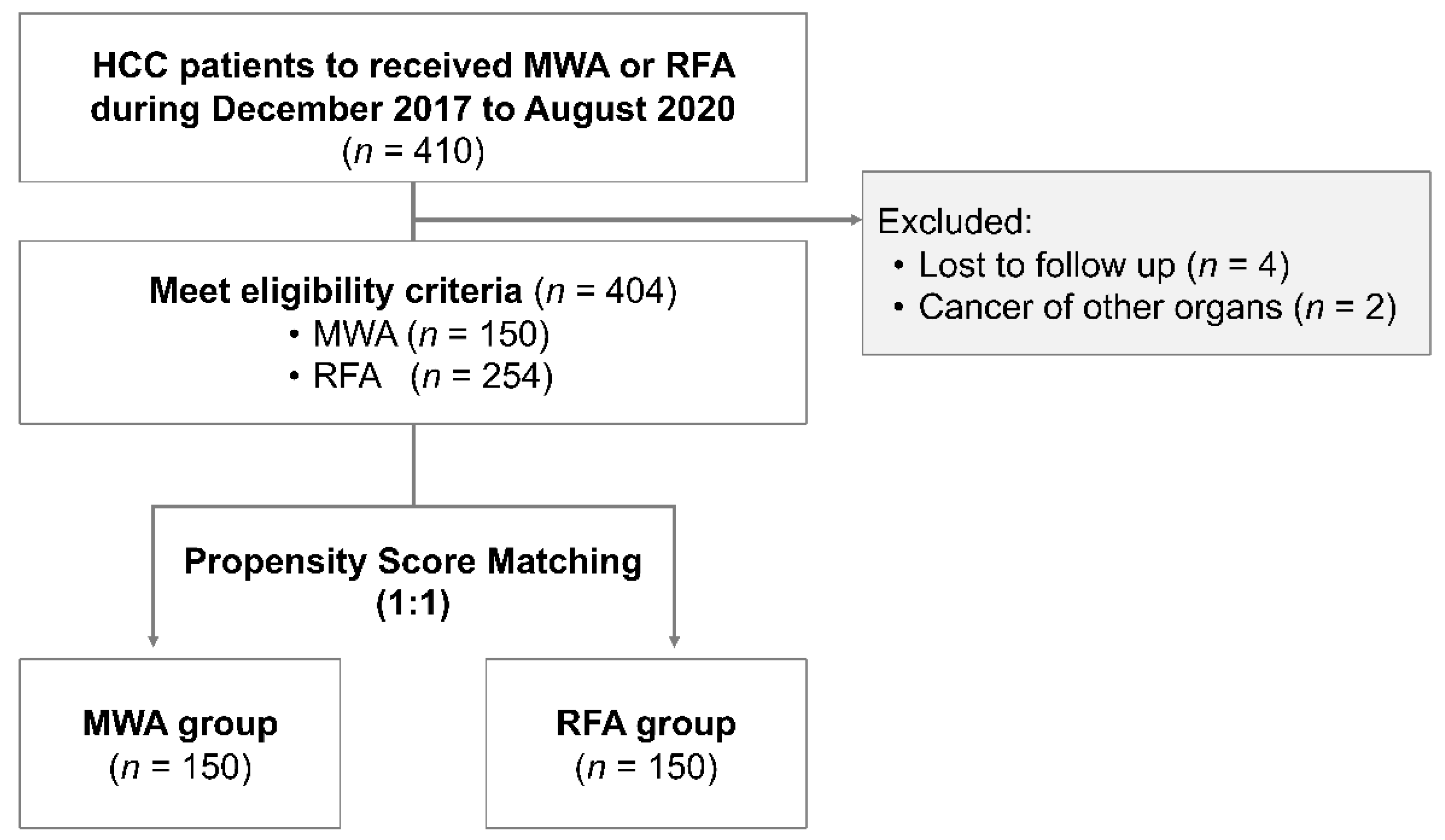
2. Materials and Methods
2.1. Patients
2.2. MWA Procedure
2.3. RFA Procedure
2.4. Assessing Treatment Efficacy and Follow-Up
2.5. PSM Analysis
2.6. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Patient Characteristics in the PSM Cohort
3.3. Treatment Efficacy in the PSM Cohort
3.4. Complications and Recurrences in the PSM Cohort
3.5. Univariate and Multivariable Analyses of OS and RFS in the PSM Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Mazzaferro, V. Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology 2016, 63, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, P.; Evans, T.R. Systemic therapy of hepatocellular carcinoma: Are we making progress? Adv. Ther. 2008, 25, 1089–1104. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef]
- Lencioni, R.; Cioni, D.; Crocetti, L.; Franchini, C.; Pina, C.D.; Lera, J.; Bartolozzi, C. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005, 234, 961–967. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lim, H.K.; Rhim, H.; Lee, M.W.; Choi, D.; Lee, W.J.; Paik, S.W.; Koh, K.C.; Lee, J.H.; Choi, M.S.; et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: Analysis of prognostic factors. J. Hepatol. 2013, 58, 89–97. [Google Scholar] [CrossRef]
- Tateishi, R.; Shiina, S.; Teratani, T.; Obi, S.; Sato, S.; Koike, Y.; Fujishima, T.; Yoshida, H.; Kawabe, T.; Omata, M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005, 103, 1201–1209. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Chen, M.H.; Yang, W.; Wang, Y.B.; Gao, W.; Hao, C.Y.; Xing, B.C.; Huang, X.F. Radiofrequency ablation of hepatocellular carcinoma: Long-term outcome and prognostic factors. Eur. J. Radiol. 2008, 67, 336–347. [Google Scholar] [CrossRef]
- Lu, D.S.; Raman, S.S.; Limanond, P.; Aziz, D.; Economou, J.; Busuttil, R.; Sayre, J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J. Vasc. Interv. Radiol. 2003, 14, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Liu, X.; Li, X.; Jiao, B.; Kang, T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: A meta-analysis. J. Gastroenterol. Hepatol. 2012, 27, 51–58. [Google Scholar] [CrossRef]
- Harari, C.M.; Magagna, M.; Bedoya, M.; Lee, F.T. Jr.; Lubner, M.G.; Hinshaw, J.L.; Ziemlewicz, T.; Brace, C.L. Microwave ablation: Comparison of simultaneous and sequential activation of multiple antennas in liver model systems. Radiology 2016, 278, 95–103. [Google Scholar] [CrossRef]
- Vietti Violi, N.; Duran, R.; Guiu, B.; Cercueil, J.P.; Aubé, C.; Digklia, A.; Pache, I.; Deltenre, P.; Knebel, J.F.; Denys, A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 317–325. [Google Scholar] [CrossRef]
- Chong, C.C.N.; Lee, K.F.; Cheung, S.Y.S.; Chu, C.C.M.; Fong, A.K.W.; Wong, J.; Hui, J.W.Y.; Fung, A.K.Y.; Lok, H.T.; Lo, E.Y.L.; et al. Prospective double-blinded randomized controlled trial of Microwave versus RadioFrequency Ablation for hepatocellular carcinoma (McRFA trial). HPB (Oxford) 2020, 22, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Bos, A.; Bennett, S.; Ferral, H. The EmprintTM ablation system with ThermosphereTM technology: One of the newer next-generation microwave ablation technologies. Semin. Intervent. Radiol. 2015, 32, 335–338. [Google Scholar] [PubMed]
- Imajo, K.; Tomeno, W.; Kanezaki, M.; Honda, Y.; Kessoku, T.; Ogawa, Y.; Yoshida, K.; Yoneda, M.; Kirikoshi, H.; Ono, M; et al. New microwave ablation system for unresectable liver tumors that forms large, spherical ablation zones. J. Gastroenterol. Hepatol. 2018, 33, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, H.; Nisiewicz, M.J.; Jayavarapu, R.; Gedaly, R.; Raissi, D. Early outcomes with single-antenna high-powered percutaneous microwave ablation for primary and secondary hepatic malignancies: Safety, effectiveness, and predictors of ablative failure. J. Clin. Imaging Sci. 2020, 10, 10. [Google Scholar] [CrossRef]
- Teratani, T.; Yoshida, H.; Shiina, S.; Obi, S.; Sato, S.; Tateishi, R.; Mine, N.; Kondo, Y.; Kawabe, T.; Omata, M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006, 43, 1101–1108. [Google Scholar] [CrossRef]
- Nishikawa, H.; Inuzuka, T.; Takeda, H.; Nakajima, J.; Sakamoto, A.; Henmi, S.; Matsuda, F.; Eso, Y.; Ishikawa, T.; Saito, S.; et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: A proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J. Gastroenterol. 2011, 46, 1418–1426. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave ablation: Principles and applications. Radiographics 2005, 25 (Suppl. 1), S69–S83. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Suwa, K.; Seki, T.; Tsuda, R.; Yamashina, M.; Murata, M.; Yamaguchi, T.; Nishio, A.; Okazaki, K. Short term treatment results of local ablation with water‑cooled microwave antenna for liver cancer: Comparison with radiofrequency ablation. Mol. Clin. Oncol. 2020, 12, 230–236. [Google Scholar] [CrossRef]
- Stauffer, P.R.; Rossetto, F.; Prakash, M.; Neuman, D.G.; Lee, T. Phantom and animal tissues for modelling the electrical properties of human liver. Int. J. Hyperthermia 2003, 19, 89–101. [Google Scholar] [CrossRef]
- Wright, A.S.; Lee, F.T., Jr.; Mahvi, D.M. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann. Surg. Oncol. 2003, 10, 275–283. [Google Scholar] [CrossRef]
- Shock, S.A.; Meredith, K.; Warner, T.F.; Sampson, L.A.; Wright, A.S.; Winter, T.C., 3rd; Mahvi, D.M.; Fine, J.P.; Lee, F.T., Jr. Microwave ablation with loop antenna: In vivo porcine liver model. Radiology 2004, 231, 143–149. [Google Scholar] [CrossRef]



| Characteristic | Unmatched Cohort | Propensity Score-Matched Cohort | ||||
|---|---|---|---|---|---|---|
| MWA (n = 150) | RFA (n = 254) | p-Value | MWA (n = 150) | RFA (n = 150) | p-Value | |
| Sex (male/female) | 109/41 | 187/67 | 0.837 | 109/41 | 112/38 | 0.694 |
| Mean age (years) | 71.6 ± 9.10 | 71.8 ± 10.3 | 0.605 | 71.6 ± 9.10 | 72.3 ± 9.60 | 0.369 |
| PS score (0/1) | 140/10 | 236/18 | 0.901 | 140/10 | 138/12 | 0.658 |
| Etiology (HBV/HCV/Alcohol/Others) | 18/62/34/36 | 37/132/55/30 | 0.195 | 18/62/34/36 | 24/64/33/29 | 0.689 |
| Naive/non-naive | 73/77 | 143/111 | 0.137 | 73/77 | 75/75 | 0.817 |
| Child-Pugh grade (A/B) | 132/18 | 208/46 | 0.104 | 132/18 | 131/19 | 0.861 |
| T.Bil (mg/dL) | 0.6 [0.5–0.9] | 0.6 [0.5–0.9] | 0.888 | 0.6 [0.5–0.9] | 0.6 [0.5–0.8] | 0.798 |
| Alb (g/L) | 3.8 [3.5–4.1] | 3.8 [3.4–4.2] | 0.942 | 3.8 [3.5–4.1] | 3.8 [3.5–4.1] | 0.684 |
| AST (U/L) | 56.8 [31.5–64.1] | 54.3 [30.5–67.1] | 0.596 | 56.8 [31.5–64.1] | 55.1 [30.9–68.3] | 0.605 |
| PT (%) | 86.0 [74.0–95.0] | 84.5 [72.7–95.6] | 0.814 | 86.0 [74.0–95.0] | 88.5 [75.0–97.0] | 0.752 |
| Plt (×104/mm3) | 11.7 [7.5–14.1] | 11.8 [7.0–15.9] | 0.851 | 11.7 [7.5–14.1] | 11.9 [7.1–14.9] | 0.765 |
| AFP > 100 ng/mL (%) | 16.0% (24/150) | 14.2% (36/254) | 0.618 | 16.0% (24/150) | 14.0% (21/150) | 0.698 |
| DCP > 40 mAU/mL (%) | 35.3% (53/150) | 38.5% (98/254) | 0.238 | 35.3% (53/150) | 36.7% (55/150) | 0.759 |
| Tumor size (mm) | 26.8 ± 11.3 * | 20.3 ± 8.60 | < 0.001 | 26.8 ± 11.3 | 24.6 ± 10.1 | 0.174 |
| Number of tumors | 1.32 ± 0.54 | 1.48 ± 0.85 | 0.082 | 1.32 ± 0.54 | 1.39 ± 0.76 | 0.926 |
| High-risk locations met (%) | 26.0% (39/150) ** | 35.4% (90/254) | 0.039 | 26.0% (39/150) | 22.7% (34/150) | 0.275 |
| TACE before ablation (%) | 37.3% (56/150) | 35.8% (91/254) | 0.761 | 37.3% (56/150) | 32.7% (49/150) | 0.432 |
| Variables | MWA (n = 150) | RFA (n = 150) | p-Value |
|---|---|---|---|
| Treatment outcome after 1 session (R grade A/B/C/D) | 61/67/20/2 | 53/76/18/3 | 0.611 |
| Final treatment outcome (R grade A/B/C/D) | 82/60/6/2 | 80/57/10/3 | 0.729 |
| Total number of ablation sessions | 1.14 ± 0.34 | 1.21 ± 0.42 | 0.123 |
| Total number of insertions | 1.22 ± 0.49 | 1.59 ± 0.94 | <0.0001 |
| Total number of complications | 5.3% (8/150) | 7.3% (11/150) | 0.477 |
| Hepatic infarction | 3 | 2 | |
| Intraperitoneal hemorrhage requiring blood transfusion | 2 | 1 | |
| Pleural effusion requiring drainage | 1 | 1 | |
| Bile peritonitis | 0 | 1 | |
| Duodenal perforation | 0 | 1 | |
| Hepatic abscess requiring drainage | 1 | 1 | |
| Neoplastic seeding | 0 | 1 | |
| Skin burn | 0 | 1 | |
| Biloma | 0 | 1 | |
| Portal vein thrombosis | 1 | 1 | |
| Recurrences | 22.7% (34/150) | 22.0% (33/150) | 0.814 |
| Intrahepatic distant recurrence | 29 | 26 | |
| Local tumor progression | 5 | 7 |
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Sex (male) | 0.979 | (0.937–1.023) | 0.349 | |||
| Age (>70) | 0.885 | (0.366–2.137) | 0.785 | |||
| PS score (1) | 1.132 | (0.601–2.121) | 0.376 | |||
| Etiology (HBV + HCV) | 0.602 | (0.269–1.347) | 0.217 | |||
| Non-naive | 2.152 | (1.236–6.583) | 0.014 | 1.322 | (0.978–5.902) | 0.057 |
| Child-Pugh (grade B) | 2.855 | (1.262–6.261) | 0.002 | 3.734 | (1.190–8.079) | 0.003 |
| T.Bil (>1.0 mg/dL) | 1.925 | (0.808–4.584) | 0.139 | |||
| Alb (>3.5 g/L) | 0.880 | (0.373–2.080) | 0.771 | |||
| AST (>50 U/L) | 0.723 | (0.457–2.258) | 0.852 | |||
| PT (>80 %) | 0.995 | (0.970–1.021) | 0.714 | |||
| Plt (<10 ×104/mm3) | 1.443 | (0.966–2.157) | 0.098 | |||
| AFP (>100 ng/mL) | 1.682 | (0.563–4.003) | 0.348 | |||
| DCP (>40 mAU/mL) | 1.136 | (0.466–2.768) | 0.802 | |||
| Tumor size (>25 mm) | 1.027 | (0.993–1.061) | 0.116 | |||
| Number of tumors (multiple) | 1.102 | (0.338–1.459) | 0.343 | |||
| High-risk tumor location (yes) | 0.301 | (0.071–1.284) | 0.105 | |||
| TACE before ablation (yes) | 1.230 | (0.537–2.715) | 0.625 | |||
| Ablation modality (MWA) | 0.867 | (0.386–1.944) | 0.729 | |||
| Final R grade (C–D) | 4.803 | (1.896–9.622) | 0.001 | 5.143 | (2.087–9.652) | 0.001 |
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Sex (male) | 0.985 | (0.960–1.010) | 0.235 | |||
| Age (>70) | 1.410 | (0.795–2.501) | 0.239 | |||
| PS score (1) | 1.231 | (0.701–2.365) | 0.321 | |||
| Etiology (HBV+HCV) | 1.298 | (0.777–2.169) | 0.319 | |||
| Non-naive | 1.171 | (0.722–1.901) | 0.522 | |||
| Child-Pugh (grade B) | 2.243 | (1.201–4.189) | 0.009 | 2.459 | (1.307–4.628) | 0.005 |
| T.Bil (>1.0 mg/dL) | 1.977 | (0.835–3.442) | 0.216 | |||
| Alb (>3.5 g/L) | 0.537 | (0.254–1.587) | 0.314 | |||
| AST (>50 U/L) | 1.052 | (0.784–1.412) | 0.734 | |||
| PT (>80 %) | 1.004 | (0.989–1.019) | 0.631 | |||
| Plt (<10 ×104/mm3) | 0.991 | (0.973–1.009) | 0.332 | |||
| AFP (>100 ng/mL) | 1.967 | (1.052–3.672) | 0.034 | 2.005 | (1.065–3.775) | 0.031 |
| DCP (>40 mAU/mL) | 1.237 | (0.995–1.813) | 0.067 | |||
| Tumor size (>25 mm) | 1.215 | (1.032–2.953) | 0.025 | 1.015 | (0.992–3.022) | 0.275 |
| Number of tumors (multiple) | 1.182 | (1.012–2.672) | 0.032 | 1.406 | (1.108–1.865) | 0.039 |
| High-risk tumor location (yes) | 0.533 | (0.279–1.017) | 0.056 | |||
| TACE before ablation (yes) | 1.473 | (0.911–2.382) | 0.114 | |||
| Ablation modality (MWA) | 0.846 | (0.526–1.361) | 0.491 | |||
| Final R grade (C–D) | 9.463 | (5.329–16.780) | <0.001 | 8.837 | (4.563–17.115) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, H.; Nagasawa, T.; Fujiwara, Y.; Sato, H.; Abe, T.; Kooka, Y.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Comparing the Safety and Efficacy of Microwave Ablation Using ThermosphereTM Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers 2021, 13, 1295. https://doi.org/10.3390/cancers13061295
Kuroda H, Nagasawa T, Fujiwara Y, Sato H, Abe T, Kooka Y, Endo K, Oikawa T, Sawara K, Takikawa Y. Comparing the Safety and Efficacy of Microwave Ablation Using ThermosphereTM Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers. 2021; 13(6):1295. https://doi.org/10.3390/cancers13061295
Chicago/Turabian StyleKuroda, Hidekatsu, Tomoaki Nagasawa, Yudai Fujiwara, Hiroki Sato, Tamami Abe, Yohei Kooka, Kei Endo, Takayoshi Oikawa, Kei Sawara, and Yasuhiro Takikawa. 2021. "Comparing the Safety and Efficacy of Microwave Ablation Using ThermosphereTM Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis" Cancers 13, no. 6: 1295. https://doi.org/10.3390/cancers13061295
APA StyleKuroda, H., Nagasawa, T., Fujiwara, Y., Sato, H., Abe, T., Kooka, Y., Endo, K., Oikawa, T., Sawara, K., & Takikawa, Y. (2021). Comparing the Safety and Efficacy of Microwave Ablation Using ThermosphereTM Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers, 13(6), 1295. https://doi.org/10.3390/cancers13061295








