Safety and Treatment Outcomes of Nivolumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Retrospective Multicenter Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Tumor Characteristics
2.2. Treatment Characteristics and Survival Data
2.3. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Treatment Characteristics
- (1)
- CDF: Nivolumab following recurrence or progression after neo-adjuvant chemotherapy (n = 1); within 6 months of completing primary/adjuvant concurrent chemoradiotherapy with platinum-based chemotherapy (n = 32); within 6 months of receiving palliative chemotherapy with platinum-based chemotherapy (n = 86).
- (2)
- Four patients did not fit the criteria above (n = 2, co-payment: one before CDF approval and the other had nasopharynx cancer; 1 compassionate access; 1 received nivolumab having progressed more than 6 months after platinum chemotherapy).
3.3. Efficacy
3.4. PD-L1 Express PD-L1 Expression Status and Response to Treatment
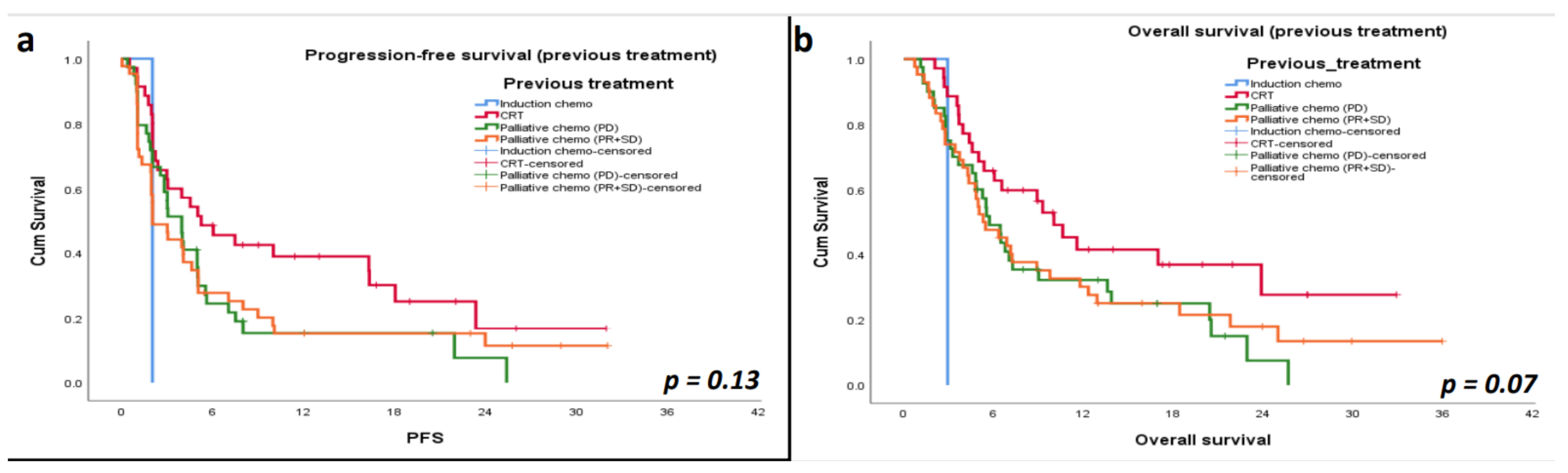
3.5. Progression-Free Survival (PFS)
3.6. Overall Survival (OS)
3.7. Reasons for Stopping Treatment
3.8. Safety and Immune-Related Toxicity (IRT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- International Agency for Research on Cancer. Global Cancer Observatory. Available online: https://gco.iarc.fr (accessed on 9 October 2020).
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients With Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Vermorken, J.B.; Specenier, P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann. Oncol. 2010, 21, vii252–vii261. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Cancer: Changing Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef]
- Pisani, P.; Airoldi, M.; Allais, A.; Aluffi Valletti, P.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic disease in head and neck oncology. Acta Otorhinolaryngol. Ital. 2020, 40, S1–S86. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- National Institue for Health and Care Excellence. Nivolumab for Treating Squamous Cell Carcinoma of the Head and Neck After Platinum-Based Chemotherapy. Available online: https://www.nice.org.uk/guidance/TA490 (accessed on 24 November 2020).
- National Institute for Health and Care Excellence. Cancer Drugs Fund. Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/cancer-drugs-fund (accessed on 24 November 2020).
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Okamoto, I.; Sato, H.; Kondo, T.; Koyama, N.; Fushimi, C.; Okada, T.; Miura, K.; Matsuki, T.; Yamashita, T.; Omura, G.; et al. Efficacy and safety of nivolumab in 100 patients with recurrent or metastatic head and neck cancer—A retrospective multicentre study. Acta Otolaryngol. 2019, 139, 918–925. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Matsuo, M.; Yasumatsu, R.; Masuda, M.; Toh, S.; Wakasaki, T.; Hashimoto, K.; Taura, M.; Uchi, R.; Nakagawa, T. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. 2020, 101, 104525. [Google Scholar] [CrossRef]
- Hori, R.; Shinohara, S.; Kojima, T.; Kagoshima, H.; Kitamura, M.; Tateya, I.; Tamaki, H.; Kumabe, Y.; Asato, R.; Harada, H.; et al. Real-World Outcomes and Prognostic Factors in Patients Receiving Nivolumab Therapy for Recurrent or Metastatic Head and Neck Carcinoma. Cancers 2019, 11, 1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skovlund, E.; Leufkens, H.G.M.; Smyth, J.F. The use of real-world data in cancer drug development. Eur. J. Cancer 2018, 101, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Cancer Therapy Evaluation Program. Available online: https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm (accessed on 12 January 2021).
- Gillison, M.L.; Blumenschein, G.R.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab (Nivo) vs investigator’s choice (IC) for platinum-refractory (PR) recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN.; Checkmate 141): Outcomes in first-line (1L) R/m patients and updated safety and efficacy. J. Clin. Oncol. 2017, 35, 6019. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Rischin, D.; Greil, R.; Soulieres, D.; Tahara, M.; Castro, G.; Psyrri, A.; Baste, N.; Neupane, P.C.; Bratland, Å.; et al. KEYNOTE-048: Progression after the next line of therapy following pembrolizumab (P) or P plus chemotherapy (P+C) vs EXTREME (E) as first-line (1L) therapy for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2020, 38, 6505. [Google Scholar] [CrossRef]
- Uppaluri, R.; Lee, N.Y.; Westra, W.; Cohen, E.E.W.; Haddad, R.I.; Temam, S.; Le Tourneau, C.; Chernock, R.; Safina, S.; Klochikhin, A.; et al. KEYNOTE-689: Phase 3 study of adjuvant and neoadjuvant pembrolizumab combined with standard of care (SOC) in patients with resectable, locally advanced head and neck squamous cell carcinoma. J. Clin. Oncol. 2019, 37, TPS6090. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [Green Version]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]


| Characteristics | Nivolumab Patients (n = 123) |
|---|---|
| Age (years) | |
| Median (range) | 64 (22–94) |
| Male sex (%) | 99 (80.5%) |
| Smoking status (%) | |
| Never | 23 (18.7%) |
| Ex-smoker | 48 (39.0%) |
| Current smoker | 38 (30.9%) |
| Not recorded | 14 (11.4%) |
| Site of primary tumor (%) | |
| Oral cavity | 33 (26.8%) |
| Nasopharynx | 1 (0.8%) |
| Oropharynx | 43 (35.0%) |
| Hypopharynx | 8 (6.5%) |
| Larynx | 25 (20.3%) |
| Paranasal sinuses | 5 (4.1%) |
| Unknown primary | 8 (6.5%) |
| Staging TNM7 at time of diagnosis | |
| 1 | 2 (1.6%) |
| 2 | 5 (4.1%) |
| 3 | 17 (13.8%) |
| 4 | |
| 4a | 75 (61.0%) |
| 4b | 7 (5.7%) |
| 4c | 15 (12.2%) |
| Not available | 2 (1.6%) |
| PD-L1 status (%) | |
| Negative | 17 (13.8%) |
| ≥1% | 12 (9.8%) |
| Not tested | 94 (76.4%) |
| Characteristics | Nivolumab Patients (n = 123) |
|---|---|
| Intent of treatment at diagnosis (%) | |
| Curative | 95 (77.2%) |
| Palliative | 28 (22.8%) |
| Primary treatment (%) | |
| Surgery +/− adjuvant (C)RT (including IC) | 38 (30.9%) |
| (C)RT +/− IC | 59 (48.0%) |
| Palliative chemotherapy | 21 (17.1%) |
| Palliative radiotherapy | 5 (4.0%) |
| Characteristics | Nivolumab Patients (n = 119) |
|---|---|
| Best response to treatment (%) | |
| PD | 75 (63.0%) |
| CR/PR | 23 (19.3%) |
| SD | 19 (16.0%) |
| Ν/A | 2 (1.7%) |
| Reason for stopping treatment (%) | |
| PD | 74 (62.2%) |
| Death | 10 (8.4%) |
| Toxicity | 8 (6.7%) |
| Ongoing treatment | 6 (5.0%) |
| Not fit for treatment | 11 (9.3%) |
| Completion of treatment (24 months) | 3 (2.5%) |
| Patient choice | 1 (0.9%) |
| n/a | 6 (5.0%) |
| IO-Related Toxicities | Total = 18 |
|---|---|
| Pneumonitis | n = 3; Grade 3 (1), suspected pneumonitis (2) |
| Hepatitis | n = 3; Grade 4 (1), grade 3 (1), grade 2 (1) |
| Colitis | n = 3; Grade 3 (2), unknown grading (1) |
| Endocrine toxicities | n = 6 (hypothyroidism, hypophysitis) |
| Other | n = 3; lichen planus (1; grade 2), hyponatremia (1; grade 4), thrombocytopenia (1; grade 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliadou, I.; Breik, O.; Baker, H.; Leslie, I.; Sim, V.R.; Hegarty, G.; Michaelidou, A.; Nathan, K.; Hartley, A.; Good, J.; et al. Safety and Treatment Outcomes of Nivolumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Retrospective Multicenter Cohort Study. Cancers 2021, 13, 1413. https://doi.org/10.3390/cancers13061413
Vasiliadou I, Breik O, Baker H, Leslie I, Sim VR, Hegarty G, Michaelidou A, Nathan K, Hartley A, Good J, et al. Safety and Treatment Outcomes of Nivolumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Retrospective Multicenter Cohort Study. Cancers. 2021; 13(6):1413. https://doi.org/10.3390/cancers13061413
Chicago/Turabian StyleVasiliadou, Ifigenia, Omar Breik, Holly Baker, Isla Leslie, Van Ren Sim, Gemma Hegarty, Andriana Michaelidou, Kannon Nathan, Andrew Hartley, James Good, and et al. 2021. "Safety and Treatment Outcomes of Nivolumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Retrospective Multicenter Cohort Study" Cancers 13, no. 6: 1413. https://doi.org/10.3390/cancers13061413






