Late Central Airway Toxicity after High-Dose Radiotherapy: Clinical Outcomes and a Proposed Bronchoscopic Classification
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
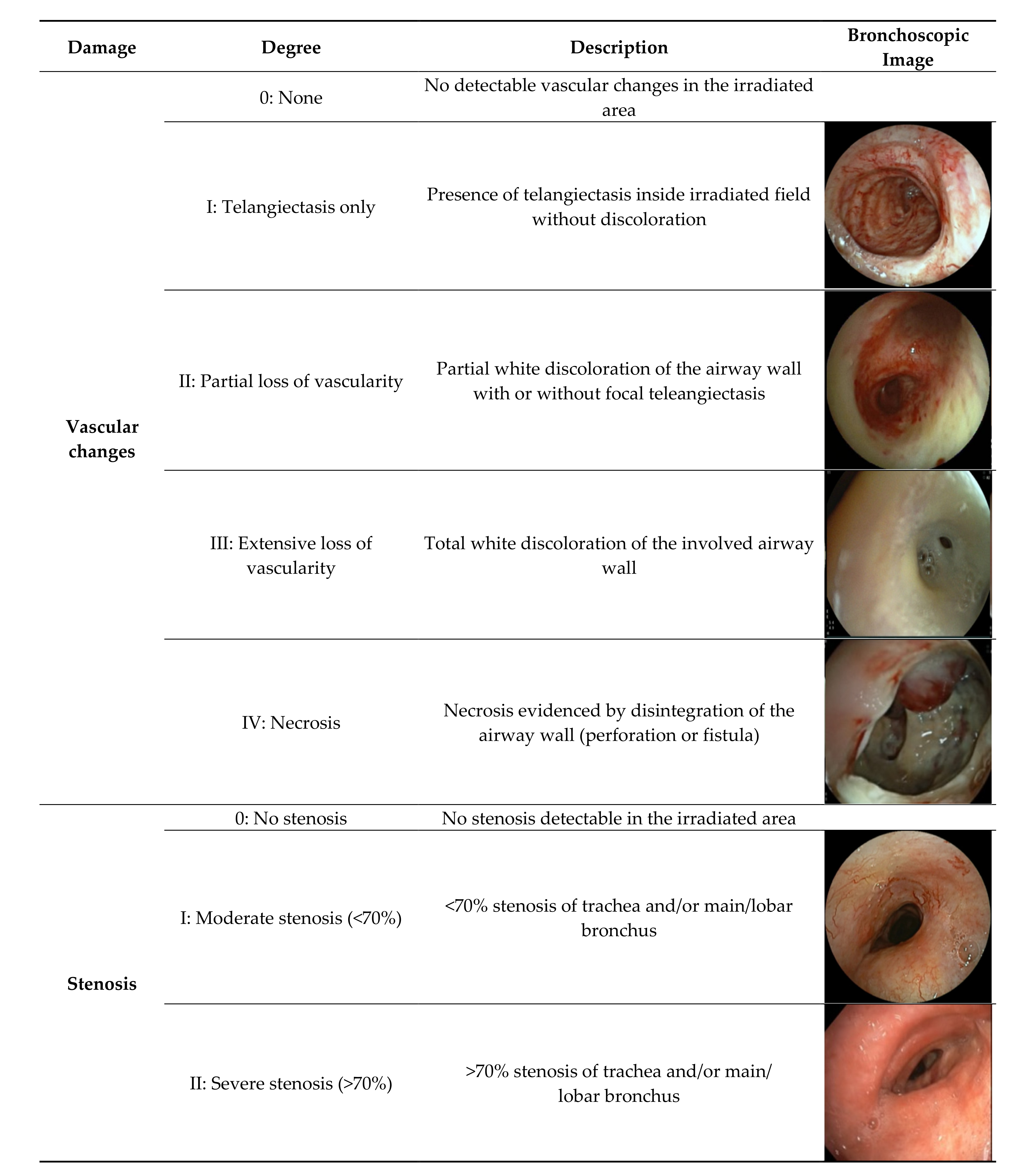
2.1. Data Collecting and Classification System
2.2. Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Bronchoscopic Findings and Proposed Classification System
3.3. Vascular Changes
3.4. Bronchial Stenosis
3.5. Combined Stenosis and Vascular Changes
4. Discussion
4.1. Mechanisms of Toxicity
4.2. Interventions to Reduce Airway Toxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Outcome for Patients with Stenosis Only
| Stenosis Degree | Indication Bronchoscopy/Symptoms | Intervention | Follow-Up after Bronchoscopy | Status |
| >70% | Post-treatment inspection | - | 42 months till last contact. | Alive |
| >70% | Impending respiratory insufficiency | Dilatation and stent placement 5 months post-RT | Died 68 months after bronchoscopy. Probable cause of death related to heart problems, not airway stenosis. | Dead |
| >70% | Severe tracheomalacia and stenosis, respiratory insufficiency | Tracheostomy, canula | Died 23 months after bronchoscopy. Repeated IC admissions for respiratory insufficiency. Palliative care for progressive dyspnea. | Dead |
| >70% | Severe dyspnea | Stent placement 2 years after CRT and immuno-therapy | Died 9 days after bronchoscopy. Palliative care for progressive dyspnea. | Dead |
| >70% | Post-treatment inspection. Dyspnea in combination with COPD exac-erbation. | Antibiotics | Died 3 months after bronchoscopy | Dead |
Appendix B. Post-Radiation Interventions for Airway Toxicity
| No intervention No intervention necessary Deemed inoperable No bronchoscopic options left (e.g., recurrent stenosis) | 51 31 12 8 |
| Dilatation | 2 |
| Dilatation and stent placement | 7 |
| Stent placement | 4 |
| Pneumonectomy/lobectomy | 2 |
| Reinforcement surgery | 2 |
| Tracheostoma/canula | 2 |
References
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, 1–21. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Faivre-Finn, C.; Moeller, D.; Nestle, U.; Hurksmans, C.W.; Le Péchoux, C.; Belderbos, J.; Guckenberger, M.; Senan, S. European Organization for Research and Treatment of Cancer (EORTC) Recommendations for Planning and Delivery of High-Dose, High Precision Radiotherapy for Lung Cancer. Radiother. Oncol. 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy Versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Sun, B.; Brooks, E.D.; Komaki, R.; Liao, Z.; Jeter, M.; McAleer, M.; Balter, P.A.; Welsh, J.D.; O’Reilly, M.; Gomez, D.; et al. Long-Term Outcomes of Salvage Stereotactic Ablative Radiotherapy for Isolated Lung Recurrence of Non–Small Cell Lung Cancer: A Phase II Clinical Trial. J. Thorac. Oncol. 2017, 12, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.; McGarry, R.; Yiannoutsos, C.; Papiez, L.; Tudor, K.; DeLuca, J.; Ewing, M.; Abdulrahman, R.; DesRosiers, C.; Williams, M.; et al. Excessive Toxicity When Treating Central Tumors in a Phase II Study of Stereotactic Body Radiation Therapy for Medically Inoperable Early-stage Lung Cancer. J. Clin. Oncol. 2006, 24, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.N.B.; Hause, D.J.; Novak, J.; Monjazeb, A.M.; Daly, M.E. Tumor Control and Toxicity after SBRT for Ultracentral, Central, and Paramediastinal Lung Tumors. Pract. Radiat. Oncol. 2019, 9, 196–202. [Google Scholar] [CrossRef]
- Atalar, B.; Mustafayev, T.Z.; Sio, T.T.; Sahin, B.; Gungor, G.; Aydin, G.; Yapici, B.; Ozyar, E. Long-Term Toxicity and Survival Outcomes After Stereotactic Ablative Radiotherapy for Patients with Centrally Located Thoracic Tumors. Radiol. Oncol. 2020, 54, 480–487. [Google Scholar] [CrossRef]
- Miller, K.L.; Shafman, T.D.; Anscher, M.S.; Zhou, S.M.; Clough, R.W.; Garst, J.L.; Crawford, J.; Rosenman, J.; Socinski, M.A.; Blackstock, W.; et al. Bronchial Stenosis: An Underreported Complication of High-Dose External Beam Radiotherapy for Lung Cancer? Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Tekatli, H.; Haasbeek, N.; Dahele, M.; De Haan, P.; Verbakel, W.; Bongers, E.; Hashemi, S.; Nossent, E.; Spoelstra, F.; de Langen, A.J. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with “Ultracentral” Non-Small Cell Lung Cancer. J. Thorac Oncol. 2016, 11, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Van Hoorn, J.E.; Dahele, M.; Daniels, J.M.A. Bronchoscopic Manifestations of Airway Toxicity After Radiotherapy. Clin. Lung Cancer 2018, 19, 875–878. [Google Scholar] [CrossRef]
- Duijm, M.; Schillemans, W.; Aerts, J.G.; Heijmen, B.; Nuyttens, J.J. Dose and Volume of the Irradiated Main Bronchi and Related Side Effects in the Treatment of Central Lung Tumors with Stereotactic Radiotherapy. Semin. Radiat. Oncol. 2016, 26, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, M.N.; Haas, A.R.; Rengan, R. Central-Airway Necrosis after Stereotactic Body-Radiation Therapy. N. Engl. J. Med. 2012, 366, 2327–2329. [Google Scholar] [CrossRef]
- Tekatli, H.; van ’t Hof, S.; Nossent, E.J.; Dahele, M.; Verbakel, W.; Slotman, B.; Senan, S. Use of Stereotactic Ablative RadioTherapy (SABR) in Non–Small Cell Lung Cancer Measuring More Than 5 cm. J. Thorac. Oncol. 2017, 12, 974–982. [Google Scholar] [CrossRef]
- Giuliani, M.; Mathew, A.S.; Bahig, H.; Bratman, S.V.; Filion, E.; Glick, D.; Louie, A.V.; Raman, S.; Swaminath, A.; Warner, A.; et al. SUNSET: Stereotactic Radiation for Ultracentral Non–Small-Cell Lung Cancer—A Safety and Efficacy Trial. Clin. Lung Cancer 2018, 19, 529–532. [Google Scholar] [CrossRef]
- Owen, D.; Sio, T.T. Stereotactic Body Radiotherapy (SBRT) for Central and Ultracentral Node-Negative Lung Tumors. J. Thorac Dis. 2020, 12, 7024–7031. [Google Scholar] [CrossRef]
- Kroeze, S.G.C.; Fritz, C.; Hoyer, M.; Lo, S.S.; Ricardi, U.; Sahgal, A.; Stahel, R.; Stupp, R.; Guckenberger, M. Toxicity of Concurrent Stereotactic Radiotherapy and Targeted Therapy or Immunotherapy: A Systematic Review. Cancer Treat. Rev. 2017, 53, 25–37. [Google Scholar] [CrossRef]
- Wirsdörfer, F.; De Leve, S.; Jendrossek, V. Combining Radiotherapy and Immunotherapy in Lung Cancer: Can we Expect Limitations Due to Altered Normal Tissue Toxicity? Int J. Mol. Sci. 2019, 20, 24. [Google Scholar] [CrossRef]
- Haseltine, J.M.; Rimner, A.; Gelblum, D.Y.; Modh, A.; Rosenzweig, K.E.; Jackson, A.; Yorke, E.D.; Wu, J.A. Fatal Complications After Stereotactic Body Radiation Therapy for Central Lung Tumors Abutting the Proximal Bronchial Tree. Pract. Radiat. Oncol. 2016, 6, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Senan, S.; Bahce, I. Pulmonary Toxicity in Patients Treated with Immune Checkpoint Inhibitors and Radiation. Ann. Oncol. 2020, 31, 1597–1598. [Google Scholar] [CrossRef]
- Shaverdian, N.; Beattie, J.; Thor, M.; Offin, M.; Shepherd, A.F.; Gelblum, D.Y.; Wu, A.J.; Simone, C.B.; Hellmann, M.D.; Chaft, J.E.; et al. Safety of Thoracic Radiotherapy in Patients with Prior Immune-Related Adverse Events from Immune Checkpoint Inhibitors. Ann. Oncol. 2020, 31, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Myer, C.M.; O’Connor, D.M.; Cotton, R.T. Proposed Grading System for Subglottic Stenosis Based on Endotracheal Tube Sizes. Ann. Otol. Rhinol. Laryngol. 1994, 103, 319–323. [Google Scholar] [CrossRef]
- Yamamoto, R.; Tada, H.; Kishi, A.; Tojo, T. Effects of Preoperative Chemotherapy and Radiation Therapy on Human Bronchial Blood Flow. J. Thorac. Cardiovasc. Surg. 2000, 119, 939–945. [Google Scholar] [CrossRef]
- Graves, P.R.; Siddiqui, F.; Anscher, M.S.; Movsas, B. Radiation Pulmonary Toxicity: From Mechanisms to Management. Semin. Radiat. Oncol. 2010, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, N.; Modiri, A.; Samanta, S.; Yan, Y.; Bland, R.; Rozario, T.; Wibowo, H.; Iyengar, P.; Ahn, C.; Timmerman, R.; et al. Virtual Bronchoscopy-Guided Treatment Planning to Map and Mitigate Radiation-Induced Airway Injury in Lung SAbR. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Modiri, A.; Kipritidis, J.; Hagan, A.; Yu, K.; Wibowo, H.; Yan, Y.; Owen, D.R.; Matuszak, M.M.; Mohindra, P.; et al. Functionally Weighted Airway Sparing (FWAS): A Functional Avoidance Method for Preserving Post-Treatment Ventilation in Lung Radiotherapy. Phys. Med. Biol. 2020, 65, 165010. [Google Scholar] [CrossRef] [PubMed]
- Dickhoff, C.; Dahele, M.; Hashemi, S.M.; Senan, S.; Smit, E.F.; Hartemink, K.J.; Paul, M.A. Surgical Treatment of Complications After High-Dose Chemoradiotherapy for Lung Cancer. Ann. Thorac. Surg. 2017, 104, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Greason, K.L.; Miller, D.L.; Clay, R.P.; Deschamps, C.; Johnson, C.H.; Allen, M.S.; Trastek, V.F.; Pairolero, P.C. Management of the Irradiated Bronchus After Lobectomy for Lung Cancer. Ann. Thorac. Surg. 2003, 76, 180–185. [Google Scholar] [CrossRef]
- Meyer, A.J.H.; Krueger, T.; Lepori, D.; Dusmet, M.; Aubert, J.D.; Pasche, P.; Ris, H.B. Closure of Large Intrathoracic Airway Defects Using Extrathoracic Muscle Flaps. Ann. Thorac. Surg. 2004, 77, 397–404. [Google Scholar] [CrossRef]
- Grønhøj, C.; Jensen, D.H.; Glovinski, P.V.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Specht, L.; Thomsen, C.; Darkner, S.; Kiss, K.; et al. First-in-Man Mesenchymal Stem Cells for Radiation-Induced Xerostomia (MESRIX): Study Protocol for a Randomized Controlled Trial. Trials 2017, 18. [Google Scholar] [CrossRef]
- Grønhøj, C.; Jensen, D.H.; Vester-Glowinski, P.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Fog, L.M.; Specht, L.; Thomsen, C.; Darkner, S.; et al. Safety and Efficacy of Mesenchymal Stem Cells for Radiation-Induced Xerostomia: A Randomized, Placebo-Controlled Phase 1/2 Trial (MESRIX). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 581–592. [Google Scholar] [CrossRef]

| Characteristics | No. |
|---|---|
| Gender | |
| Male | 42 |
| Female | 28 |
| Age (years) | |
| Median (range) | 64 (42–80) |
| Location of malignancy | |
| Trachea | 8 |
| Central (up to and segmental bronchi) | 21 |
| Peripheral | 36 |
| Esophagus | 3 |
| Mediastinal lymph node | 1 |
| Pathology | |
| NSCLC | 64 |
| Metastases | 2 |
| Other | 4 |
| Type of RT | |
| SBRT | 10 |
| Conventional | 14 |
| Chemoradiotherapy (CRT) | 46 |
| Indication for bronchoscopy | |
| Airway symptoms | 28 |
| Assessment post CRT/surgery | 12 |
| Suspected tumor recurrence | 21 |
| Radiological findings | 9 |
| Recurrence at time of bronchoscopy | |
| Free of recurrence | 47 |
| High suspicion of recurrence on imaging | 9 |
| Proven recurrence with biopsy | 14 |
| Damage | Degree | SBRT | RT | CRT |
|---|---|---|---|---|
| Vascular changes | 0: None | 0 | 2 | 3 |
| I: Telangiectasis only | 5 | 3 | 17 | |
| II: Partial loss of vascularity | 3 | 3 | 12 | |
| III: Extensive loss of vascularity | 1 | 4 | 6 | |
| IV: Necrosis | 1 | 2 | 8 | |
| Stenosis | 0: No stenosis | 3 | 5 | 17 |
| I: Moderate stenosis (<70%) | 1 | 1 | 11 | |
| II: Severe stenosis (>70%) | 6 | 8 | 18 | |
| Total | 10 | 14 | 46 |
| Indication for Bronchoscopy | Vascular Changes Only N = 25 | <70% Stenosis N = 13 | >70% Stenosis N = 32 |
|---|---|---|---|
| Hemoptysis | 5 | 3 | 3 |
| Dyspnea | 1 | 1 | 9 |
| Respiratory insufficiency | 1 | 1 | 2 |
| Recurrent infections | 0 | 0 | 2 |
| Assessment post-CRT or surgery | 3 | 2 | 7 |
| Assessment (suspected) recurrence | 11 | 1 | 3 |
| Intervention recurrence (diathermy/debulking) | 2 | 2 | 2 |
| Assessment of radiological findings suspicious for RT damage | 2 | 3 | 4 |
| Damage | Degree | RT-Toxicity | Follow-Up |
|---|---|---|---|
| Vascular changes | 0: None (N = 5) | 10.0 (4.0–23.5) | 23.0 (1.5–55.0) |
| I: Telangiectasis only (N = 14) | 47.0 (26.0–88.0) | 10.0 (5.0–16.0) | |
| II: Partial loss of vascularity (N = 10) | 23.0 (12.5–84.5) | 13.5 (4.0–31.0) | |
| III: Extensive loss of vascularity (N = 9) | 19.0 (12.0–37.0) | 1.0 (0–11.0) | |
| IV: Necrosis (N = 10) | 13.0 (6.0–23.0) | 5.0 (1.0–10.0) | |
| Stenosis | 0: No stenosis (N = 19) | 29.0 (10.5–48.5) | 9.0 (2.5–19.5) |
| I: Moderate stenosis (<70%) (N = 8) | 28.0 (14.5–70.0) | 5.0 (0–11.0) | |
| II: Severe stenosis (>70%) (N = 21) | 22.5 (13.0–60.0) | 11.0 (2.0–23.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Hoorn, J.E.; Dahele, M.; Daniels, J.M.A. Late Central Airway Toxicity after High-Dose Radiotherapy: Clinical Outcomes and a Proposed Bronchoscopic Classification. Cancers 2021, 13, 1313. https://doi.org/10.3390/cancers13061313
van Hoorn JE, Dahele M, Daniels JMA. Late Central Airway Toxicity after High-Dose Radiotherapy: Clinical Outcomes and a Proposed Bronchoscopic Classification. Cancers. 2021; 13(6):1313. https://doi.org/10.3390/cancers13061313
Chicago/Turabian Stylevan Hoorn, Juliët E., Max Dahele, and Johannes M. A. Daniels. 2021. "Late Central Airway Toxicity after High-Dose Radiotherapy: Clinical Outcomes and a Proposed Bronchoscopic Classification" Cancers 13, no. 6: 1313. https://doi.org/10.3390/cancers13061313
APA Stylevan Hoorn, J. E., Dahele, M., & Daniels, J. M. A. (2021). Late Central Airway Toxicity after High-Dose Radiotherapy: Clinical Outcomes and a Proposed Bronchoscopic Classification. Cancers, 13(6), 1313. https://doi.org/10.3390/cancers13061313






