Immune Checkpoint Inhibitors in Urothelial Carcinoma: Recommendations for Practical Approaches to PD-L1 and Other Potential Predictive Biomarker Testing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Current Treatment Landscape for UC
3. The Role of PD-L1 in UC
4. Evaluation of PD-L1 in UC
5. Methodological Considerations
5.1. Pre-Analytics
5.2. Analytics
6. Reporting of Results
7. Emerging Biomarkers in UC
8. Conclusions/Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalasani, V.; Chin, J.L.; Izawa, J.I. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can. Urol. Assoc. J. 2009, 3, S193–S198. [Google Scholar] [CrossRef] [Green Version]
- Audenet, F.; Attalla, K.; Sfakianos, J.P. The evolution of bladder cancer genomics: What have we learned and how can we use it? Urol. Oncol. 2018, 36, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, P.; Hamilou, Z.; Loriot, Y.; Massard, C. Durvalumab in urothelial cancers. Expert Rev. Anticancer Ther. 2018, 18, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Henriques, V.; Montironi, R.; Cimadamore, A.; Raspollini, M.R.; Cheng, L. Variants and new entities of bladder cancer. Histopathology 2019, 74, 77–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghate, K.; Amir, E.; Kuksis, M.; Hernandez-Barajas, D.; Rodriguez-Romo, L.; Booth, C.M.; Vera-Badillo, F.E. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: A meta-analysis. Cancer Treat. Rev. 2019, 76, 51–56. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija JÁ, A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Powles, T.; Walker, J.; Andrew Williams, J.; Bellmunt, J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat. Rev. 2020, 82, 101925. [Google Scholar] [CrossRef] [Green Version]
- Farina, M.S.; Lundgren, K.T.; Bellmunt, J. Immunotherapy in urothelial cancer: Recent results and future perspectives. Drugs 2017, 77, 1077–1089. [Google Scholar] [CrossRef]
- Veeratterapillay, R.; Heer, R.; Johnson, M.I.; Persad, R.; Bach, C. High-risk non-muscle-invasive bladder cancer-therapy options during intravesical BCG shortage. Curr. Urol. Rep. 2016, 17, 68. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, C.N.; Donat, S.M.; Bellmunt, J.; Millikan, R.E.; Stadler, W.; De Mulder, P.; Sherif, A.; von der Maase, H.; Tsukamoto, T.; Soloway, M.S. Chemotherapy for bladder cancer: Treatment guidelines for neoadjuvant chemotherapy, bladder preservation, adjuvant chemotherapy, and metastatic cancer. Urology 2007, 69, 62–79. [Google Scholar] [CrossRef]
- Bukhari, N.; Al-Shamsi, H.O.; Azam, F. Update on the treatment of metastatic urothelial carcinoma. Sci. World J. 2018, 2018, 5682078. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Strissel, P.; Strick, R.; Weyerer, V.; Wirtz, R.; Pfannstiel, C.; Wullweber, A.; Lange, F.; Erben, P.; Stoehr, R.; et al. Cytotoxic T-cell-related gene expression signature predicts improved survival in muscle-invasive urothelial bladder cancer patients after radical cystectomy and adjuvant chemotherapy. J. Immunother. Cancer 2020, 8, e000162. [Google Scholar] [CrossRef] [PubMed]
- Pfannstiel, C.; Strissel, P.L.; Chiappinelli, K.B.; Sikic, D.; Wach, S.; Wirtz, R.M.; Wullweber, A.; Taubert, H.; Breyer, J.; Otto, W.; et al. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol. Res. 2019, 7, 923–938. [Google Scholar] [CrossRef]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjödahl, G.; Lövgren, K.; Lauss, M.; Chebil, G.; Patschan, O.; Gudjonsson, S.; Månsson, W.; Fernö, M.; Leandersson, K.; Lindgren, D.; et al. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol. Oncol. 2014, 32, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Jóźwicki, W.; Brożyna, A.A.; Siekiera, J.; Slominski, A.T. Changes in immunogenicity during the development of urinary bladder cancer: A preliminary study. Int. J. Mol. Sci. 2016, 17, 285. [Google Scholar] [CrossRef] [Green Version]
- TECENTRIQ® (Atezolizumab); [Prescribing Information]; Genentech, Inc., A Member of the Roche Group: San Francisco, CA, USA, 2019.
- EMD Serono, Inc.; Pfizer Inc. BAVENCIO® (Avelumab); [Prescribing, Information]; EMD Serono, Inc.: Rockland, MA, USA; Pfizer Inc.: New York, NY, USA, 2019. [Google Scholar]
- AstraZeneca. IMFINZI™ (Durvalumab); [Prescribing Information]; AstraZeneca: Wilmington, DE, USA, 2017. [Google Scholar]
- Merck & Co, Inc. KEYTRUDA® (Pembrolizumab); [Prescribing Information]; Merck & Co, Inc.: Whitehouse Station, NJ, USA, 2019. [Google Scholar]
- Bristol-Myers Squibb. OPDIVO (Nivolumab); [Prescribing Information]; Bristol-Myers Squibb: Princeton, NJ, USA, 2020. [Google Scholar]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullén, A.; et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. J. Clin. Oncol. 2020, 38 (Suppl. 18). [Google Scholar] [CrossRef]
- Hussain, M.H.A.; Powles, T.; Albers, P.; Castellano, D.; Daneshmand, S.; Gschwend, J.; Nishiyama, H.; Oudard, S.; Tayama, D.; Davarpanah, N.N.; et al. IMvigor010: Primary analysis from a phase III randomized study of adjuvant atezolizumab (atezo) versus observation (obs) in high-risk muscle-invasive urothelial carcinoma (MIUC). J. Clin. Oncol. 2020, 38 (Suppl. 15), 5000. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): An open-label, single-arm, phase II study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.S.; Ballas, L.K.; Skinner, E.C.; Dorff, T.B.; Sadeghi, S.; Quinn, D.I. Immunotherapy in urothelial cancer, part 2: Adjuvant, neoadjuvant, and adjunctive treatment. Clin. Adv. Hematol. Oncol. 2017, 15, 543–551. [Google Scholar] [PubMed]
- Gibbons Johnson, R.M.; Dong, H. Functional expression of programmed death-ligand 1 (B7-H1) by immune cells and tumor cells. Front. Immunol. 2017, 8, 961. [Google Scholar] [CrossRef]
- Noguchi, T.; Ward, J.P.; Gubin, M.M.; Arthur, C.D.; Lee, S.H.; Hundal, J.; Selby, M.J.; Graziano, R.F.; Mardis, E.R.; Korman, A.J.; et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol. Res. 2017, 5, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micevic, G.; Thakral, D.; McGeary, M.; Bosenberg, M.W. PD-L1 methylation regulates PD-L1 expression and is associated with melanoma survival. Pigment. Cell Melanoma Res. 2019, 32, 435–440. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, C.; Wang, Y.; Duan, Y.; Liu, C.; Zhang, Y. PD-L1 promoter methylation mediates the resistance response to anti-PD-1 therapy in NSCLC patients with EGFR-TKI resistance. Oncotarget 2017, 8, 101535–101544. [Google Scholar] [CrossRef] [Green Version]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Sheinin, Y.; Crispen, P.L.; Farmer, S.A.; Lohse, C.M.; Kuntz, S.M.; Leibovich, B.C.; Kwon, E.D.; Frank, I. T-cell coregulatory molecule expression in urothelial cell carcinoma: Clinicopathologic correlations and association with survival. Clin. Cancer Res. 2008, 14, 4800–4808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inman, B.A.; Sebo, T.J.; Frigola, X.; Dong, H.; Bergstralh, E.J.; Frank, I.; Fradet, Y.; Lacombe, L.; Kwon, E.D. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: Associations with localized stage progression. Cancer 2007, 109, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, L.M.; Heitplatz, B.; Preuss, S.; Hutchinson, R.C.; Woldu, S.L.; Singla, N.; Boegemann, M.; Wood, C.G.; Karam, J.A.; Weizer, A.Z.; et al. Prognostic value of PD-1 and PD-L1 expression in patients with high grade upper tract urothelial carcinoma. J. Urol. 2017, 198, 1253–1262. [Google Scholar] [CrossRef]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Q.; Zhou, S.; Hu, Z.; Lan, R. Costimulatory molecule B7-H1 on the immune escape of bladder cancer and its clinical significance. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Wirtz, R.M.; Pfannstil, C.; Wach, S.; Stoehr, R.; Breyer, J.; Erlmeier, F.; Gunes, C.; Nitschke, K.; Weichert, W.; et al. A multicenter round robin test of PD-L1 expression assessment in urothelial bladder cancer by immunohistochemistry and RT-qPCR with emphasis on prognosis prediction after radical cystectomy. Oncotarget 2018, 9, 15001–15014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galsky, M.D.; Banchereau, R.; Hamidi, H.R.; Leng, N.; Harris, W.; O’Donnell, P.H.; Kadel, E.E.; Yuen, K.C.Y.; Jin, D.; Koeppen, H.; et al. Tumor, immune, and stromal characteristics associated with clinical outcomes with atezolizumab (atezo) + platinum-based chemotherapy (PBC) or atezo monotherapy (mono) versus PBC in metastatic urothelial cancer (mUC) from the phase III IMvigor130 study. J. Clin. Oncol. 2020, 38 (Suppl. 15), 5011. [Google Scholar] [CrossRef]
- Grande, E.; Galsky, M.; Arranz Arija, J.A.; De Santis, M.; Davis, I.D.; De Giorgi, U.F.F.; Mencinger, M.; Kikuchi, E.; Garcia del Muro, X. IMvigor130: Efficacy and safety from a phase 3 study of atezolizumab (atezo) as monotherapy or combined with platinum-based chemotherapy (PBC) vs. placebo + PBC in previously untreated locally advanced or metastatic urothelial carcinoma (mUC). Ann. Oncol. 2019, 30 (Suppl. 5), v888–v889. [Google Scholar] [CrossRef]
- Vuky, J.; Balar, A.V.; Castellano, D.E.; O’Donnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.F.; Hahn, N.M.; Wit, R.D.; et al. Updated efficacy and safety of KEYNOTE-052: A single-arm phase 2 study investigating first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J. Clin. Oncol. 2018, 36 (Suppl. 15), 4524. [Google Scholar] [CrossRef]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.L.; et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Zajac, M.; Boothman, A.M.; Ben, Y.; Gupta, A.; Jin, X.; Mistry, A.; Sabalos, C.; Nielsen, A.; Manriquez, G.; Barker, C.; et al. Analytical validation and clinical utility of an immunohistochemical programmed death ligand-1 diagnostic assay and combined tumor and immune cell scoring algorithm for durvalumab in urothelial carcinoma. Arch. Pathol. Lab. Med. 2019, 143, 722–731. [Google Scholar] [CrossRef] [Green Version]
- Zajac, M.; Scott, M.; Ratcliffe, M.; Scorer, P.; Barker, C.; Al-Masri, H.; Rebelatto, M.C.; Walker, J. Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma. Diagn. Pathol. 2019, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zajac, M.; Ye, J.; Mukhopadhyay, P.; Jin, X.; Ben, Y.; Antal, J.; Gupta, A.K.; Rebelatto, M.C.; Williams, J.A.; Walker, J. Optimal PD-L1-high cutoff for association with overall survival in patients with urothelial cancer treated with durvalumab monotherapy. PLoS ONE 2020, 15, e0231936. [Google Scholar] [CrossRef] [PubMed]
- Dako Agilent Pathology Solutions. PD-L1 IHC 28-8 pharmDx Interpretation Manual, Urothelial Carcinoma; Publication Part Number: 29188; Dako Agilent Pathology Solutions: Santa Clara, CA, USA, 2017. [Google Scholar]
- Dako Agilent Pathology Solutions. PD-L1 IHC 22C3 pharmDx Interpretation Manual, Urothelial Carcinoma; Publication Part Number: 29271; Dako Agilent Pathology Solutions: Santa Clara, CA, USA, 2019. [Google Scholar]
- Ventana Medical Systems Inc. VENTANA PD-L1 (SP142) Assay Staining in Urothelial Carcinoma Interpretation Guide; Ventana Medical Systems Inc: Tucson, AZ, USA, 2016. [Google Scholar]
- Ventana Medical Systems Inc. VENTANA PD-L1 (SP263) Assay Staining in Urothelial Carcinoma Interpretation Guide; Ventana Medical Systems Inc: Tucson, AZ, USA, 2017. [Google Scholar]
- Bergmann, S.; Coym, A.; Ott, L.; Soave, A.; Rink, M.; Janning, M.; Stoupiec, M.; Coith, C.; Peine, S.; von Amsberg, G.; et al. Evaluation of PD-L1 expression on circulating tumor cells (CTCs) in patients with advanced urothelial carcinoma (UC). Oncoimmunology 2020, 9, 1738798. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef]
- Eckstein, M.; Erben, P.; Kriegmair, M.C.; Worst, T.S.; Weiss, C.A.; Wirtz, R.M.; Wach, S.; Stoehr, R.; Sikic, D.; Geppert, C.I.; et al. Performance of the Food and Drug Administration/EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of atezolizumab and pembrolizumab. Eur. J. Cancer 2019, 106, 234–243. [Google Scholar] [CrossRef]
- Ventana Medical Systems, Inc. VENTANA PD-L1 SP142; [Package Insert]; Ventana Medical Systems, Inc.: Tucson, AZ, USA, 2016. [Google Scholar]
- Ventana Medical Systems, Inc. VENTANA PD-L1 SP263; [Package Insert]; Ventana Medical Systems, Inc.: Tucson, AZ, USA, 2017. [Google Scholar]
- Vennapusa, B.; Baker, B.; Kowanetz, M.; Boone, J.; Menzl, I.; Bruey, J.M.; Fine, G.; Mariathasan, S.; McCaffery, I.; Mocci, S.; et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 92–100. [Google Scholar] [CrossRef] [PubMed]
- PD-L1 IHC 22C3 pharmDx [Package Insert] 2018. Dako Agilent Pathology Solutions. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150013S011C.pdf (accessed on 10 March 2021).
- PD-L1 IHC 28-8 pharmDx [Package Insert] 2017. Dako Agilent Pathology Solutions. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150025S003B.pdf (accessed on 10 March 2021).
- Torlakovic, E.; Lim, H.J.; Adam, J.; Barnes, P.; Bigras, G.; Chan, A.W.H.; Cheung, C.C.; Chung, J.H.; Couture, C.; Fiset, P.O.; et al. “Interchangeability” of PD-L1 immunohistochemistry assays: A meta-analysis of diagnostic accuracy. Mod. Pathol. 2020, 33, 4–17. [Google Scholar] [CrossRef]
- Lawson, N.L.; Dix, C.I.; Scorer, P.W.; Stubbs, C.J.; Wong, E.; Hutchinson, L.; McCall, E.J.; Schimpl, M.; DeVries, E.; Walker, J.; et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies. Mod. Pathol. 2020, 33, 518–530. [Google Scholar] [CrossRef] [Green Version]
- National External Quality Assessment Service (NEQAS). UK NEQAS. Available online: https://ukneqas.org.uk/ (accessed on 6 August 2020).
- College of American Pathologists. External Quality Assurance/Proficiency Testing for International Laboratories. Available online: https://www.cap.org/laboratory-improvement/international-laboratories/external-quality-assurance-proficiency-testing-for-international-laboratories (accessed on 6 August 2020).
- International Quality Network for Pathology. Proficiency Testing CBQA Readout. Available online: http://www.iqnpath.org/proficiency_testing_cbqareadout/ (accessed on 1 December 2020).
- Buttner, R.; Longshore, J.W.; Lopez-Rios, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: A perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- Krieger, T.; Pearson, I.; Bell, J.; Doherty, J.; Robbins, P. Targeted literature review on use of tumor mutational burden status and programmed cell death ligand 1 expression to predict outcomes of checkpoint inhibitor treatment. Diagn. Pathol. 2020, 15, 6. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Allgauer, M.; Budczies, J.; Christopoulos, P.; Endris, V.; Lier, A.; Rempel, E.; Volckmar, A.L.; Kirchner, M.; von Winterfeld, M.; Leichsenring, J.; et al. Implementing tumor mutational burden (TMB) analysis in routine diagnostics-a primer for molecular pathologists and clinicians. Transl. Lung Cancer Res. 2018, 7, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Vanderwalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Rinaldetti, S.; Rempel, E.; Worst, T.S.; Eckstein, M.; Steidler, A.; Weiss, C.A.; Bolenz, C.; Hartmann, A.; Erben, P. Subclassification, survival prediction and drug target analyses of chemotherapy-naïve muscle-invasive bladder cancer with a molecular screening. Oncotarget 2018, 9, 25935–25945. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- McConkey, D.J.; Choi, W.; Ochoa, A.; Siefker-Radtke, A.; Czerniak, B.; Dinney, C.P. Therapeutic opportunities in the intrinsic subtypes of muscle-invasive bladder cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 377–394, x–xi. [Google Scholar] [CrossRef]
- Choi, W.; Ochoa, A.; McConkey, D.J.; Aine, M.; Hoglund, M.; Kim, W.Y.; Real, F.X.; Kiltie, A.E.; Milsom, I.; Dyrskjot, L.; et al. Genetic alterations in the molecular subtypes of bladder cancer: Illustration in the Cancer Genome Atlas Dataset. Eur. Urol. 2017, 72, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Conde, E.; Caminoa, A.; Dominguez, C.; Calles, A.; Walter, S.; Angulo, B.; Sanchez, E.; Alonso, M.; Jimenez, L.; Madrigal, L.; et al. Aligning digital CD8(+) scoring and targeted next-generation sequencing with programmed death ligand 1 expression: A pragmatic approach in early-stage squamous cell lung carcinoma. Histopathology 2018, 72, 270–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yost, K.E.; Satpathy, A.T.; Wells, D.K.; Qi, Y.; Wang, C.; Kageyama, R.; McNamara, K.L.; Granja, J.M.; Sarin, K.Y.; Brown, R.A.; et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019, 25, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef]

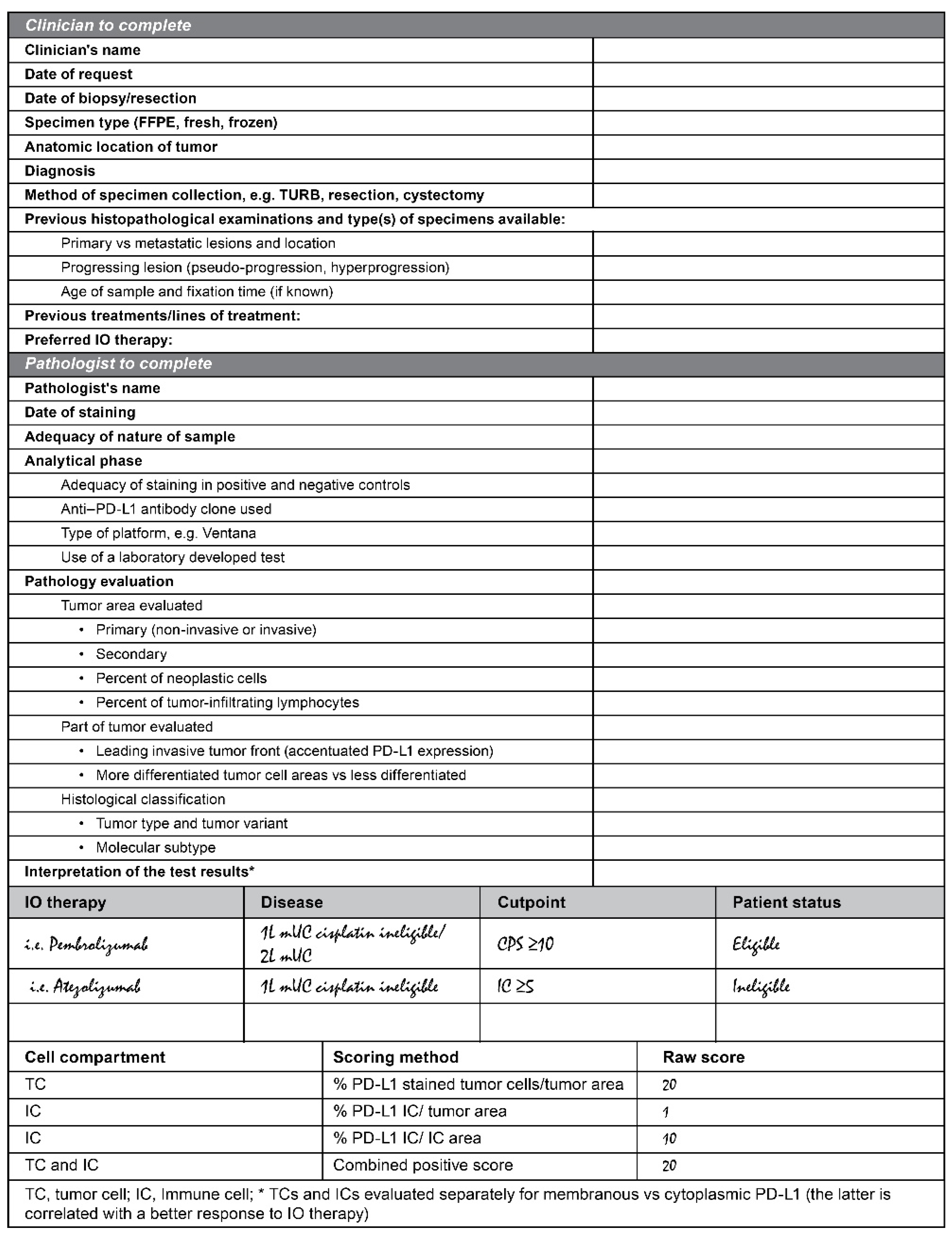


| Anti–PD-1/PD-L1 Therapeutics | Atezolizumab | Avelumab | Durvalumab, Avelumab, and Tislelizumab | Nivolumab | Pembrolizumab |
|---|---|---|---|---|---|
| Assay | VENTANA PD-L1 (SP142) Assay [52] | PD-L1 IHC pharmDx 73-10 [not FDA approved] | VENTANA PD-L1 (SP263) Assay [53] | PD-L1 IHC pharmDx 28-8 [50] | PD-L1 IHC pharmDx 22C3 [51] |
| Scoring algorithm | IC ≥ 5%; number of PD-L1–positive tumor-infiltrating ICs as a proportion of the total TC and IC area | TC ≥ 5%; number of PD-L1–positive TCs as a proportion of the total TC area | TC or IC ≥ 25%; number of PD-L1–positive TCs with membrane staining as a proportion of the total TC area or PD-L1–positive ICs with membrane, cytoplasm, or punctate as a proportion of the total IC area. | TC ≥ 1%; number of PD-L1–positive TCs as a proportion of the total TC area | Number (Count) of PD-L1–positive TCs and number of PD-L1–positive ICs as a proportion of the total TC area |
| Typical staining characteristics | Dot-/ant-like staining pattern Low tumor cell staining Strong IC staining Developed for immune cell scoring | Homogenous tumor cell staining Mostly strong staining intensity for TCs and ICs | Homogenous tumor cell staining Moderate-strong staining intensity | Homogenous tumor cell staining Mostly weak staining intensity | |
| Design Considerations | Plasma cells have to be excluded from scoring All immune cells are included (incl. neutrophil granulocytes) | Immune cell positivity is scored according to the area occupied by all immune cells (IC-“Area”-score) TC and IC are scored independently. Patients are positive when exceeding one of the two cutoffs or both PD-L1 can also be considered high if: ICP * > 1% and IC+ ≥ 25%; or, ICP * = 1% and IC+ = 100%. Plasma cells have to be excluded from scoring All immune cells are included (incl. neutrophil granulocytes) | Combined positive score including immune cells and tumor cells Plasma cells have to be excluded from scoring Neutrophil granulocytes not included |
| Specimen Selection | Recommendations for Optimum Conditions * |
|---|---|
| Site of biopsy | Use biopsies from a sample relevant to the disease stage, e.g., TURBS for MIBC, primary/metastatic for mUC. |
| Specimen age | Use formalin-fixed, paraffin-embedded sample blocks which have not been subjected to warm or fluctuating temperatures. Use the most recent sample proximal to starting therapy (maximum of 1 year old) [55]. When using approved assays follow the manufacturer’s instructions as cut slide stability can range from 1 to 6 months (PD-L1 IHC pharmDx 22C3 PI [51] and VENTANA PD-L1 (SP263) PI [58]). |
| Specimen type | Score the whole slide. Select a tissue specimen with invasive disease. ** TURB samples may be considered, however, only if they contain the invasive disease. Avoid highly necrotic samples where possible. Use positive controls for PD-L1; lymphatic tonsil tissue is recommended as optimal, with positive staining for macrophages, dendritic cells, and lymphocytes. Do not use cytology/smears for scoring ICs, as tissue architecture is necessary to understand if ICs are tumor infiltrating. It is currently not generally advised to use samples of bone metastases. There is however some evidence that bone decalcification using EDTA solution may yield good results and could possibly be used for PD-L1 IHC. Validation is required. |
| Sample preparation/fixation | Use 10% neutral buffered formalin in a quantity > 10 times the volume of the specimen. Sample should be placed in formalin as soon as possible (<30 min) and for a period of 12–24 h. Longer fixation may cause diffuse staining patterns. For immunohistochemistry, fixation should be performed for a minimum of 6 h and no more than 72 h. Large samples should be excised to allow for sufficient penetration of the fixative. Fixative penetrates about 1 mm/h with slight variation across different types of tissues. i.e., for cystectomy samples, the tumor should be excised, or the bladder opened to allow fixative to penetrate. Avoid decalcified tissue or tissue processed with other fixatives. Use on-slide positive and negative controls from the same institution or manufacturer, especially if an automated stainer is not used. Section specimens into a thickness of 3 or 4 µmm (as specified in manufacturers instruction). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Beltran, A.; López-Rios, F.; Montironi, R.; Wildsmith, S.; Eckstein, M. Immune Checkpoint Inhibitors in Urothelial Carcinoma: Recommendations for Practical Approaches to PD-L1 and Other Potential Predictive Biomarker Testing. Cancers 2021, 13, 1424. https://doi.org/10.3390/cancers13061424
Lopez-Beltran A, López-Rios F, Montironi R, Wildsmith S, Eckstein M. Immune Checkpoint Inhibitors in Urothelial Carcinoma: Recommendations for Practical Approaches to PD-L1 and Other Potential Predictive Biomarker Testing. Cancers. 2021; 13(6):1424. https://doi.org/10.3390/cancers13061424
Chicago/Turabian StyleLopez-Beltran, Antonio, Fernando López-Rios, Rodolfo Montironi, Sophie Wildsmith, and Markus Eckstein. 2021. "Immune Checkpoint Inhibitors in Urothelial Carcinoma: Recommendations for Practical Approaches to PD-L1 and Other Potential Predictive Biomarker Testing" Cancers 13, no. 6: 1424. https://doi.org/10.3390/cancers13061424
APA StyleLopez-Beltran, A., López-Rios, F., Montironi, R., Wildsmith, S., & Eckstein, M. (2021). Immune Checkpoint Inhibitors in Urothelial Carcinoma: Recommendations for Practical Approaches to PD-L1 and Other Potential Predictive Biomarker Testing. Cancers, 13(6), 1424. https://doi.org/10.3390/cancers13061424








