Triggers for Palliative Care Referral in Pediatric Oncology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demographics
3.2. PC Consultation Findings
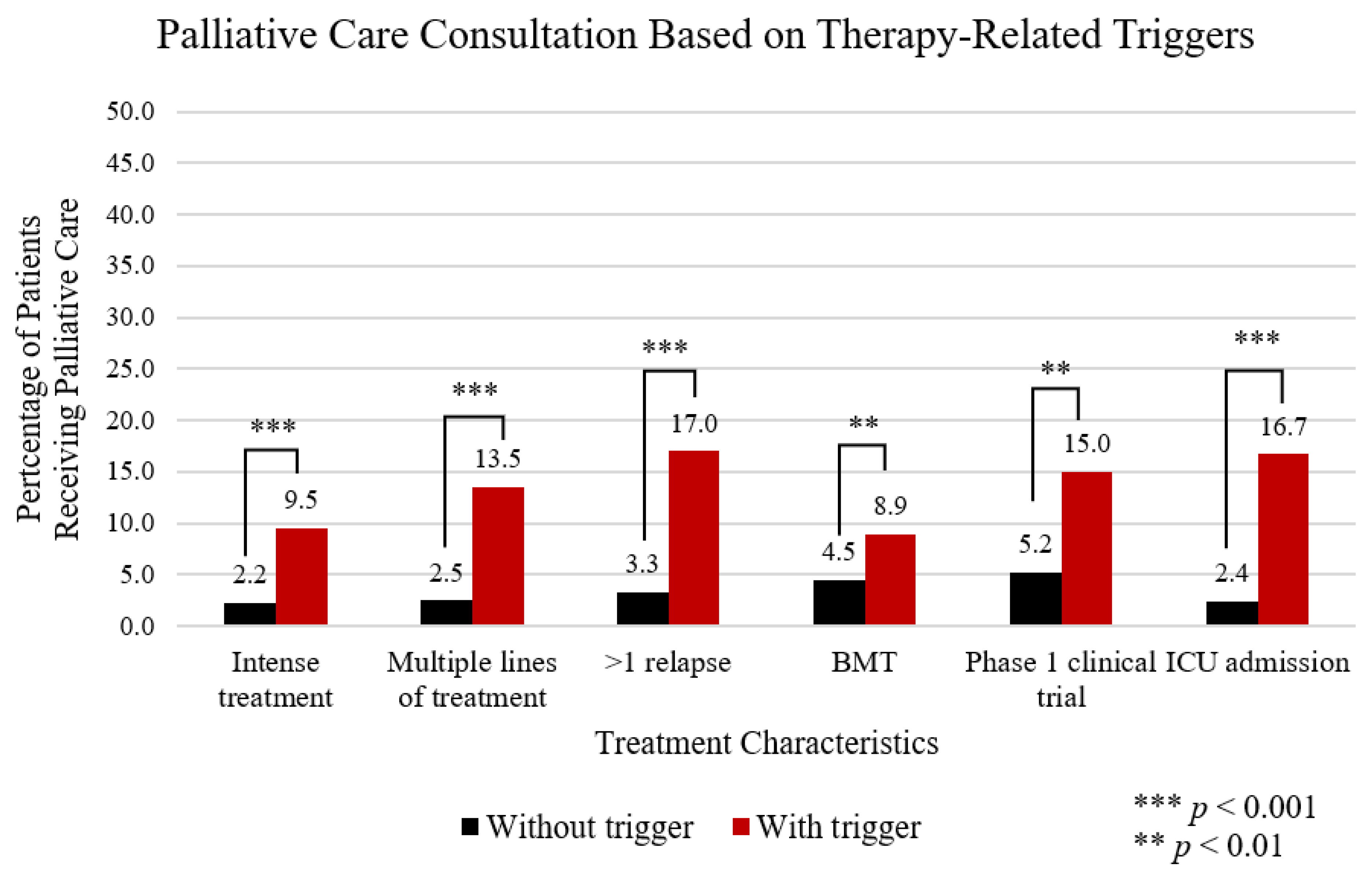
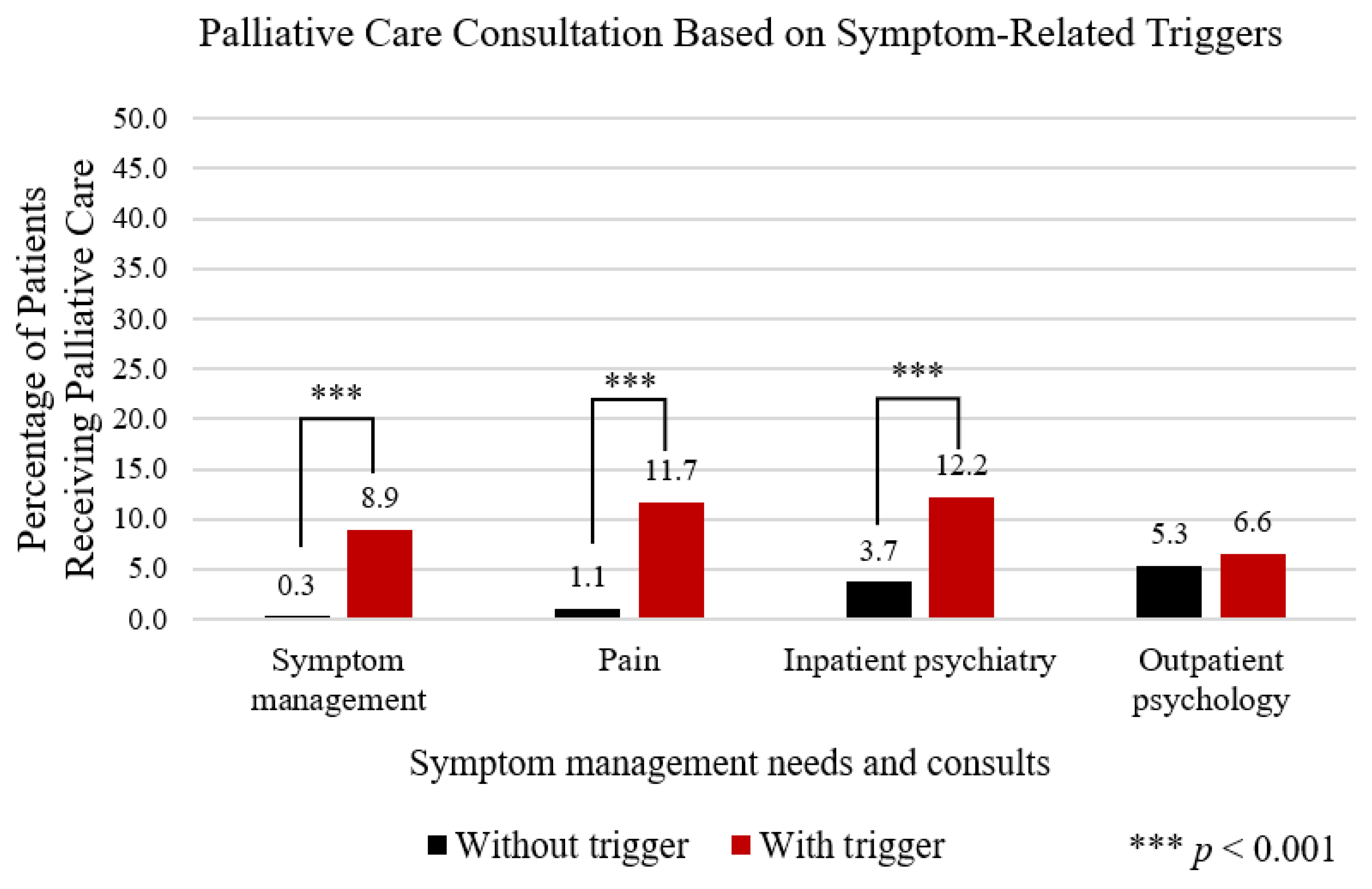
3.3. Overall Trigger Associated PC Consultation Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhukovsky, D.S.; Herzog, C.E.; Kaur, G.; Palmer, J.L.; Bruera, E. The impact of palliative care consultation on symptom assessment, communication needs, and palliative interventions in pediatric patients with cancer. J. Palliat. Med. 2009, 12, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.R.; Mandrell, B.N.; Sykes, A.; Pritchard, M.; Gibson, D.; Symons, H.J.; Wendler, D.; Baker, J.N. Patients’ and Parents’ Needs, Attitudes, and Perceptions About Early Palliative Care Integration in Pediatric Oncology. JAMA Oncol. 2017, 3, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.; Hammel, J.F.; Edwards, K.E.; Duncan, J.; Comeau, M.; Breyer, J.; Aldridge, S.A.; Grier, H.E.; Berde, C.; Dussel, V.; et al. Easing of suffering in children with cancer at the end of life: Is care changing? J. Clin. Oncol. 2008, 26, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Vern-Gross, T.Z.; Lam, C.G.; Graff, Z.; Singhal, S.; Levine, D.R.; Gibson, D.; Sykes, A.; Anghelescu, D.L.; Yuan, Y.; Baker, J.N. Patterns of End-of-Life Care in Children With Advanced Solid Tumor Malignancies Enrolled on a Palliative Care Service. J. Pain Symptom Manag. 2015, 50, 305–312. [Google Scholar] [CrossRef]
- Cuviello, A.; Raisanen, J.C.; Donohue, P.K.; Wiener, L.; Boss, R.D. Defining the Boundaries of Palliative Care in Pediatric Oncology. J. Pain Symptom Manag. 2020, 59, 1033–1042, in press. [Google Scholar] [CrossRef]
- Johnston, D.L.; Vadeboncoeur, C. Palliative care consultation in pediatric oncology. Support Care Cancer 2012, 20, 799–803. [Google Scholar] [CrossRef]
- Snaman, J.M.; Kaye, E.C.; Baker, J.N.; Wolfe, J. Pediatric palliative oncology: The state of the science and art of caring for children with cancer. Curr. Opin. Pediatr. 2018, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.E.; Meier, D.E. Identifying Patients in Need of a Palliative Care Assessment in the Hospital Setting. J. Palliat. Med. 2011, 14, 17–23. [Google Scholar] [CrossRef]
- Cherny, N.I.; Catane, R.; European Society of Medical Oncology Taskforce on Palliative and Supportive Care. Attitudes of medical oncologists toward palliative care for patients with advanced and incurable cancer: Report on a survery by the European Society of Medical Oncology Taskforce on Palliative and Supportive Care. Cancer 2003, 98, 2502–2510. [Google Scholar] [CrossRef]
- Haines, E.; Frost, A.; Kane, H.; Rokoske, F. Barriers to accessing palliative care for pediatric patients with cancer: A review of the literature. Cancer 2018, 124, 2278–2288. [Google Scholar] [CrossRef]
- Knapp, C.; Thompson, L. Factors associated with perceived barriers to pediatric palliative care: A survey of pediatricians in Florida and California. Palliat. Med. 2012, 26, 268–274. [Google Scholar] [CrossRef]
- Rost, M.; De Clercq, E.; Rakic, M.; Wangmo, T.; Elger, B. Barriers to Palliative Care in Pediatric Oncology in Switzerland: A Focus Group Study. J. Pediatr. Oncol. Nurs. 2019, 37, 35–45. [Google Scholar] [CrossRef]
- Wentlandt, K.; Krzyzanowska, M.K.; Swami, N.; Rodin, G.; Le, L.W.; Sung, L.; Zimmermann, C. Referral practices of pediatric oncologists to specialized palliative care. Support Care Cancer 2014, 22, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, B.; Dietrich, J.; Du, Q.; Morrison, R.S. Variability in access to hospital palliative care in the United States. J. Palliat. Med. 2008, 11, 1094–1102. [Google Scholar] [CrossRef]
- Cuviello, A.; Raisanen, J.C.; Donohue, P.K.; Wiener, L.; Boss, R.D. Initiating Palliative Care Referrals in Pediatric Oncology. J. Pain Symptom Manag. 2021, 61, 81–89. [Google Scholar] [CrossRef]
- Kaye, E.C.; Rubenstein, J.; Levine, D.; Baker, J.N.; Dabbs, D.; Friebert, S.E. Pediatric Palliative Care in the Community. Cancer J. Clin. 2015, 65, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Kaye, E.C.; Friebert, S.; Baker, J.N. Early Integration of Palliative Care for Children with High-Risk Cancer and Their Families. Pediatr. Blood Cancer 2016, 63, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.L.; Nagel, K.; Friedman, D.L.; Meza, J.L.; Hurwitz, C.A.; Friebert, S. Availability and use of palliative care and end-of-life services for pediatric oncology patients. J. Clin. Oncol. 2008, 26, 4646–4650. [Google Scholar] [CrossRef]
- Brock, K.E.; Steineck, A.; Twist, C.J. Trends in End-of-Life Care in Pediatric Hematology, Oncology, and Stem Cell Transplant Patients. Pediatr. Blood Cancer 2016, 63, 516–522. [Google Scholar] [CrossRef]
- Weaver, M.S.; Rosenberg, A.R.; Tager, J.; Wichman, C.S.; Wiener, L. A Summary of Pediatric Palliative Care Team Structure and Services as Reported by Centers Caring for Children with Cancer. J. Palliat. Med. 2018, 21, 452–462. [Google Scholar] [CrossRef]
- Thompson, L.; Knapp, C.; Madden, V.; Shenkman, E. Pediatricians’ Perceptions of and Preferred Timing for Pediatric Palliative Care. Pediatrics 2009, 123, 777–782. [Google Scholar] [CrossRef]
- Cheng, B.T.; Rost, M.; De Clercq, E.; Arnold, L.; Elger, B.S.; Wangmo, T. Palliative care initiation in pediatric oncology patients: A systematic review. Cancer Med. 2019, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ananth, P.; Monsereenusorn, C.; Ma, C.; Al-Sayegh, H.; Wolfe, J.; Rodriguez-Galindo, C. Influence of early phase clinical trial enrollment on patterns of end-of-life care for children with advanced cancer. Pediatr. Blood Cancer 2018, 65, e26748. [Google Scholar] [CrossRef] [PubMed]
- Dalberg, T.; Jacob-Files, E.; Carney, P.A.; Meyrowitz, J.; Fromme, E.K.; Thomas, G. Pediatric oncology providers’ perceptions of barriers and facilitators to early integration of pediatric palliative care. Pediatr. Blood Cancer 2013, 60, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Dharmasena, H.P.; Forbes, K. Palliative care for patients with non-malignant disease: Will hospital physicians refer? Palliat. Med. 2001, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Sammer, C.E.; Lykens, K.; Singh, K.P. Physician characteristics and the reported effect of evidence-based practice guidelines. Health Serv. Res. 2008, 43, 569–581. [Google Scholar] [CrossRef]
- Ghoshal, A.; Salins, N.; Damani, A.; Deodhar, J.; Muckaden, M. Specialist pediatric palliative care referral practices in pediatric oncology: A large 5-year retrospective audit. Indian J. Palliat. Care 2016, 22, 266. [Google Scholar] [PubMed]
- Snaman, J.M.; Feraco, A.M.; Wolfe, J.; Baker, J.N. “What if?”: Addressing uncertainty with families. Pediatr. Blood Cancer 2019, 66, e27699. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Chung, V.M.; Koczywas, M.; Williams, A.C.; Hurria, A.; Borneman, T.R.; Cooper, R.; Knight, L.; Fischer, P.; Gallagher, D.; et al. Integration of palliative care for patients with solid tumors on phase I clinical trials. J. Clin. Oncol. 2016, 34, 138. [Google Scholar] [CrossRef]
- Davies, B.; Sehring, S.A.; Partridge, J.C.; Cooper, B.A.; Hughes, A.; Philp, J.C.; Amidi-Nouri, A.; Kramer, R.F. Barriers to palliative care for children: Perceptions of pediatric health care providers. Pediatrics 2008, 121, 282–288. [Google Scholar] [CrossRef]
- Mack, J.W.; Wolfe, J.; Cook, E.F.; Grier, H.E.; Cleary, P.D.; Weeks, J.C. Hope and prognostic disclosure. J. Clin. Oncol. 2007, 25, 5636–5642. [Google Scholar] [CrossRef]
- Wolfe, J.; Klar, N.; Grier, H.E.; Duncan, J.; Salem-Schatz, S.; Emanuel, E.J.; Weeks, J.C. Understanding of prognosis among parents of children who died of cancer. JAMA 2000, 284, 2469–2475. [Google Scholar] [CrossRef]
- Levy, M.; Back, A.; Benedetti, C.; Billings, J.A.; Block, S.; Boston, B.; Bruera, E.; Dy, S.; Eberle, C.; Foley, K.M.; et al. NCCN clinical practice guidelines in oncology: Palliative care. J. Natl. Compr. Cancer Netw. JNCCN 2009, 7, 436. [Google Scholar] [CrossRef]
- Glare, P.A.; Chow, K. Validation of a Simple Screening Tool for Identifying Unmet Palliative Care Needs in Patients with Cancer. J. Oncol. Pract. 2015, 11, e81–e86. [Google Scholar] [CrossRef]
- Back, A.L.; Arnold, R.M.; Baile, W.F.; Tulsky, J.A.; Fryer-Edwards, K.A. Approaching difficult communication tasks in oncology. CA Cancer J. Clin. 2005, 55, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Wiener, L.; Kazak, A.E.; Noll, R.B.; Patenaude, A.F.; Kupst, M.J. Standards for the Psychosocial Care of Children With Cancer and Their Families: An Introduction to the Special Issue. Pediatr. Blood Cancer 2015, 62 (Suppl. S5), S419–S424. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.B. Palliative care in children with cancer: Which child and when? J. Natl. Cancer Inst. Monogr. 2004, 2004, 144–149. [Google Scholar] [CrossRef]
- Mack, J.W.; Wolfe, J. Early integration of pediatric palliative care: For some children, palliative care starts at diagnosis. Curr. Opin. Pediatr. 2006, 18, 10–14. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Section on Hospice and Palliative Medicine and Committee on Hospital Care: Pediatric palliative care and hospice care commitments, guidelines, and recommendations. Pediatrics 2013, 132, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Temin, S.; Alesi, E.R.; Abernethy, A.P.; Balboni, T.A.; Basch, E.M.; Ferrell, B.R.; Loscalzo, M.; Meier, D.E.; Paice, J.A.; et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J. Clin. Oncol. 2012, 30, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.; Marlin, A.; Yoshida, T.; Ullrich, A. Palliative care: The World Health Organization’s global perspective. J. Pain Symptom Manag. 2002, 24, 91–96. [Google Scholar] [CrossRef]
- Committee on Bioethics. Palliative Care for Children. Pediatrics 2000, 106, 351–357. [Google Scholar] [CrossRef]
- Whiting, P.; Wolff, R.; Mallett, S.; Simera, I.; Savovic, J. A proposed framework for developing quality assessment tools. Syst. Rev. 2017, 6, 204. [Google Scholar] [CrossRef]
| General Demographics | Patients without PC Consult (%) n = 879 | Patients with PC Consult (%) n = 52 |
|---|---|---|
| Sex | ||
| Male | 518 (58.9) | 36 (69.2) |
| Female | 361 (41.1) | 16 (30.8) |
| Age | ||
| <18 year | 566 (64.4) | 29 (55.8) |
| ≥18 year | 313 (35.6) | 23 (44.2) |
| Diagnosis Category | ||
| Hematological malignancies | 343 (39.0) | 15 (28.8) |
| Sarcomas | 140 (15.9) | 22 (42.3) |
| CNS tumors | 150 (17.1) | 2 (3.8) |
| Histiocytic disorders | 32 (3.6) | 0 (0) |
| Red cell disorders | 59 (6.7) | 3 (5.8) |
| Immunodeficiencies | 28 (3.2) | 1 (1.9) |
| Neuroendocrine tumors | 127 (14.4) | 9 (17.3) |
| Vital Status | ||
| Alive | 805 (91.6) | 18 (34.6) |
| Deceased | 74 (8.4) | 34 (65.4) |
| High Risk Diagnosis | ||
| Yes | 479 (54.5) | 45 (86.5) |
| No | 400 (45.5) | 7 (13.5) |
| Poor prognosis | ||
| Yes | 231 (26.3) | 43 (82.7) |
| No | 648 (73.7) | 9 (17.3) |
| Comorbidities | ||
| Yes | 525 (59.7) | 30 (57.7) |
| No | 354 (40.3) | 22 (42.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuviello, A.; Yip, C.; Battles, H.; Wiener, L.; Boss, R. Triggers for Palliative Care Referral in Pediatric Oncology. Cancers 2021, 13, 1419. https://doi.org/10.3390/cancers13061419
Cuviello A, Yip C, Battles H, Wiener L, Boss R. Triggers for Palliative Care Referral in Pediatric Oncology. Cancers. 2021; 13(6):1419. https://doi.org/10.3390/cancers13061419
Chicago/Turabian StyleCuviello, Andrea, Catherine Yip, Haven Battles, Lori Wiener, and Renee Boss. 2021. "Triggers for Palliative Care Referral in Pediatric Oncology" Cancers 13, no. 6: 1419. https://doi.org/10.3390/cancers13061419
APA StyleCuviello, A., Yip, C., Battles, H., Wiener, L., & Boss, R. (2021). Triggers for Palliative Care Referral in Pediatric Oncology. Cancers, 13(6), 1419. https://doi.org/10.3390/cancers13061419






