Impact of Gastrointestinal Side Effects on Patients’ Reported Quality of Life Trajectories after Radiotherapy for Prostate Cancer: Data from the Prospective, Observational Pros-IT CNR Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
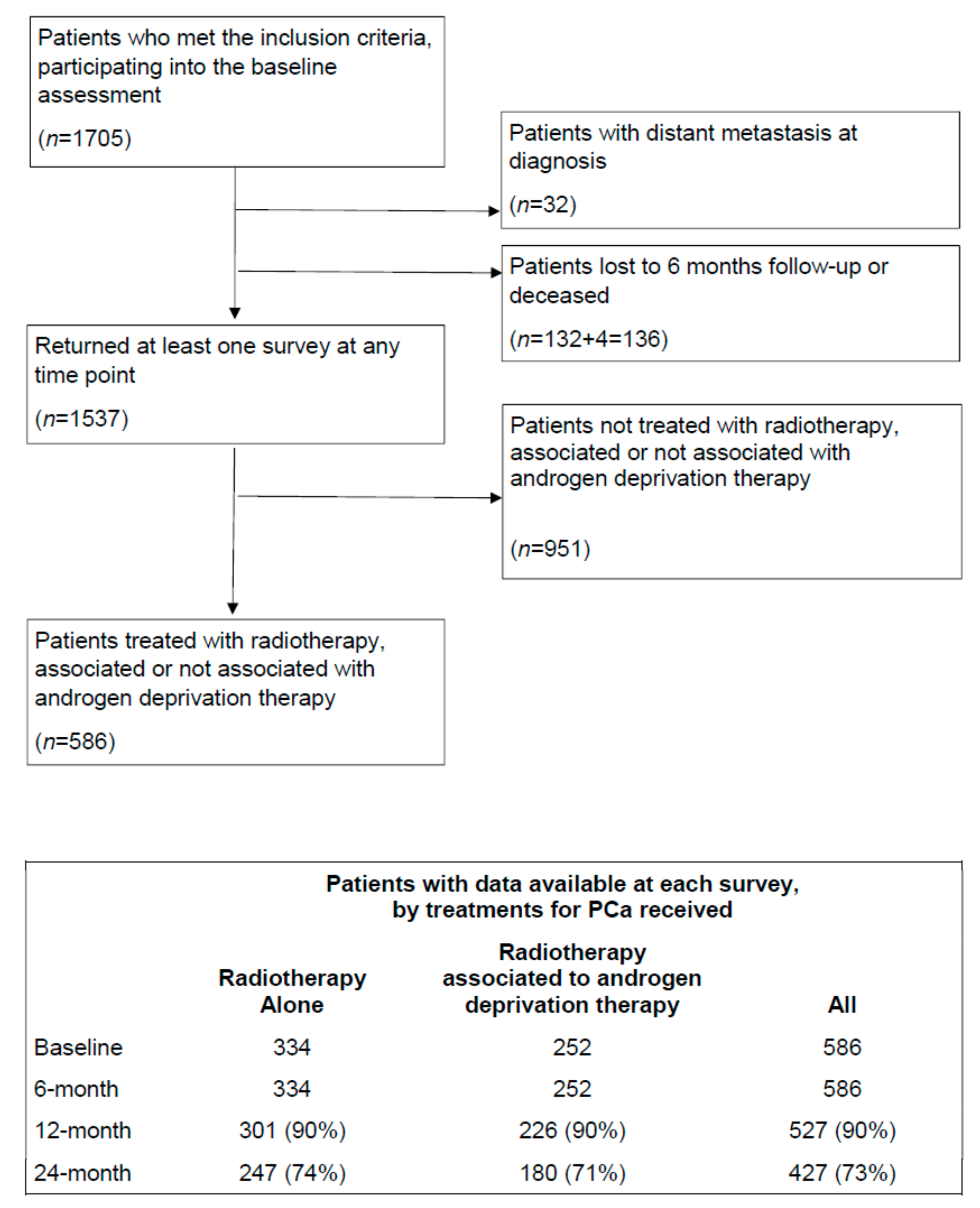
2.1. Participants
2.2. Outcome Variables
2.3. Predictor Variables
2.4. Statistical Analysis
- The model fit statistics in terms of the Bayesian Information Criterion (BIC); the magnitude of the BIC difference was utilized to choose between more complex and simpler models (2ΔBIC > 10 [13]);
- the significance of polynomial terms, starting with a cubic specification for the trajectory shape and dropping non-significant polynomial terms [14];
- the value of group membership probability of at least 5%;
- the value of average posterior probability within each group greater than 0.7.
3. Results
- Trajectory 3 referred to the largest group of patients (67% of the population considered in the present analysis) who had high BF scores at diagnosis (the mean baseline score was 100 ± 0) that fell gradually throughout the 24-month follow-up (mean score at the 6-month follow-up: 93.2 ± 13.1, at the 12-month follow-up: 84.4 ± 14.3, at the 24-month follow-up: 85.3 ± 12.8). The posterior group membership probability was 0.9;
- Trajectory 2 referred to the second largest group of patients (23%) whose BF scores were lower at diagnosis (the mean baseline score was 78 ± 14), but higher at the 6-month follow-up assessment (mean score: 95.6 ± 7.6); they fell again (mean score at the 12-month follow-up: 85.9 ± 10.2) and were lower by the time the 24-month follow-up assessment was reached (mean score: 86.5 ± 10.2). The posterior group membership probability was 0.9;
- Trajectory 1 referred to the smallest group of patients (10%) who started with lower scores (the mean baseline score was 71 ± 23); it initially fell (mean score at the 6-month follow-up: 52.1 ± 23.2) and then rose remaining at a plateau until the 24-month follow-up assessment was reached (mean score at the 12-month follow-up: 62.9 ± 23.7, at the 24-month follow-up: 63.2 ± 23.5). The posterior group membership probability was 0.9.
- Trajectory 2 referred to the largest proportion of the patients (71% of those considered) who showed constantly high BB scores throughout the 24-month follow-up period studied (96 ± 13 was the mean baseline BB score, 98 ± 9 was the score at the 6-and 98 ± 8 at the 12-month follow-ups, and 96 ± 14 at the 24-month follow-up). The posterior group membership probability was 0.9;
- Trajectory 1 referred to a smaller group of patients (29%) who showed lower BB scores at diagnosis (the mean baseline score was 81 ± 29) and even lower scores at the 6-month follow-up (61 ± 30); then the curve stabilized or rose slightly until the 24-month follow-up assessment was reached (64 ± 30 at the 12-month follow-up; 73 ± 31 at the 24-month follow-up). The posterior group membership probability was 0.8.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buglione, M.; Noale, M.; Bruni, A.; Antonelli, A.; Bertoni, F.; Corvo’, R.; Ricardi, U.; Borghetti, P.; Maddalo, M.; Simeone, C.; et al. Treatment paths for localised prostate cancer in Italy: The results of a multidisciplinary, observational, prospective study (Pros-IT CNR). PLoS ONE 2019, 14, e0224151. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, R.W.M.; Cremers, R.G.H.M.; Jansen, H.; Somford, D.M.; Kiemeney, L.A.; van Andel, G.; Wijsman, B.P.; Busstra, M.B.; van Moorselaar, R.J.A.; Wijnen, E.M.; et al. Urinary incontinence and erectile dysfunction in patients with localized or locally advanced prostate cancer: A nationwide observational study. Urol. Oncol. 2020. [Google Scholar] [CrossRef]
- Hoffman, K.E.; Penson, D.F.; Zhao, Z.; Huang, L.C.; Conwill, R.; Laviana, A.A.; Joyce, D.D.; Luckenbaugh, A.N.; Goodman, M.; Hamilton, A.S.; et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2020, 323, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Budaus, L.; Bolla, M.; Bossi, A.; Cozzarini, C.; Crook, J.; Widmark, A.; Wiegel, T. Functional outcomes and complications following radiation therapy for prostate cancer: A critical analysis of the literature. Eur. Urol. 2012, 61, 112–127. [Google Scholar] [CrossRef]
- Barocas, D.A.; Alvarez, J.; Resnick, M.J.; Koyama, T.; Hoffman, K.E.; Tyson, M.D.; Conwill, R.; McCollum, D.; Cooperberg, M.R.; Goodman, M.; et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017, 317, 1126–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, N.; Grimm, K.J. Growth Mixture Modeling: A method for identifying differences in longitudinal change among unobserved groups. Int. J. Behav. Dev. 2009, 33, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palumbo, C.; Bruni, A.; Antonelli, A.; Artibani, W.; Bassi, P.F.; Bertoni, F.; Borghetti, P.; Bracarda, S.; Cicchetti, A.; Corvò, R.; et al. Health-related quality of life 24-month after prostate cancer diagnosis: An update from the Pros-IT CNR prospective observational study. Health-related quality of life 24-month after prostate cancer diagnosis: An update from the Pros-IT CNR prospective observational study. Minerva Urol. Nefrol. 2021. [Google Scholar] [CrossRef]
- Noale, M.; the Pros-IT CNR study group; Maggi, S.; Artibani, W.; Bassi, P.F.; Bertoni, F.; Bracarda, S.; Conti, G.N.; Corvò, R.; Gacci, M.; et al. Pros-IT CNR: An Italian prostate cancer monitoring project. Aging Clin. Exp. Res. 2017, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Porreca, A.; the Pros-IT CNR study group; Noale, M.; Artibani, W.; Bassi, P.F.; Bertoni, F.; Bracarda, S.; Conti, G.N.; Corvò, R.; Gacci, M.; et al. Disease-specific and general health-related quality of life in newly diagnosed prostate cancer patients: The Pros-IT CNR study. Health Qual. Life Outcomes 2018, 16, 122. [Google Scholar] [CrossRef]
- Gacci, M.; Noale, M.; Artibani, W.; Bassi, P.F.; Bertoni, F.; Bracarda, S.; Conti, G.N.; Corvò, R.; Graziotti, P.; Magrini, S.M.; et al. Quality of Life After Prostate Cancer Diagnosis: Data from the Pros-IT CNR. Eur. Urol. Focus 2017, 3, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Livi, L.; Paiar, F.; Detti, B.; Litwin, M.; Bartoletti, R.; Giubilei, G.; Cai, T.; Mariani, M.; Carini, M. Quality of life after radical treatment of prostate cancer: Validation of the Italian version of the University of California-Los Angeles Prostate Cancer Index. Urology 2005, 66, 338–343. [Google Scholar] [CrossRef]
- Conwell, Y.; Forbes, N.T.; Cox, C.; Caine, E.D. Validation of a measure of physical illness burden at autopsy: The Cumulative Illness Rating Scale. J. Am. Geriatr. Soc. 1993, 41, 38–41. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S.; Roeder, K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Methods Res. 2001, 29, 374–393. [Google Scholar] [CrossRef]
- Andruff, H.; Carraro, N.; Thompson, A.; Gaudreau, P.; Louvet, P. Latent class growth modelling: A tutorial. Tutor. Quant. Methods Psychol. 2009, 5, 11–24. [Google Scholar] [CrossRef]
- Geinitz, H.; Thamm, R.; Scholz, C.; Heinrich, C.; Prause, N.; Kerndl, S.; Keller, M.; Busch, R.; Molls, M.; Zimmermann, F.B. Longitudinal analysis of quality of life in patients receiving conformal radiation therapy for prostate cancer. Strahlenther Onkol. 2010, 186, 46–52. [Google Scholar] [CrossRef]
- Antonelli, A.; Palumbo, C.; Noale, M.; Artibani, W.; Bassi, P.; Bertoni, F.; Bracarda, S.; Bruni, A.; Corvò, R.; Gacci, M.; et al. Overview of potential determinants of radical prostatectomy versus radiation therapy in management of clinically localized prostate cancer: Results from an Italian, prospective, observational study (the Pros-IT CNR study). Minerva Urol. Nefrol. 2020, 72, 595–604. [Google Scholar] [CrossRef]
- Hamstra, D.A.; Conlon, A.S.; Daignault, S.; Dunn, R.L.; Sandler, H.M.; Hembroff, A.L.; Zietman, A.L.; Kaplan, I.; Ciezki, J.; Kuban, D.A.; et al. Multi-institutional prospective evaluation of bowel quality of life after prostate external beam radiation therapy identifies patient and treatment factors associated with patient-reported outcomes: The PROSTQA experience. Int J. Radiat. Oncol. Biol. Phys. 2013, 86, 546–553. [Google Scholar] [CrossRef]
- Parinaz, M.; Behzad, B.; Fatemeh, V.; Sousan, N. Functional response difference between diabetic/normal cancerous patients to inflammatory cytokines and oxidative stresses after radiotherapy. Rep. Pract. Oncol. Radiother 2020, 25, 730–737. [Google Scholar] [CrossRef]
- Özkan, E.E.; Erdemoğlu, E.; Raoufi, J. Turk Impact of diabetes on gastrointestinal and urinary toxicity after radiotherapy for gynecologic malignancy. J. Obstet. Gynecol. 2019, 16, 260–265. [Google Scholar] [CrossRef]
- Alashkham, A.; Paterson, C.; Hubbard, S.; Nabi, G. What is the impact of diabetes mellitus on radiation induced acute proctitis after radical radiotherapy for adenocarcinoma prostate? A prospective longitudinal study. Clin. Transl. Radiat. Oncol. 2017, 14, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.; Griffin, C.; Gulliford, S.; Syndikus, I.; Staffurth, J.; Panades, M.; Scrase, C.; Parker, C.; Khoo, V.; Dean, J.; et al. CHHiP Investigators A randomised assessment of image guided radiotherapy within a phase 3 trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2020, 142, 62–71. [Google Scholar] [CrossRef]
- Mangar, S.A.; Huddart, R.A.; Parker, C.C.; Dearnaley, D.P.; Khoo, V.S.; Horwich, A. Technological advances in radiotherapy for the treatment of localised prostate cancer. Eur. J. Cancer 2005, 41, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.T.; Xia, P.; Chan, L.W.; Park-Somers, E.; Roach, M., 3rd. Does imageguided radiotherapy improve toxicity profile in whole pelvic-treated high-risk prostate cancer? Comparison between IG-IMRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 53–60. [Google Scholar] [CrossRef] [PubMed]
- De Crevoisier, R.; Bayar, M.A.; Pommier, P.; Muracciole, X.; Pêne, F.; Dudouet, P.; Latorzeff, I.; Beckendorf, V.; Bachaud, J.-M.; Laplanche, A.; et al. Daily versus weekly prostate cancer image guided radiation therapy: Phase 3 multicenter randomised trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Tøndel, H.; Lund, J.-Å.; Lydersen, S.; Wanderås, A.D.; Aksnessæther, B.; Jensen, C.A.; Kaasa, S.; Solberg, A. Radiotherapy for prostate cancer—Does daily image guidance with tighter margins improve patient reported outcomes compared to weekly orthogonal verified irradiation? Results from a randomized controlled trial. Radiother Oncol. 2018, 126, 229–235. [Google Scholar] [CrossRef] [Green Version]


| Characteristics of Patients | Overall (n = 586) | Trajectory 1 (n = 60) | Trajectory 2 (n = 134) | Trajectory 3 (n = 392) | p-Value |
|---|---|---|---|---|---|
| Age at diagnosis, years, mean ± SD | 72.7 ± 5.5 | 71.9 ± 5.6 | 72.5 ± 6.3 | 72.9 ± 5.2 | 0.4244 |
| Education > lower secondary school, n (%) | 241 (42.0) | 24 (40.0) | 56 (42.1) | 161 (42.3) | 0.9468 |
| BMI ≥ 30 kg/m2, n (%) | 106 (18.8) | 14 (23.3) | 28 (21.4) | 64 (17.1) | 0.4072 |
| Current smoker, n (%) | 72 (12.5) | 7 (11.9) | 18 (13.7) | 47 (12.2) | 0.8922 |
| Diabetes mellitus, n (%) | 116 (19.9) | 15 (25.0) | 28 (21.1) | 73 (18.7) | 0.4887 |
| 3+ moderate/severe comorbidities *, n (%) | 109 (18.6) | 22 (36.7) | 29 (21.8) | 58 (14.8) | 0.0002 |
| Family history of prostate cancer, n (%) | 76 (13.3) | 7 (11.7) | 20 (15.0) | 49 (12.7) | 0.7363 |
| T staging at diagnosis, n (%) | 0.2689 | ||||
| T1 | 194 (34.8) | 15 (26.8) | 44 (35.8) | 135 (35.6) | |
| T2 | 266 (47.7) | 32 (57.2) | 63 (51.2) | 171 (45.1) | |
| T3 or T4 | 98 (17.6) | 9 (16.1) | 16 (13.0) | 73 (19.3) | |
| Gleason score at diagnosis, n (%) | 0.8773 | ||||
| ≤6 | 203 (35.0) | 23 (39.7) | 45 (33.6) | 135 (34.8) | |
| 3 + 4 | 151 (26.0) | 14 (24.1) | 34 (25.4) | 103 (26.6) | |
| 4 + 3 | 93 (16.0) | 11 (19.0) | 20 (14.9) | 62 (16.0) | |
| ≥8 | 133 (22.9) | 10 (17.2) | 35 (16.1) | 88 (22.7) | |
| PSA at diagnosis, ng/mL, median (Q1, Q3) | 7.9 (5.4, 11.5) | 8.4 (5.5, 10.9) | 8 (5.3, 12.4) | 7.8 (5.4, 11.3) | 0.2366 |
| D’Amico risk class, n (%) | 0.9117 | ||||
| Low | 80 (13.9) | 6 (10.3) | 20 (15.3) | 54 (14.0) | |
| Intermediate | 235 (40.8) | 26 (44.8) | 52 (39.7) | 157 (40.6) | |
| High | 261 (45.3) | 26 (44.8) | 59 (45.0) | 176 (45.4) | |
| RT method, n (%) | 0.0445 | ||||
| IGRT | 423 (74.7) | 38 (63.3) | 95 (72.0) | 290 (77.5) | |
| Non-IGRT | 143 (25.3) | 22 (36.7) | 37 (28.0) | 84 (22.5) | |
| RT technique, n (%) | 0.0006 | ||||
| 3D-CRT | 186 (32.8) | 28 (46.7) | 47 (35.6) | 111 (29.6) | |
| IMRT | 229 (40.4) | 18 (30.0) | 48 (36.4) | 163 (43.5) | |
| VMAT | 143 (25.2) | 14 (23.3) | 30 (22.7) | 99 (26.4) | |
| SBRT | 9 (1.5) | 0 (0.0) | 7 (5.3) | 2 (0.5) | |
| Volume treated with RT, n (%) | 0.4779 | ||||
| Prostate alone | 143 (25.9) | 14 (23.3) | 30 (23.4) | 99 (27.1) | |
| Prostate plus seminal vesicles | 324 (58.6) | 35 (58.3) | 83 (64.8) | 206 (56.4) | |
| Prostate, seminal vesicles and pelvic nodes | 86 (15.5) | 11 (18.3) | 15 (11.7) | 60 (16.4) | |
| Dose Gy, n (%) | 0.1798 | ||||
| <70 | 105 (21.3) | 7 (12.1) | 27 (22.9) | 71 (22.4) | |
| 70–75 | 210 (42.6) | 26 (44.8) | 43 (36.4) | 141 (44.5) | |
| >75 | 178 (36.1) | 25 (43.1) | 48 (40.7) | 105 (33.1) | |
| Association RT and ADT, n (%) | 0.3554 | ||||
| Only RT | 333 (57.9) | 41 (68.3) | 73 (54.5) | 227 (58.4) | |
| ADT before or during RT | 107 (18.6) | 10 (16.7) | 24 (17.9) | 73 (18.8) | |
| ADT after RT | 135 (23.5) | 9 (15.0) | 37 (27.6) | 89 (22.9) |
| Characteristics of Patients | Overall (n = 586) | Trajectory 1 (n = 170) | Trajectory 2 (n = 416) | p-Value |
|---|---|---|---|---|
| Age at diagnosis, years, mean ± SD | 72.7 ± 5.5 | 72.3 ± 6.0 | 72.5 ± 6.3 | 0.4543 |
| Education > lower secondary school, n (%) | 241 (42.0) | 79 (47.0) | 162 (39.9) | 0.1157 |
| BMI ≥ 30 kg/m2, n (%) | 106 (18.8) | 31 (18.5) | 75 (18.9) | 0.3255 |
| Current smoker, n (%) | 72 (12.5) | 18 (10.8) | 54 (13.3) | 0.4134 |
| Diabetes mellitus, n (%) | 116 (19.9) | 43 (25.0) | 73 (17.6) | 0.0321 |
| 3+ moderate/severe comorbidities *, n (%) | 109 (18.6) | 39 (22.9) | 70 (16.9) | 0.0867 |
| Family history of prostate cancer, n (%) | 76 (13.3) | 22 (12.9) | 20 (13.2) | 0.9406 |
| T staging at diagnosis, n (%) | 0.1748 | |||
| T1 | 194 (34.8) | 55 (33.7) | 139 (35.2) | |
| T2 | 266 (47.7) | 86 (52.8) | 180 (45.6) | |
| T3 or T4 | 98 (17.6) | 22 (13.5) | 76 (19.2) | |
| Gleason score at diagnosis, n (%) | 0.781 | |||
| ≤6 | 203 (35.0) | 53 (31.9) | 150 (36.2) | |
| 3 + 4 | 151 (26.0) | 44 (26.5) | 107 (25.9) | |
| 4 + 3 | 93 (16.0) | 29 (17.5) | 64 (15.5) | |
| ≥8 | 133 (22.9) | 40 (24.1) | 93 (22.5) | |
| PSA at diagnosis, ng/mL, median (Q1, Q3) | 7.9 (5.4, 11.5) | 7.8 (5.4, 11) | 7.9 (5.4, 11.8) | 0.8856 |
| D’Amico risk class, n (%) | 0.8596 | |||
| Low | 80 (13.9) | 21 (12.7) | 59 (14.4) | |
| Intermediate | 235 (40.8) | 69 (41.6) | 166 (40.5) | |
| High | 261 (45.3) | 76 (45.8) | 185 (45.1) | |
| RT method, n (%) | <0.0001 | |||
| IGRT | 423 (74.7) | 101 (61.6) | 322 (80.1) | |
| Non-IGRT | 143 (25.3) | 63 (38.4) | 80 (19.9) | |
| RT technique, n (%) | 0.1615 | |||
| 3D-CRT | 186 (32.8) | 61 (36.5) | 125 (31.3) | |
| IMRT | 229 (40.4) | 56 (33.5) | 173 (43.3) | |
| VMAT | 143 (25.2) | 48 (28.7) | 95 (23.8) | |
| SBRT | 9 (1.5) | 2 (1.2) | 7 (1.8) | |
| Volume treated with RT, n (%) | 0.3743 | |||
| Prostate alone | 143 (25.9) | 49 (29.9) | 94 (24.2) | |
| Prostate plus seminal vesicles | 324 (58.6) | 91 (55.5) | 233 (59.9) | |
| Prostate, seminal vesicles and pelvic nodes | 86 (15.5) | 24 (14.6) | 62 (15.9) | |
| Dose Gy, n (%) | 0.6125 | |||
| <70 | 105 (21.3) | 33 (22.0) | 72 (21.0) | |
| 70–75 | 210 (42.6) | 59 (39.3) | 151 (44.0) | |
| >75 | 178 (36.1) | 58 (38.7) | 120 (35.0) | |
| Association RT and ADT, n (%) | 0.8388 | |||
| Only RT | 333 (57.9) | 100 (58.8) | 241 (58.4) | |
| ADT before or during RT | 107 (18.6) | 33 (19.4) | 74 (17.9) | |
| ADT after RT | 135 (23.5) | 37 (21.8) | 98 (23.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noale, M.; Bruni, A.; Triggiani, L.; Buglione, M.; Bertoni, F.; Frassinelli, L.; Montironi, R.; Corvò, R.; Zagonel, V.; Porreca, A.; et al. Impact of Gastrointestinal Side Effects on Patients’ Reported Quality of Life Trajectories after Radiotherapy for Prostate Cancer: Data from the Prospective, Observational Pros-IT CNR Study. Cancers 2021, 13, 1479. https://doi.org/10.3390/cancers13061479
Noale M, Bruni A, Triggiani L, Buglione M, Bertoni F, Frassinelli L, Montironi R, Corvò R, Zagonel V, Porreca A, et al. Impact of Gastrointestinal Side Effects on Patients’ Reported Quality of Life Trajectories after Radiotherapy for Prostate Cancer: Data from the Prospective, Observational Pros-IT CNR Study. Cancers. 2021; 13(6):1479. https://doi.org/10.3390/cancers13061479
Chicago/Turabian StyleNoale, Marianna, Alessio Bruni, Luca Triggiani, Michela Buglione, Filippo Bertoni, Luca Frassinelli, Rodolfo Montironi, Renzo Corvò, Vittorina Zagonel, Angelo Porreca, and et al. 2021. "Impact of Gastrointestinal Side Effects on Patients’ Reported Quality of Life Trajectories after Radiotherapy for Prostate Cancer: Data from the Prospective, Observational Pros-IT CNR Study" Cancers 13, no. 6: 1479. https://doi.org/10.3390/cancers13061479







