Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patients with Lung Cancer and Healthy Volunteers
2.2. VOCs for SIFT-MS Analysis
2.3. XGBoost Prediction Model
2.4. Adjust Algorithm for Environmental VOCs
3. Discussion
4. Materials and Methods
4.1. Study Participants and Data Collection
4.2. Breath Sampling Methodology
4.3. Measurements of VOCs in Exhaled Air
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Compound | No. | Compound | No. | Compound | No. | Compound |
|---|---|---|---|---|---|---|---|
| 1 *,† | beta-caryophyllene (87-44-5) | 30 | 2-pentanone (107-87-9) | 59 | diethyl ether (60-29-7) | 88 † | 1,4-diaminobutane (110-60-1) |
| 2 | pyrrole (109-97-7) | 31 *,† | (E)-2-heptenal (18829-55-5) | 60 | isobutyl alcohol (78-83-1) | 89 | o-xylene (95-47-6) |
| 3 * | benzoic acid (65-85-0) | 32 † | 3-buten-2-one (78-94-4) | 61 † | 2-methylpentane (107-83-5) | 90 † | cyclopentane (287-92-3) |
| 4 *,† | 2,5-dimethylfuran (625-86-5) | 33 † | butanone (78-93-3) | 62 | methylcyclopentane (96-37-7) | 91 | propane (74-98-6) |
| 5 * | acetophenone (98-86-2) | 34 *,† | 1,5-diaminopentane (462-94-2) | 63 † | heptanal (111-71-7) | 92 | heptane (142-82-5) |
| 6 | pyridine (110-86-1) | 35 *,† | alpha-terpinene (99-86-5) | 64 | 1-butanol (71-36-3) | 93 | propanal (123-38-6) |
| 7 * | 2-methylpyrazine (109-08-0) | 36 * | 1-butyne (107-00-6) | 65 | 3-methyl-2-butenal (107-86-8) | 94 * | 2-propanol (67-63-0) |
| 8 † | tridecane (629-50-5) | 37 | 1-methyl-2-pyrrolidinone (872-50-4) | 66 † | pentanoic acid (109-52-4) | 95 *,† | cyclohexane (110-82-7) |
| 9 † | 2,5-dimethylpyrazine (123-32-0) | 38 † | diisopropyl ether (108-20-3) | 67 * | ethylbenzene (100-41-4) | 96 | ethane (74-84-0) |
| 10 † | 1,3-butadiene (106-99-0) | 39 | 2-pentanone new (107-87-9) | 68 *,† | 1-heptene (592-76-7) | 97 | carbon disulfide (75-15-0) |
| 11 *,† | dodecane (112-40-3) | 40 * | 1,2,4-trimethylbenzene (95-63-6) | 69 *,† | dimethyl sulfide (75-18-3) | 98 *,† | trimethylamine (75-50-3) |
| 12 | propyne (74-99-7) | 41 † | nonane (111-84-2) | 70 *,† | propanoic acid (79-09-4) | 99 | acetaldehyde (75-07-0) |
| 13 † | (E)-2-nonenal (18829-56-6) | 42 * | propylbenzene (103-65-1) | 71 | toluene (108-88-3) | 100 | dimethyl ether (115-10-6) |
| 14 * | 4-isopropyl toluene (99-87-6) | 43 | 3-butyn-2-ol new (2028-63-9) | 72 | p-xylene (106-42-3) | 101 † | acetic acid (64-19-7) |
| 15 † | 2-hexanone (591-78-6) | 44 *,† | cyclohexanone (108-94-1) | 73 † | 3-methylbutanal (590-86-3) | 102 | propene (115-07-1) |
| 16 | undecane (1120-21-4) | 45 *,† | ethylcyclohexane (1678-91-7) | 74 | butanal (123-72-8) | 103 | formaldehyde (50-00-0) |
| 17 * | benzaldehyde (100-52-7) | 46 † | 2-methylbutanal (96-17-3) | 75 | xylenes + ethylbenzene (1330-20-7) | 104 | furan (110-00-9) |
| 18 * | styrene (100-42-5) | 47 * | nonanal (124-19-6) | 76 * | isopropylamine (75-31-0) | 105 * | 1-propanol (71-23-8) |
| 19 *,† | eucalyptol (470-82-6) | 48 *,† | limonene (138-86-3; 7705-14-8) | 77 *,† | methyl acetate (79-20-9) | 106 † | isobutane (75-28-5) |
| 20 † | furfural (98-01-1) | 49 † | 2-pentene (109-68-2) | 78 *,† | 1-hexene (592-41-6) | 107 | isoprene (78-79-5) |
| 21 * | 1-pentanol (71-41-0) | 50 | decane (124-18-5) | 79 *,† | 1-butene (106-98-9) | 108 * | formic acid (64-18-6) |
| 22 *,† | butyl acetate (123-86-4) | 51 | methyl n-propyl sulfide (3877-15-4) | 80 † | pentanal (110-62-3) | 109 | pentane (109-66-0) |
| 23 * | octanal (124-13-0) | 53 † | 2-methylpropanal (78-84-2) | 81 | 1-methoxy-2-propanol (107-98-2) | 110 * | acetonitrile (75-05-8) |
| 24 * | 3-methyl-1-butanol (123-51-3) | 53 *,† | acetoin (513-86-0) | 82 | 2,3-butanediol (513-85-9; 513-89-3) | 111 * | ethanol (64-17-5) |
| 25 † | (E)-2-hexenal (6728-26-3) | 54 *,† | alpha-pinene (80-56-8; 2437-95-8) | 83 † | hexanal (66-25-1) | 112 † | hexane (110-54-3) |
| 26 † | 1,4-butyrolactone (96-48-0) | 55 * | acrylonitrile (107-13-1) | 84 *,† | acrolein (107-02-8) | 113 * | methanol (67-56-1) |
| 27 † | 6-methyl-5-hepten-2-one (110-93-0) | 56 *,† | ethyl acetate (141-78-6) | 85 † | acetic anhydride (108-24-7) | 114 * | acetone (67-64-1) |
| 28 | benzene (71-43-2) | 57 *,† | 2,3-butanedione (431-03-8) | 86 † | 3-methylpentane (96-14-0) | 115 * | butane (106-97-8) |
| 29 † | decanal (112-31-2) | 58 *,† | 2-methyl-2-propenal (78-85-3) | 87 *,† | octane (111-65-9) | 116 * | ethanedial (107-22-2) |
References
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid Partition Chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and Methodologic Challenges of Volatile Organic Compound–Based Exhaled Breath Tests for Cancer Diagnosis. JAMA Oncol. 2019, 5, e182815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, R.A.; Weinberg, R.A. The Biology of Cancer; Garland Science: New York, NY, USA, 2013. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Marzorati, D.; Mainardi, L.; Sedda, G.; Gasparri, R.; Spaggiari, L.; Cerveri, P. A review of exhaled breath: A key role in lung cancer diagnosis. J. Breath Res. 2019, 13, e034001. [Google Scholar] [CrossRef]
- Syft Technologies Ltd. Technical Comparison SIFT-MS and GCMS full. Available online: https://www.lqa.com/wp-content/uploads/2017/08/SIFTMS-vs-GCMS.pdf (accessed on 7 December 2020).
- Španěl, P.; Smith, D. Progress in SIFT-MS: Breath analysis and other applications. Mass Spectrom. Rev. 2010, 30, 236–267. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. Selected Ion Flow Tube Mass Spectrometry for On-Line Trace Gas Analysis in Biology and Medicine. Eur. J. Mass Spectrom. 2007, 13, 77–82. [Google Scholar] [CrossRef]
- Markar, S.R.; Chin, S.-T.; Romano, A.; Wiggins, T.; Antonowicz, S.; Paraskeva, P.; Ziprin, P.; Darzi, A.; Hanna, G.B. Breath Volatile Organic Compound Profiling of Colorectal Cancer Using Selected Ion Flow-tube Mass Spectrometry. Ann. Surg. 2019, 269, 903–910. [Google Scholar] [CrossRef]
- Markar, S.R.; Wiggins, T.; Antonowicz, S.; Chin, S.-T.; Romano, A.; Nikolic, K.; Evans, B.; Cunningham, D.; Mughal, M.; Lagergren, J.; et al. Assessment of a Noninvasive Exhaled Breath Test for the Diagnosis of Oesophagogastric Cancer. JAMA Oncol. 2018, 4, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, F.; Yoneda, K.; Kondo, N.; Hashimoto, M.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Rahman, S.; Tsubota, N.; Tsujimura, T.; et al. Circulating Tumor Cell as a Diagnostic Marker in Primary Lung Cancer. Clin. Cancer Res. 2009, 15, 6980–6986. [Google Scholar] [CrossRef] [Green Version]
- Sonn, C.-H.; Cho, J.H.; Kim, J.-W.; Kang, M.S.; Lee, J.; Kim, J. Detection of circulating tumor cells in patients with non-small cell lung cancer using a size-based platform. Oncol. Lett. 2017, 13, 2717–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, K.; Takayama, K.; Izumi, M.; Harada, T.; Furuyama, K.; Nakanishi, Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer 2013, 80, 45–49. [Google Scholar] [CrossRef]
- Nolen, B.M.; Lomakin, A.; Marrangoni, A.; Velikokhatnaya, L.; Prosser, D.; Lokshin, A.E. Urinary Protein Biomarkers in the Early Detection of Lung Cancer. Cancer Prev. Res. 2015, 8, 111–119. [Google Scholar] [CrossRef] [Green Version]
- López-Sánchez, L.M.; Jurado-Gámez, B.; Feu-Collado, N.; Valverde, A.; Cañas, A.; Fernández-Rueda, J.L.; Aranda, E.; Rodríguez-Ariza, A. Exhaled breath condensate biomarkers for the early diagnosis of lung cancer using proteomics. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, 664–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagita, K.; Nagashio, R.; Jiang, S.-X.; Kuchitsu, Y.; Hachimura, K.; Ichinoe, M.; Igawa, S.; Fukuda, E.; Goshima, N.; Satoh, Y.; et al. Cytoskeleton-Associated Protein 4 Is a Novel Serodiagnostic Marker for Lung Cancer. Am. J. Pathol. 2018, 188, 1328–1333. [Google Scholar] [CrossRef]
- Kim, H.; Yang, J.M.; Jin, Y.; Jheon, S.; Kim, K.; Lee, C.T.; Chung, J.-H.; Paik, J.H. MicroRNA expression profiles and clinicopathological implications in lung adenocarcinoma according to EGFR, KRAS, and ALK status. Oncotarget 2017, 8, 8484–8498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Wei, Y.; Wang, N.; Gao, H.; Liu, K. MicroRNA-26b suppresses the metastasis of non-small cell lung cancer by targeting MIEN1 via NF-κB/MMP-9/VEGF pathways. Biochem. Biophys. Res. Commun. 2016, 472, 465–470. [Google Scholar] [CrossRef]
- Dacic, S.; Kelly, L.; Shuai, Y.; Nikiforova, M.N. miRNA expression profiling of lung adenocarcinomas: Correlation with mutational status. Mod. Pathol. 2010, 23, 1577–1582. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Kong, H.; Hou, Y.; Ge, D.; Huang, W.; Ou, J.; Yang, D.; Zhang, L.; Wu, G.; Song, Y.; et al. Two plasma microRNA panels for diagnosis and subtype discrimination of lung cancer. Lung Cancer 2018, 123, 44–51. [Google Scholar] [CrossRef]
- Leng, Q.; Lin, Y.; Jiang, F.; Lee, C.-J.; Zhan, M.; Fang, H.; Wang, Y.; Jiang, F. A plasma miRNA signature for lung cancer early detection. Oncotarget 2017, 8, 111902–111911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielscher, M.; Vierlinger, K.; Kegler, U.; Ziesche, R.; Gsur, A.; Weinhäusel, A. Diagnostic Performance of Plasma DNA Methylation Profiles in Lung Cancer, Pulmonary Fibrosis and COPD. EBioMedicine 2015, 2, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Ilse, P.; Biesterfeld, S.; Pomjanski, N.; Wrobel, C.; Schramm, M. Analysis of SHOX2 methylation as an aid to cytology in lung cancer diagnosis. Cancer Genom. Proteom. 2014, 11, 251–258. [Google Scholar]
- Duan, G.-C.; Zhao, Q.-T.; Guo, T.; Wang, H.-E.; Zhang, X.-P.; Zhang, H.; Wang, Z.-K.; Yuan, Z. Diagnostic value of SHOX2 DNA methylation in lung cancer: A meta-analysis. OncoTargets Ther. 2015, 8, 3433–3439. [Google Scholar] [CrossRef] [Green Version]
- Fiala, C.; Diamandis, E.P. Circulating tumor DNA for personalized lung cancer monitoring. BMC Med. 2017, 15, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Erratum: Corrigendum: Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nat. Cell Biol. 2018, 554, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolism 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- D’Mello, J.; Butani, M. Capnography. Indian J. Anaesth. 2002, 46, 269–278. [Google Scholar]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of Lung Cancer With Volatile Markers in the Breatha. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehinger, A.; Schmid, A.; Mechtcheriakov, S.; Ledochowski, M.; Grabmer, C.; Gastl, G.A.; Amann, A. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int. J. Mass Spectrom. 2007, 265, 49–59. [Google Scholar] [CrossRef]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hu, Y.; Wang, D.; Yu, K.; Wang, L.; Zou, Y.; Zhao, C.; Zhang, X.; Wang, P.; Ying, K. The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomark. 2012, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Doran, S.L.F.; Romano, A.; Hanna, G.B. Optimisation of sampling parameters for standardised exhaled breath sampling. J. Breath Res. 2017, 12, 016007. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, e014001. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Greenberg, J.; Awad, J. Metabolic and environmental origins of volatile organic compounds in breath. J. Clin. Pathol. 1994, 47, 1052–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchamp, J. Inhaled today, not gone tomorrow: Pharmacokinetics and environmental exposure of volatiles in exhaled breath. J. Breath Res. 2011, 5, e037103. [Google Scholar] [CrossRef]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME–GC–TOF/MS and chemometrics. J. Chromatogr. B 2011, 879, 3360–3366. [Google Scholar] [CrossRef]
- Machado, R.F.; Laskowski, D.; Deffenderfer, O.; Burch, T.; Zheng, S.; Mazzone, P.J.; Mekhail, T.; Jennings, C.; Stoller, J.K.; Pyle, J.; et al. Detection of Lung Cancer by Sensor Array Analyses of Exhaled Breath. Am. J. Respir. Crit. Care Med. 2005, 171, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Lechner, M.; Moser, B.; Niederseer, D.; Karlseder, A.; Holzknecht, B.; Fuchs, M.; Colvin, S.; Tilg, H.; Rieder, J. Gender and age specific differences in exhaled isoprene levels. Respir. Physiol. Neurobiol. 2006, 154, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Greenberg, J.; Cataneo, R.N. Effect of age on the profile of alkanes in normal human breath. Free Radic. Res. 2000, 33, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Krilaviciute, A.; Heiss, J.A.; Leja, M.; Kupcinskas, J.; Haick, H.; Brenner, H. Detection of cancer through exhaled breath: A systematic review. Oncotarget 2015, 6, 38643–38657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saalberg, Y.; Wolff, M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Altorki, N.; Austin, J.H.; Cameron, R.B.; Cataneo, R.N.; Greenberg, J.; Kloss, R.; Maxfield, R.A.; Munawar, M.I.; Pass, H.I.; et al. Prediction of lung cancer using volatile biomarkers in breath1. Cancer Biomark. 2007, 3, 95–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakumura, Y.; Koyama, Y.; Tokutake, H.; Hida, T.; Sato, K.; Itoh, T.; Akamatsu, T.; Shin, W. Diagnosis by Volatile Organic Compounds in Exhaled Breath from Lung Cancer Patients Using Support Vector Machine Algorithm. Sensors 2017, 17, 287. [Google Scholar] [CrossRef] [Green Version]
- Rudnicka, J.; Kowalkowski, T.; Buszewski, B. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer 2019, 135, 123–129. [Google Scholar] [CrossRef]
- Wang, C.; Long, Y.; Li, W.; Dai, W.; Xie, S.; Liu, Y.; Zhang, Y.; Liu, M.; Tian, Y.; Li, Q.; et al. Exploratory study on classification of lung cancer subtypes through a combined K-nearest neighbor classifier in breathomics. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Mochalski, P.; King, J.; Unterkofler, K.; Amann, A. Stability of selected volatile breath constituents in Tedlar, Kynar and Flexfilm sampling bags. Analyst 2013, 138, 1405–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Harreveld, A. (Ton) Odor Concentration Decay and Stability in Gas Sampling Bags. J. Air Waste Manag. Assoc. 2003, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath Analysis: Comparison among Methodological Approaches for Breath Sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef] [PubMed]
- Heat Map—Wikipedia. Available online: https://en.wikipedia.org/wiki/Heat_map (accessed on 22 February 2021).
- Rosner, B. Fundamentals of Biostatistics, 6th ed.; Thomson-Brooks/Cole: Belmont, CA, USA, 2006. [Google Scholar]
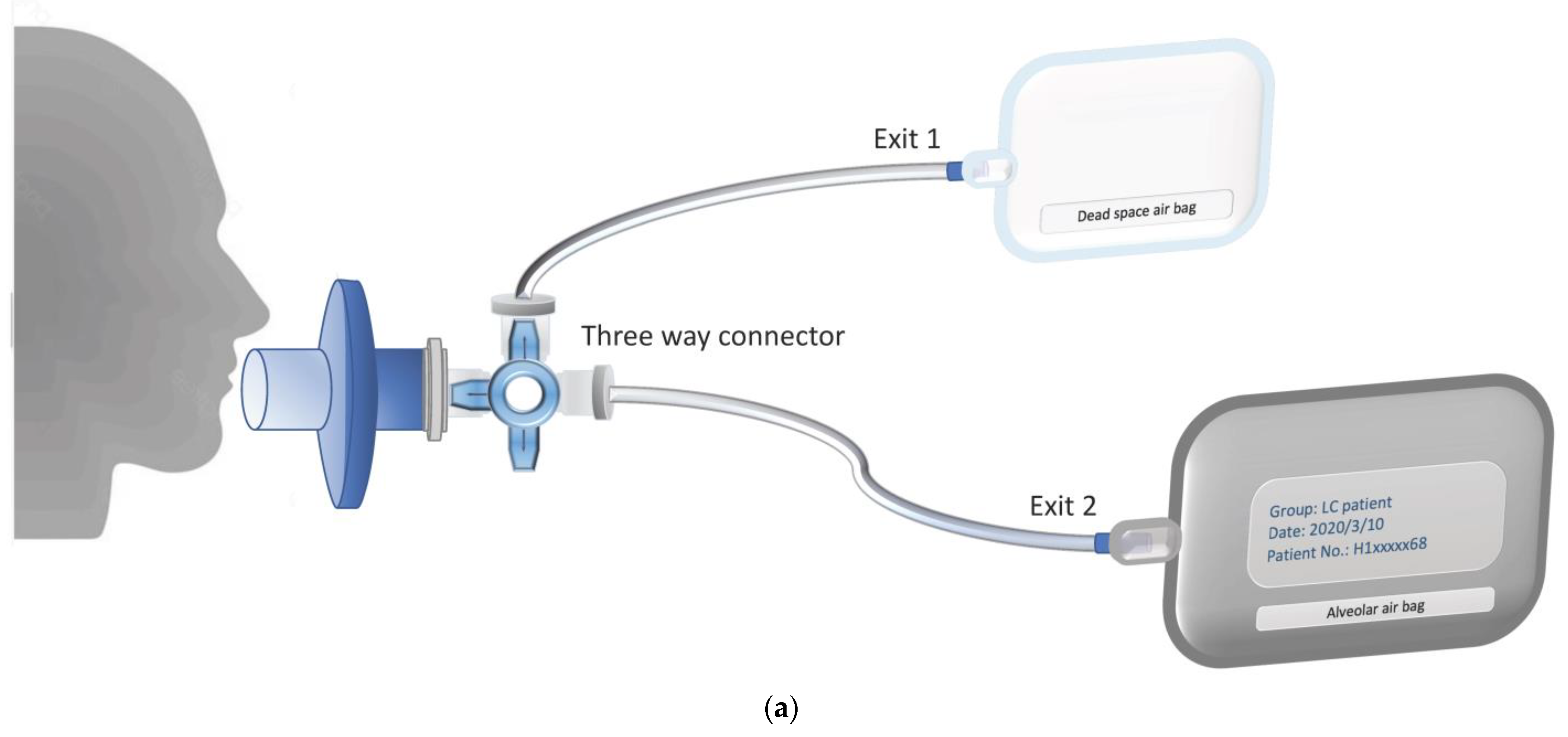
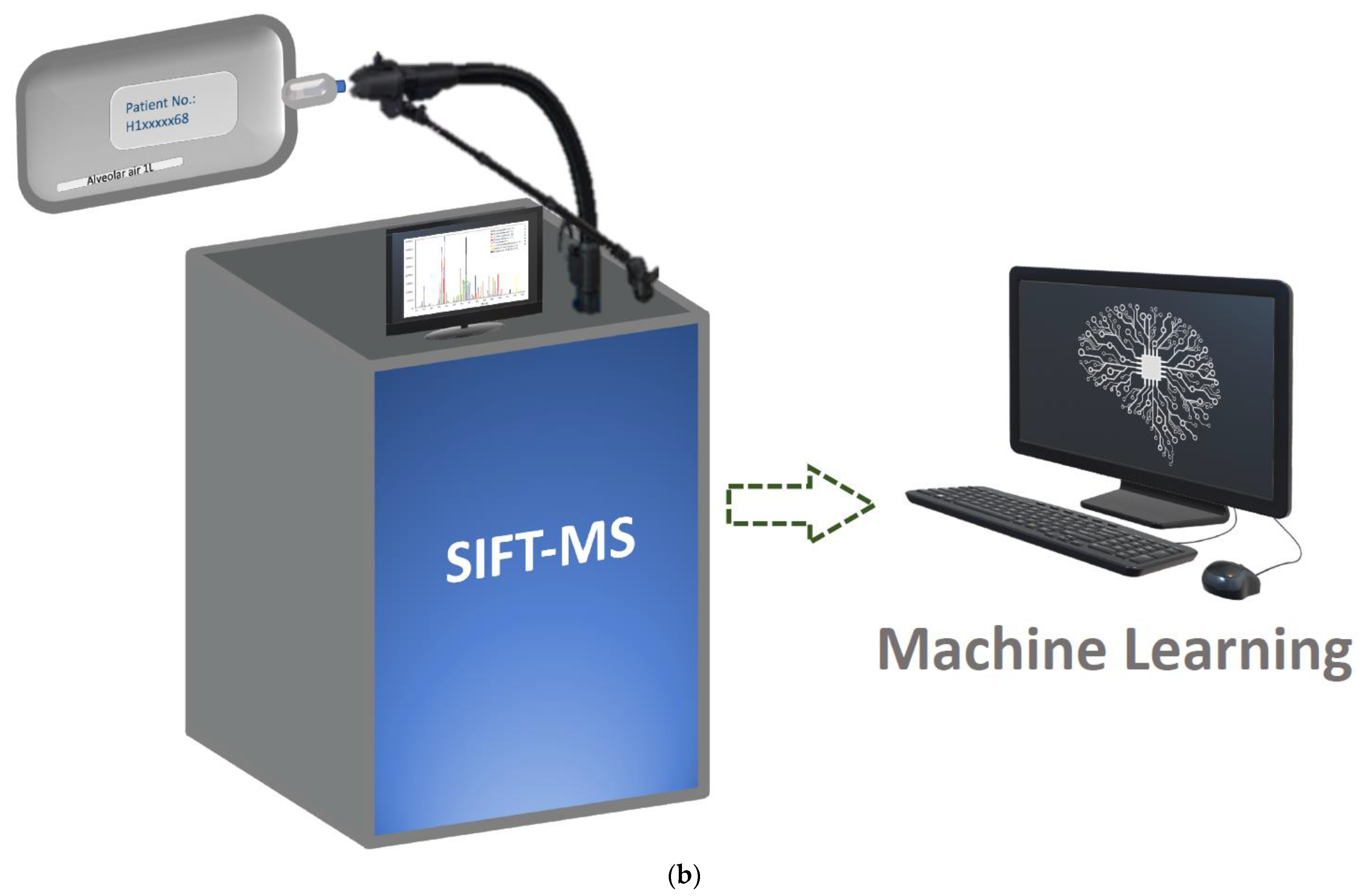

| Biomarkers/Specimen | Analytic Platform | Detection Target | Sensitivity (%) | Advantages | Deficiencies | Ref. |
|---|---|---|---|---|---|---|
| CTCs/Blood | IF; FISH | EpCAM, Size-based cells | 30.0–69.5 | Viable cell, high specificity, high throughput | Limited sensitivity; require enrichment; only detect advanced cancers | [14,15] |
| Traditional Proteins/Blood | ECLIA | CEA, CYFRA 21-1 | 22–69 | Rapid and common | Limited sensitivity and specificity | [16] |
| Novel Proteins/EBC, Saliva, Urine, Blood | Microarray; LC-MS/MS | CKAP4, exosomal proteins (NFX1, PKG1, GPC1) | 70.0–84.0 | Higher sensitivity; high throughput; rapid | Quantity required (MS); validation required | [17,18,19] |
| microRNA/Blood | Microarray; RT-PCR; NGS | miRNAs-126, -145, -210 and -205-5p, -17, -190b, -19a, -19b, -26b, -375 | 80.0–91.5 | High throughput, stable | Specialized abilities and facilities are required | [20,21,22,23,24] |
| Methylated DNA/Blood | NGS; PCR | HOXD10, PAX9, PTPRN2, STAG3, SHOX2 | 70.0–87.8 | High sensitivity and specificity | Require standardization | [25,26,27] |
| ctDNA/Blood | NGS; Multiplex-PCR | Genetic mutation, SNVs | 48.0–59.0 | Target for precision medicine; early detection (~70 days prior to CT image) | Limited sensitivity, require expensive equipment | [28,29,30] |
| VOCs/Exhaled Breath | E-Nose sensors; GC-MS; PTR-MS, IMS; LPPI-MS | propanol, isoprene, acetone, pentane, hexanal, toluene, benzene, ethylbenzene, and others | 81.0–96.5 | Rapid, simple, noninvasive; inexpensive | Require standardization | [7,31,32] |
| Characteristic | Lung Cancer Patients (n = 148) | Health Controls (n = 168) |
|---|---|---|
| Age (years), y * | ||
| Mean ± SD | 64.5 ± 11 | 31.4 ± 10.4 |
| Rage | 37–90 | 20–74 |
| Sex, n (%) † | ||
| Female | 75 (50.7) | 101 (60.1) |
| Male | 73 (49.3) | 67 (39.9) |
| Smoking status, n (%) * | ||
| Current smoker | 9 (6) | 0 |
| Former smoker | 47 (31.2) | 1 |
| Nonsmoker | 92 (62.1) | 167 (99) |
| Lung cancer type, n (%) | - | |
| Adenocarcinoma | 108 (72.9) | |
| Squamous cell carcinoma | 17 (11.5) | |
| Small cell lung cancer | 14 (9.5) | |
| Other lung cancer | 8 (5.4) | |
| Targetable driver mutation, n (%) | ||
| EGFR | - | |
| Exon 19 deletion | 33 (22.3) | |
| Exon 21 point mutation | 30 (20.3) | |
| T790M | 6 (4.1) | |
| ALK | 7 (4.7) | |
| ROS1 | 3 (2.0) | |
| Wild type | 75 (50.7) | |
| PD-L1 expression, n (%) | ||
| >50% | 18 (12.1) | - |
| 1–49% | 57 (39.0) | |
| <1% | 29 (19.6) | |
| Clinical stage status, n (%) | ||
| IA and B | 4 (2.7) | - |
| IIA and B | 4 (2.7) | |
| IIIA | 8 (5.4) | |
| III B and C | 27 (18.2) | |
| IVA | 65 (43.9) | |
| IVB | 40 (27.0) |
| Algorithms | Analytical Platform | Patients with Cancer No. | Analyzed VOC No. | Sensitivity % | Specificity % | AUC | Reference/(Year) |
|---|---|---|---|---|---|---|---|
| Stepwise Discriminant Analysis | GC-MS | 67 | 9 | 85.1 | 80.5 | NR | [35]/(2003) |
| Logistic Regression | GC-MS | 193 | 16 | 84.6 | 80.0 | 0.88 | [50]/(2007) |
| Weighted Digital Sum Discriminator | GC-MS | 193 | 30 | 84.5 | 81 | 0.9 | [32]/(2008) |
| Support Vector Machine | GS-MS | 107 | 5 | 95 | 89 | NR * | [51]/(2016) |
| Artificial Neural Networks | GC-MS | 108 | 88 | 86.36 | 86.36 | 0.86 | [52]/(2019) |
| K-nearest Neighbor | GC-MS | 325 | NR | NR | NR | 0.63 † | [53]/(2020) |
| Extreme Gradient Boosting | SIFT-MS | 148 | 116 | 82 | 94 | 0.95 | This WorkConsidering only participants’ VOCs |
| 96 | 88 | 0.98 | Considering both participants’ VOCs and environmental VOCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsou, P.-H.; Lin, Z.-L.; Pan, Y.-C.; Yang, H.-C.; Chang, C.-J.; Liang, S.-K.; Wen, Y.-F.; Chang, C.-H.; Chang, L.-Y.; Yu, K.-L.; et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers 2021, 13, 1431. https://doi.org/10.3390/cancers13061431
Tsou P-H, Lin Z-L, Pan Y-C, Yang H-C, Chang C-J, Liang S-K, Wen Y-F, Chang C-H, Chang L-Y, Yu K-L, et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers. 2021; 13(6):1431. https://doi.org/10.3390/cancers13061431
Chicago/Turabian StyleTsou, Ping-Hsien, Zong-Lin Lin, Yu-Chiang Pan, Hui-Chen Yang, Chien-Jen Chang, Sheng-Kai Liang, Yueh-Feng Wen, Chia-Hao Chang, Lih-Yu Chang, Kai-Lun Yu, and et al. 2021. "Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer" Cancers 13, no. 6: 1431. https://doi.org/10.3390/cancers13061431
APA StyleTsou, P.-H., Lin, Z.-L., Pan, Y.-C., Yang, H.-C., Chang, C.-J., Liang, S.-K., Wen, Y.-F., Chang, C.-H., Chang, L.-Y., Yu, K.-L., Liu, C.-J., Keng, L.-T., Lee, M.-R., Ko, J.-C., Huang, G.-H., & Li, Y.-K. (2021). Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers, 13(6), 1431. https://doi.org/10.3390/cancers13061431









