Imaging and Management of Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathology and Risk Factors
3. Diagnosis
4. Staging
5. Role of Imaging in Staging:
5.1. Computed Tomography (CT)
5.2. Magnetic Resonance Imaging (MRI)
5.3. Positron Emission Tomography (PET)
5.4. Ultrasound
5.5. Fluoroscopic/Radiographic Techniques
6. Management
7. Imaging Surveillance
8. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef] [Green Version]
- Sloan, F.A.; Yashkin, A.P.; Akushevich, I.; Inman, B.A. The Cost to Medicare of Bladder Cancer Care. Eur. Urol. Oncol. 2020, 3, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Al-Husseini, M.J.; Kunbaz, A.; Saad, A.M.; Santos, J.V.; Salahia, S.; Iqbal, M.; Alahdab, F. Trends in the incidence and mortality of transitional cell carcinoma of the bladder for the last four decades in the USA: A SEER-based analysis. BMC Cancer 2019, 19, 46. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Willis, D.; Kamat, A.M. Nonurothelial bladder cancer and rare variant histologies. Hematol. Oncol. Clin. N. Am. 2015, 29, 237–252. [Google Scholar] [CrossRef]
- Wasco, M.J.; Daignault, S.; Zhang, Y.; Kunju, L.P.; Kinnaman, M.; Braun, T.; Lee, C.T.; Shah, R.B. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology 2007, 70, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.; Rajesh, A.; Prasad, S.R.; Gaitonde, K.; Lall, C.G.; Mouraviev, V.; Aeron, G.; Bracken, R.B.; Sandrasegaran, K. Urinary bladder cancer: Role of MR imaging. Radiographics 2012, 32, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Wong-You-Cheong, J.J.; Woodward, P.J.; Manning, M.A.; Sesterhenn, I.A. From the Archives of the AFIP: Neoplasms of the urinary bladder: Radiologic-pathologic correlation. Radiographics 2006, 26, 553–580. [Google Scholar] [CrossRef] [PubMed]
- Barocas, D.A.; Boorjian, S.A.; Alvarez, R.D.; Downs, T.M.; Gross, C.P.; Hamilton, B.D.; Kobashi, K.C.; Lipman, R.R.; Lotan, Y.; Ng, C.K.; et al. Microhematuria: AUA/SUFU Guideline. J. Urol. 2020, 204, 778–786. [Google Scholar] [CrossRef]
- Cattaneo, F.; Motterle, G.; Zattoni, F.; Morlacco, A.; Dal Moro, F. The Role of Lymph Node Dissection in the Treatment of Bladder Cancer. Front. Surg. 2018, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Vazina, A.; Dugi, D.; Shariat, S.F.; Evans, J.; Link, R.; Lerner, S.P. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J. Urol. 2004, 171, 1830–1834. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 28 December 2020).
- Browne, R.F.; Meehan, C.P.; Colville, J.; Power, R.; Torreggiani, W.C. Transitional cell carcinoma of the upper urinary tract: Spectrum of imaging findings. Radiographics 2005, 25, 1609–1627. [Google Scholar] [CrossRef]
- Dillman, J.R.; Caoili, E.M.; Cohan, R.H.; Ellis, J.H.; Francis, I.R.; Nan, B.; Zhang, Y. Comparison of urinary tract distension and opacification using single-bolus 3-Phase vs split-bolus 2-phase multidetector row CT urography. J. Comput. Assist. Tomogr. 2007, 31, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Froemming, A.; Potretzke, T.; Takahashi, N.; Kim, B. Upper tract urothelial cancer. Eur. J. Radiol. 2018, 98, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sadow, C.A.; Silverman, S.G.; O’Leary, M.P.; Signorovitch, J.E. Bladder cancer detection with CT urography in an Academic Medical Center. Radiology 2008, 249, 195–202. [Google Scholar] [CrossRef]
- Trinh, T.W.; Glazer, D.I.; Sadow, C.A.; Sahni, V.A.; Geller, N.L.; Silverman, S.G. Bladder cancer diagnosis with CT urography: Test characteristics and reasons for false-positive and false-negative results. Abdom. Radiol. 2018, 43, 663–671. [Google Scholar] [CrossRef]
- Lee, C.H.; Tan, C.H.; Faria, S.C.; Kundra, V. Role of Imaging in the Local Staging of Urothelial Carcinoma of the Bladder. AJR Am. J. Roentgenol. 2017, 208, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Vikram, R.; Sandler, C.M.; Ng, C.S. Imaging and staging of transitional cell carcinoma: Part 1, lower urinary tract. AJR Am. J. Roentgenol. 2009, 192, 1481–1487. [Google Scholar] [CrossRef]
- Metser, U.; Goldstein, M.A.; Chawla, T.P.; Fleshner, N.E.; Jacks, L.M.; O’Malley, M.E. Detection of urothelial tumors: Comparison of urothelial phase with excretory phase CT urography—A prospective study. Radiology 2012, 264, 110–118. [Google Scholar] [CrossRef]
- Girard, A.; Vila Reyes, H.; Shaish, H.; Grellier, J.F.; Dercle, L.; Salaun, P.Y.; Delcroix, O.; Rouanne, M. The Role of 18F-FDG PET/CT in Guiding Precision Medicine for Invasive Bladder Carcinoma. Front. Oncol. 2020, 10, 565086. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Takeuchi, M.; Vargas, H.A.; Muglia, V.F.; Cipollari, S.; Catalano, C.; Panebianco, V. Overview of VI-RADS in Bladder Cancer. AJR Am. J. Roentgenol. 2020, 214, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, S.W.E.; Veenboer, P.W.; Wessels, F.J.; Meijer, R.P. Diagnostic Accuracy of Multiparametric MRI for Local Staging of Bladder Cancer: A Systematic Review and Meta-Analysis. Urology 2020, 145, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.; Krishna, S.; Booth, C.M.; Breau, R.H.; Flood, T.A.; Morgan, S.C.; Schieda, N.; Salameh, J.P.; McGrath, T.A.; McInnes, M.D.F. Diagnostic accuracy of magnetic resonance imaging for tumour staging of bladder cancer: Systematic review and meta-analysis. BJU Int. 2018, 122, 744–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Koga, F.; Kawakami, S.; Ishii, C.; Tanaka, H.; Numao, N.; Sakai, Y.; Saito, K.; Masuda, H.; Fujii, Y.; et al. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology 2010, 75, 387–391. [Google Scholar] [CrossRef]
- Necchi, A.; Bandini, M.; Calareso, G.; Raggi, D.; Pederzoli, F.; Fare, E.; Colecchia, M.; Marandino, L.; Bianchi, M.; Gallina, A.; et al. Multiparametric Magnetic Resonance Imaging as a Noninvasive Assessment of Tumor Response to Neoadjuvant Pembrolizumab in Muscle-Invasive Bladder Cancer: Preliminary Findings from the PURE-01 Study. Eur. Urol. 2020, 77, 636–643. [Google Scholar] [CrossRef]
- Sudah, M.; Masarwah, A.; Kainulainen, S.; Pitkanen, M.; Matikka, H.; Dabravolskaite, V.; Aaltomaa, S.; Vanninen, R. Comprehensive MR Urography Protocol: Equally Good Diagnostic Performance and Enhanced Visibility of the Upper Urinary Tract Compared to Triple-Phase CT Urography. PLoS ONE 2016, 11, e0158673. [Google Scholar] [CrossRef]
- Martingano, P.; Cavallaro, M.F.; Bertolotto, M.; Stacul, F.; Ukmar, M.; Cova, M.A. Magnetic resonance urography vs computed tomography urography in the evaluation of patients with haematuria. Radiol. Med. 2013, 118, 1184–1198. [Google Scholar] [CrossRef]
- Rouviere, O.; Cornelis, F.; Brunelle, S.; Roy, C.; Andre, M.; Bellin, M.F.; Boulay, I.; Eiss, D.; Girouin, N.; Grenier, N.; et al. Imaging protocols for renal multiparametric MRI and MR urography: Results of a consensus conference from the French Society of Genitourinary Imaging. Eur. Radiol. 2020, 30, 2103–2114. [Google Scholar] [CrossRef]
- Apolo, A.B.; Riches, J.; Schoder, H.; Akin, O.; Trout, A.; Milowsky, M.I.; Bajorin, D.F. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J. Clin. Oncol. 2010, 28, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Chen, J.H.; Liang, J.A.; Wang, H.Y.; Lin, C.C.; Lin, W.Y.; Kao, C.H. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic review and meta-analysis. Eur. J. Radiol. 2012, 81, 2411–2416. [Google Scholar] [CrossRef]
- Witjes, J.A.; Babjuk, M.; Bellmunt, J.; Bruins, H.M.; De Reijke, T.M.; De Santis, M.; Gillessen, S.; James, N.; Maclennan, S.; Palou, J.; et al. EAU-ESMO Consensus Statements on the Management of Advanced and Variant Bladder Cancer—An International Collaborative Multistakeholder Effort(dagger): Under the Auspices of the EAU-ESMO Guidelines Committees. Eur. Urol. 2020, 77, 223–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eulitt, P.J.; Altun, E.; Sheikh, A.; Wong, T.Z.; Woods, M.E.; Rose, T.L.; Wallen, E.M.; Pruthi, R.S.; Smith, A.B.; Nielsen, M.E.; et al. Pilot Study of [18F] Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)/Magnetic Resonance Imaging (MRI) for Staging of Muscle-invasive Bladder Cancer (MIBC). Clin. Genitourin. Cancer 2020, 18, 378–386.e1. [Google Scholar] [CrossRef]
- Jeong, I.G.; Hong, S.; You, D.; Hong, J.H.; Ahn, H.; Kim, C.S. FDG PET-CT for lymph node staging of bladder cancer: A prospective study of patients with extended pelvic lymphadenectomy. Ann. Surg. Oncol. 2015, 22, 3150–3156. [Google Scholar] [CrossRef]
- Golan, S.; Sopov, V.; Baniel, J.; Groshar, D. Comparison of 11C-choline with 18F-FDG in positron emission tomography/computerized tomography for staging urothelial carcinoma: A prospective study. J. Urol. 2011, 186, 436–441. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Friedman, K.P.; Ponzo, F.; Raad, R.A.; Jackson, K.; Huang, W.C.; Balar, A.V. Prospective Pilot Study to Evaluate the Incremental Value of PET Information in Patients with Bladder Cancer Undergoing 18F-FDG Simultaneous PET/MRI. Clin. Nucl. Med. 2017, 42, e8–e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaman, O.; Baltaci, S.; Arikan, N.; Yilmaz, E.; Gogus, O. Staging with computed tomography, transrectal ultrasonography and transurethral resection of bladder tumour: Comparison with final pathological stage in invasive bladder carcinoma. Br. J. Urol. 1996, 78, 197–200. [Google Scholar] [CrossRef]
- Datta, S.N.; Allen, G.M.; Evans, R.; Vaughton, K.C.; Lucas, M.G. Urinary tract ultrasonography in the evaluation of haematuria—A report of over 1,000 cases. Ann. R. Coll. Surg. Engl. 2002, 84, 203–205. [Google Scholar] [PubMed]
- Caruso, G.; Salvaggio, G.; Campisi, A.; Melloni, D.; Midiri, M.; Bertolotto, M.; Lagalla, R. Bladder tumor staging: Comparison of contrast-enhanced and gray-scale ultrasound. AJR Am. J. Roentgenol. 2010, 194, 151–156. [Google Scholar] [CrossRef]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R.J. Intravenous urography: Technique and interpretation. Radiographics 2001, 21, 799–821; discussion 822–824. [Google Scholar] [CrossRef]
- Jinzaki, M.; Matsumoto, K.; Kikuchi, E.; Sato, K.; Horiguchi, Y.; Nishiwaki, Y.; Silverman, S.G. Comparison of CT urography and excretory urography in the detection and localization of urothelial carcinoma of the upper urinary tract. AJR Am. J. Roentgenol. 2011, 196, 1102–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.L.; El-Gabry, E.A.; Bagley, D.H. Surveillance of Upper Urinary Tract Transitional Cell Carcinoma: The Role of Ureteroscopy, Retrograde Pyelography, Cytology and Urinalysis. J. Urol. 2000, 164, 1901–1904. [Google Scholar] [CrossRef]
- Aldousari, S.; Kassouf, W. Update on the management of non-muscle invasive bladder cancer. Can. Urol. Assoc. J. 2010, 4, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.-U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Hexaminolevulinate. Available online: https://www.drugs.com/ppa/hexaminolevulinate.html (accessed on 19 February 2021).
- Daneshmand, S.; Bazargani, S.T.; Bivalacqua, T.J.; Holzbeierlein, J.M.; Willard, B.; Taylor, J.M.; Liao, J.C.; Pohar, K.; Tierney, J.; Konety, B.; et al. Blue light cystoscopy for the diagnosis of bladder cancer: Results from the US prospective multicenter registry. Urol. Oncol. 2018, 36, 361.e1–361.e6. [Google Scholar] [CrossRef] [PubMed]
- Cauberg, E.C.; Kloen, S.; Visser, M.; de la Rosette, J.J.; Babjuk, M.; Soukup, V.; Pesl, M.; Duskova, J.; de Reijke, T.M. Narrow band imaging cystoscopy improves the detection of non-muscle-invasive bladder cancer. Urology 2010, 76, 658–663. [Google Scholar] [CrossRef]
- Mukherjee, P.; George, A.J.P.; Yadav, B.K.; Jeyaseelan, L.; Kumar, R.M.; Mukha, R.P.; Chandrasingh, J.; Kumar, S.; Kekre, N.S.; Devasia, A. The Impact of Narrow Band Imaging in the Detection and Resection of Bladder Tumor in Transitional Cell Carcinoma of the Bladder: A Prospective, Blinded, Sequential Intervention Randomized Controlled Trial. Urology 2019, 128, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Cho, K.S.; Kang, D.H.; Jung, H.D.; Kwon, J.K.; Oh, C.K.; Ham, W.S.; Choi, Y.D. A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection for non-muscle invasive bladder cancer: 5-Aminolaevulinic acid fluorescence vs hexylaminolevulinate fluorescence vs narrow band imaging. BMC Cancer 2015, 15, 566. [Google Scholar] [CrossRef] [Green Version]
- Mowatt, G.; N’Dow, J.; Vale, L.; Nabi, G.; Boachie, C.; Cook, J.A.; Fraser, C.; Griffiths, T.R.L. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: Systematic review and meta-analysis. Int. J. Technol. Assess. Health Care 2011, 27, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Cumberbatch, M.G.K.; Foerster, B.; Catto, J.W.F.; Kamat, A.M.; Kassouf, W.; Jubber, I.; Shariat, S.F.; Sylvester, R.J.; Gontero, P. Repeat Transurethral Resection in Non-Muscle-Invasive Bladder Cancer: A Systematic Review. Eur. Urol. 2018, 73, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W. Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to bacillus Calmette-Guerin therapy. J. Urol. 2005, 174, 2134–2137. [Google Scholar] [CrossRef]
- Kamat, A.M.; Sylvester, R.J.; Bohle, A.; Palou, J.; Lamm, D.L.; Brausi, M.; Soloway, M.; Persad, R.; Buckley, R.; Colombel, M.; et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations from the International Bladder Cancer Group. J. Clin. Oncol. 2016, 34, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Tully, K.H.; Cole, A.P.; Krimphove, M.J.; Friedlander, D.F.; Mossanen, M.; Herzog, P.; Noldus, J.; Sonpavde, G.P.; Trinh, Q.D. Contemporary Treatment Patterns for Non-Muscle-Invasive Bladder Cancer: Has the Use of Radical Cystectomy Changed in the BCG Shortage Era? Urology 2021, 147, 199–204. [Google Scholar] [CrossRef]
- Gontero, P.; Sylvester, R.; Pisano, F.; Joniau, S.; Vander Eeckt, K.; Serretta, V.; Larre, S.; Di Stasi, S.; Van Rhijn, B.; Witjes, A.J.; et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: Results of a retrospective multicenter study of 2451 patients. Eur. Urol. 2015, 67, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Doyle, W.; Leow, J.J.; Orsola, A.; Chang, S.L.; Bellmunt, J. Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: A meta-analysis of 15,215 patients. J. Clin. Oncol. 2015, 33, 643–650. [Google Scholar] [CrossRef]
- Raphael, M.J.; Booth, C.M. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: Underused across the 49th parallel. Can. Urol. Assoc. J. 2019, 13, 29–31. [Google Scholar] [CrossRef]
- Culine, S.; Gravis, G.; Flechon, A.; Soulie, M.; Guy, L.; Laguerre, B.; Mottet, N.; Joly, F.; Allory, Y.; Harter, V.; et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (dd-MVAC) or gemcitabine and cisplatin (GC) as perioperative chemotherapy for muscle invasive urothelial bladder cancer (MIUBC): Preliminary results of the GETUG/AFU V05 VESPER trial on toxicity and pathological responses. J. Clin. Oncol. 2020, 38, 437. [Google Scholar] [CrossRef]
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- Bandini, M.; Gibb, E.A.; Gallina, A.; Raggi, D.; Marandino, L.; Bianchi, M.; Ross, J.S.; Colecchia, M.; Gandaglia, G.; Fossati, N.; et al. Does the administration of preoperative pembrolizumab lead to sustained remission post-cystectomy? First survival outcomes from the PURE-01 study. Ann. Oncol. 2020, 31, 1755–1763. [Google Scholar] [CrossRef]
- Mills, R.D.; Turner, W.H.; Fleischmann, A.; Markwalder, R.; Thalmann, G.N.; Studer, U.E. Pelvic lymph node metastases from bladder cancer: Outcome in 83 patients after radical cystectomy and pelvic lymphadenectomy. J. Urol. 2001, 166, 19–23. [Google Scholar] [CrossRef]
- Bellmunt, J.; Orsola, A.; Leow, J.J.; Wiegel, T.; De Santis, M.; Horwich, A.; Group, E.G.W. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii40–iii48. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T. Lymph node dissection for bladder cancer: Current standards and the latest evidence. Int. J. Urol. 2021, 28, 7–15. [Google Scholar] [CrossRef]
- Gschwend, J.E.; Heck, M.M.; Lehmann, J.; Rubben, H.; Albers, P.; Wolff, J.M.; Frohneberg, D.; de Geeter, P.; Heidenreich, A.; Kalble, T.; et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur. Urol. 2019, 75, 604–611. [Google Scholar] [CrossRef]
- Lerner, S.P.; Svatek, R.S. What is the Standard of Care for Pelvic Lymphadenectomy Performed at the Time of Radical Cystectomy? Eur. Urol. 2019, 75, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Ching, J.B.; Werntz, R.P.; Zietman, A.L.; Steinberg, G.D. Multidisciplinary Management of Muscle-Invasive Bladder Cancer: Current Challenges and Future Directions. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, J.A.; Spiegel, D.Y.; Shipley, W.U.; Heney, N.M.; Kaufman, D.S.; Niemierko, A.; Coen, J.J.; Skowronski, R.Y.; Paly, J.J.; McGovern, F.J.; et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur. Urol. 2012, 61, 705–711. [Google Scholar] [CrossRef]
- Mak, R.H.; Hunt, D.; Shipley, W.U.; Efstathiou, J.A.; Tester, W.J.; Hagan, M.P.; Kaufman, D.S.; Heney, N.M.; Zietman, A.L. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 2014, 32, 3801–3809. [Google Scholar] [CrossRef]
- Rodel, C.; Grabenbauer, G.G.; Kuhn, R.; Papadopoulos, T.; Dunst, J.; Meyer, M.; Schrott, K.M.; Sauer, R. Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J. Clin. Oncol. 2002, 20, 3061–3071. [Google Scholar] [CrossRef]
- James, N.D.; Hussain, S.A.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 2012, 366, 1477–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafeez, S.; Webster, A.; Hansen, V.N.; McNair, H.A.; Warren-Oseni, K.; Patel, E.; Choudhury, A.; Creswell, J.; Foroudi, F.; Henry, A.; et al. Protocol for tumour-focused dose-escalated adaptive radiotherapy for the radical treatment of bladder cancer in a multicentre phase II randomised controlled trial (RAIDER): Radiotherapy planning and delivery guidance. BMJ Open 2020, 10, e041005. [Google Scholar] [CrossRef]
- Wilhite, T.J.; Routman, D.; Arnett, A.L.H.; Glasgow, A.E.; Habermann, E.B.; Boorjian, S.A.; Jethwa, K.R.; Pisansky, T.M.; Mynderse, L.A.; Roberts, K.; et al. Increased Utilization of External Beam Radiation Therapy Relative to Cystectomy for Localized, Muscle-Invasive Bladder Cancer: A SEER Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E274–E275. [Google Scholar] [CrossRef]
- Delaney, G.; Jacob, S.; Barton, M. Estimating the optimal external-beam radiotherapy utilization rate for genitourinary malignancies. Cancer 2005, 103, 462–473. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Skoneczna, I.; Kerst, J.M.; Albers, P.; Fossa, S.D.; Agerbaek, M.; Dumez, H.; de Santis, M.; Théodore, C.; Leahy, M.G.; et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3–pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): An intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015, 16, 76–86. [Google Scholar] [CrossRef]
- Bidnur, S.; Savdie, R.; Black, P.C. Inhibiting Immune Checkpoints for the Treatment of Bladder Cancer. Bladder Cancer 2016, 2, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Ghatalia, P.; Zibelman, M.; Geynisman, D.M.; Plimack, E. Approved checkpoint inhibitors in bladder cancer: Which drug should be used when? Ther. Adv. Med. Oncol. 2018, 10, 1758835918788310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullen, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients with Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2020, 38, 1041–1049. [Google Scholar] [CrossRef]
- Bochner, B.H.; Montie, J.E.; Lee, C.T. Follow-up strategies and management of recurrence in urologic oncology bladder cancer. Urol. Clin. N. Am. 2003, 30, 777–789. [Google Scholar] [CrossRef]
- Expert Panel on Urologic Imaging; Allen, B.C.; Oto, A.; Akin, O.; Alexander, L.F.; Chong, J.; Froemming, A.T.; Fulgham, P.F.; Lloyd, S.; Maranchie, J.K.; et al. ACR Appropriateness Criteria® Post-Treatment Surveillance of Bladder Cancer. J. Am. Coll. Radiol. 2019, 16, S417–S427. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, I.A.; Keren Paz, G.E.; Chen, L.Y.; Herr, H.W.; Donat, S.M.; Bochner, B.H.; Dalbagni, G. Upper tract imaging surveillance is not effective in diagnosing upper tract recurrence in patients followed for nonmuscle invasive bladder cancer. J. Urol. 2013, 190, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
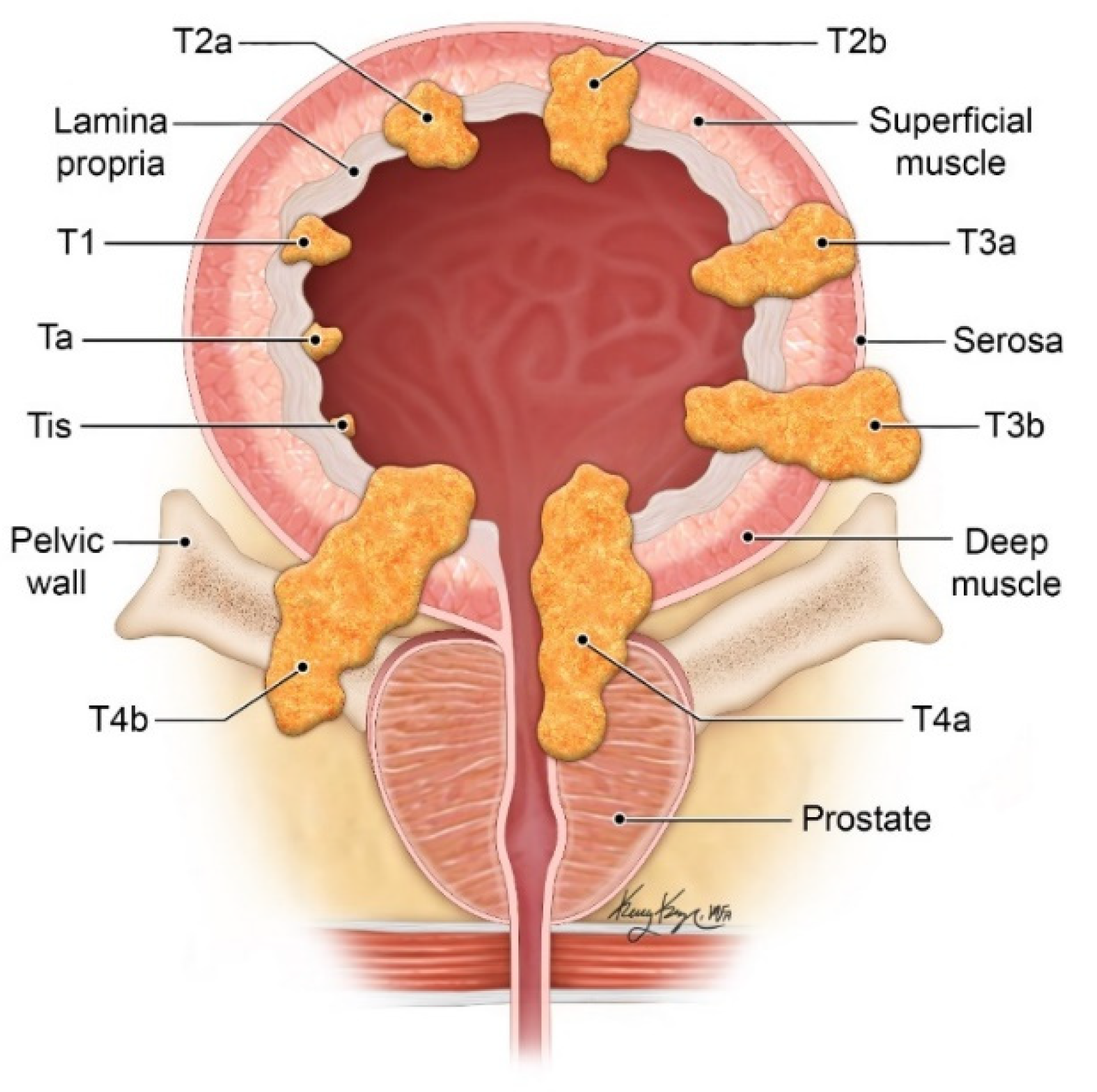









| T Stage | Description |
|---|---|
| Tx | Primary tumor unable to be evaluated |
| T0 | No evidence of primary tumor |
| Ta | Noninvasive papillary carcinoma |
| Tis | Carcinoma in situ |
| T1 | Tumor invades lamina propria but does not involve bladder muscle |
| T2 | Tumor invades bladder muscle |
| T2a | Tumor invades superficial muscle (inner half) |
| T2b | Tumor invades deep muscle (outer half) |
| T3 | Tumor invades perivesical tissue |
| T3a | Microscopic perivesical invasion |
| T3b | Macroscopic perivesical invasion |
| T4 | Tumor invades adjacent organs |
| T4a | Tumor invades prostate, seminal vesicles, uterus, or vagina |
| T4b | Tumor invades pelvic wall or abdominal wall |
| N stage | |
| Nx | Regional lymph nodes cannot be evaluated |
| N0 | Single regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral) |
| N1 | 2+ regional lymph node metastases in the true pelvis |
| N3 | Lymph node metastasis to common iliac lymph nodes |
| M stage | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Non-regional lymph node metastasis |
| M1b | Non-lymph node distant metastasis |
| Score | Description |
|---|---|
| T2WI Score Structural category (SC) | Muscularis propria is T2 hypointense Tumor is T2 intermediate in signal |
| SC 1 | Uninterrupted low signal intensity (SI) line representing the muscularis propria. Lesion < 1 cm Exophytic tumor
|
| SC 2 | Uninterrupted low signal intensity (SI) line representing the muscularis propria Lesion > 1 cm Exophytic tumor with stalk +/− high SI thickened inner layer, when present. Sessile/broad-based tumor with high SI thickened inner layer, when present |
| SC 3 | Lack of category 2 findings with no clear disruption of low SI muscularis propria. Associated presence of
|
| SC 4 | Intermediate SI tumor interrupts low SI line (muscularis propria) |
| SC 5 | Intermediate SI tumor extends into extravesical fat |
| DCE Score Contrast-enhanced category (CE) | Tumor and inner layer enhance early Muscle should not enhance early |
| CE 1 | No early enhancement of muscularis propria |
| CE 2 | No early enhancement of muscularis propria with early enhancement of inner layer |
| CE 3 | Lack of category 2 findings with no clear disruption of muscularis propria |
| CE 4 | Early enhancing tumor extends into muscularis propria |
| CE 5 | Early enhancing tumor extends to entire bladder wall and to extravesical fat |
| DWI/ADC score Diffusion weighted category (DW) | Tumor is hyperintense on DWI, hypointense on ADC Muscularis propria is intermediate SI on DWI Stalk and inner layer are low SI on DWI |
| DW 1 | Intact intermediate SI muscularis propria on DWI Lesion < 1 cm |
| DW 2 | Intact intermediate SI muscularis propria on DWI Lesion > 1 cm |
| DW 3 | Lack of category 2 findings with no clear disruption of muscularis propria |
| DW 4 | Tumor (high SI on DWI/low SI on ADC) extends into muscularis propria |
| DW 5 | Tumor (high SI on DWI/low SI on ADC) extends to entire bladder wall and extravesical fat |
| Final Score | T2WI helpful especially for VI-RADS 1-3 DWI and DCE are dominant sequences for risk estimate, especially for VI-RADS 4-5 |
| VI-RADS 1 | Muscle invasion highly unlikely SC 1, CE 1, and DW 1 |
| VI-RADS 2 | Muscle invasion unlikely SC 2 + CE 2 and DW 2 SC 3 + CE 2 and DW 2 |
| VI-RADS 3 | Muscle invasion equivocal SC 3 + CE 3 and/or DW 3 (or below) |
| VI-RADS 4 | Muscle invasion likely SC 4 + CE 4 and/or DW 4 (or below) SC 5 + CE 4 and/or DW 4 (or below) |
| VI-RADS 5 | SC 4 + CE 5 and/or DW 5 SC 5 + CE 5 and/or DW 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, V.K.; Ganeshan, D.; Jensen, C.T.; Devine, C.E. Imaging and Management of Bladder Cancer. Cancers 2021, 13, 1396. https://doi.org/10.3390/cancers13061396
Wong VK, Ganeshan D, Jensen CT, Devine CE. Imaging and Management of Bladder Cancer. Cancers. 2021; 13(6):1396. https://doi.org/10.3390/cancers13061396
Chicago/Turabian StyleWong, Vincenzo K., Dhakshinamoorthy Ganeshan, Corey T. Jensen, and Catherine E. Devine. 2021. "Imaging and Management of Bladder Cancer" Cancers 13, no. 6: 1396. https://doi.org/10.3390/cancers13061396






