Predicting the Risk of Metastases by PSMA-PET/CT—Evaluation of 335 Men with Treatment-Naïve Prostate Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
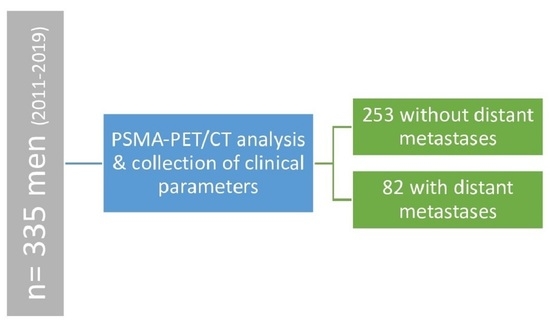
2.1. Study Design
2.2. Prostate-Specific Membrane Antigen-Positron Emission Tomography/Computed Tomography (PSMA PET/CT) Imaging and Image Evaluation
2.3. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Imaging Evaluation
3.3. Association between PSMA-PET/CT and the Presence of Metastases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Zagars, G.K.; Ayala, A.G.; von Eschenbach, A.C.; Pollack, A. The prognostic importance of Gleason grade in prostatic adenocarcinoma: A long-term follow-up study of 648 patients treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 237–245. [Google Scholar] [CrossRef]
- Bott, S.R.; Freeman, A.A.; Stenning, S.; Cohen, J.; Parkinson, M.C. Radical prostatectomy: Pathology findings in 1001 cases compared with other major series and over time. BJU Int. 2005, 95, 34–39. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Cowan, J.; Broering, J.M.; Carroll, P.R. High-risk prostate cancer in the United States, 1990-2007. World J. Urol. 2008, 26, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Eastham, J.A.; Cronin, A.M.; Fuks, Z.; Zhang, Z.; Yamada, Y.; Vickers, A.; Scardino, P.T. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: A comparison of clinical cohorts adjusted for case mix. J. Clin. Oncol. 2010, 28, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, S.A.; Hunt, D.; Sartor, A.O.; Pienta, K.J.; Gomella, L.; Grignon, D.; Rajan, R.; Kerlin, K.J.; Jones, C.U.; Dobelbower, M.; et al. A phase 3 trial of 2 years of androgen suppression and radiation therapy with or without adjuvant chemotherapy for high-risk prostate cancer: Final results of radiation therapy oncology group phase 3 randomized trial NRG oncology RTOG 9902. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 294–302. [Google Scholar] [CrossRef]
- Ciezki, J.P.; Weller, M.; Reddy, C.A.; Kittel, J.; Singh, H.; Tendulkar, R.; Stephans, K.L.; Ulchaker, J.; Angermeier, K.; Stephenson, A.; et al. A Comparison Between Low-Dose-Rate Brachytherapy With or Without Androgen Deprivation, External Beam Radiation Therapy With or Without Androgen Deprivation, and Radical Prostatectomy With or Without Adjuvant or Salvage Radiation Therapy for High-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 962–975. [Google Scholar] [CrossRef]
- Reichard, C.A.; Hoffman, K.E.; Tang, C.; Williams, S.B.; Allen, P.K.; Achim, M.F.; Kuban, D.A.; Chapin, B.F. Radical prostatectomy or radiotherapy for high- and very high-risk prostate cancer: A multidisciplinary prostate cancer clinic experience of patients eligible for either treatment. BJU Int. 2019, 124, 811–819. [Google Scholar] [CrossRef]
- Ahlgren, G.M.; Flodgren, P.; Tammela, T.L.J.; Kellokumpu-Lehtinen, P.; Borre, M.; Angelsen, A.; Iversen, J.R.; Sverrisdottir, A.; Jonsson, E.; Sengelov, L. Docetaxel Versus Surveillance After Radical Prostatectomy for High-risk Prostate Cancer: Results from the Prospective Randomised, Open-label Phase 3 Scandinavian Prostate Cancer Group 12 Trial. Eur. Urol. 2018, 73, 870–876. [Google Scholar] [CrossRef]
- Kellokumpu-Lehtinen, P.-L.; Hjälm-Eriksson, M.; Thellenberg-Karlsson, C.; Åström, L.; Franzen, L.; Fransson, A.-S.; Leskinen, M.J.; Zeke, M.; Huttunen, T.; Ginman, C. Docetaxel Versus Surveillance After Radical Radiotherapy for Intermediate-or High-risk Prostate Cancer—Results from the Prospective, Randomised, Open-label Phase III SPCG-13 Trial. Eur. Urol. 2019, 76, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Trover, J.K.; Beckett, M.L.; Wright Jr, G.L. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer 1995, 62, 552–558. [Google Scholar] [CrossRef]
- Budäus, L.; Leyh-Bannurah, S.-R.; Salomon, G.; Michl, U.; Heinzer, H.; Huland, H.; Graefen, M.; Steuber, T.; Rosenbaum, C. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur. Urol. 2016, 69, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA–PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef]
- Oh, S.W.; Cheon, G.J. Prostate-specific membrane antigen PET imaging in prostate cancer: Opportunities and challenges. Korean J. Radiol. 2018, 19, 819. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Fendler, W.P.; Rowe, S.P.; Calais, J.; Hofman, M.S.; Maurer, T.; Schwarzenboeck, S.M.; Kratowchil, C.; Herrmann, K.; Giesel, F.L. Prostate-specific membrane antigen ligands for imaging and therapy. J. Nucl. Med. 2017, 58, 67S–76S. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Forth, W. Allgemeine und Spezielle Pharmakologie und Toxikologie: Für Studenten der Medizin, Veterinärmedizin, Pharmazie, Chemie, und Biologie sowie für Ärzte, Tierärzte und Apotheker; mit 303 Tabellen; Elsevier, Urban&FischerVerlag: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Koerber, S.A.; Stach, G.; Kratochwil, C.; Haefner, M.F.; Rathke, H.; Herfarth, K.; Kopka, K.; Holland-Letz, T.; Choyke, P.L.; Haberkorn, U.; et al. Lymph Node Involvement in Treatment-Naïve Prostate Cancer Patients: Correlation of PSMA PET/CT Imaging and Roach Formula in 280 Men in Radiotherapeutic Management. J. Nucl. Med. 2019, 61, 46–50. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.; Hadaschik, B.; Holland-Letz, T.; Giesel, F.; Kratochwil, C.; Haufe, S. PET imaging with a [68 Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Wondergem, M.; van der Zant, F.M.; Knol, R.J.; Lazarenko, S.V.; Pruim, J.; de Jong, I.J. 18F-DCFPyL PET/CT in the detection of prostate cancer at 60 and 120 minutes: Detection rate, image quality, activity kinetics, and biodistribution. J. Nucl. Med. 2017, 58, 1797–1804. [Google Scholar] [CrossRef]
- Sperandei, S. Understanding logistic regression analysis. Biochem. Med. 2014, 24, 12–18. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Zelic, R.; Garmo, H.; Zugna, D.; Stattin, P.; Richiardi, L.; Akre, O.; Pettersson, A. Predicting prostate cancer death with different pretreatment risk stratification tools: A head-to-head comparison in a nationwide cohort study. Eur. Urol. 2020, 77, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Koerber, S.A.; Utzinger, M.T.; Kratochwil, C.; Kesch, C.; Haefner, M.F.; Katayama, S.; Mier, W.; Iagaru, A.H.; Herfarth, K.; Haberkorn, U. 68Ga-PSMA-11 PET/CT in newly diagnosed carcinoma of the prostate: Correlation of intraprostatic PSMA uptake with several clinical parameters. J. Nucl. Med. 2017, 58, 1943–1948. [Google Scholar] [CrossRef]
- Ried, K.; Tamanna, T.; Matthews, S.; Eng, P.; Sali, A. New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers. Front. Oncol. 2020, 10, 582. [Google Scholar] [CrossRef]
- Komek, H.; Can, C.; Yilmaz, U.; Altindag, S. Prognostic value of 68 Ga PSMA I&T PET/CT SUV parameters on survival outcome in advanced prostat cancer. Ann. Nucl. Med. 2018, 32, 542–552. [Google Scholar]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J. Nucl. Med. 2020, 61, 527–532. [Google Scholar] [CrossRef]
- Shetty, D.; Patel, D.; Le, K.; Bui, C.; Mansberg, R. Pitfalls in gallium-68 PSMA PET/CT interpretation—A pictorial review. Tomography 2018, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C. The diagnostic value of PET/CT imaging with the 68 Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 197–209. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Fendler, W.P.; Sommer, W.H.; Schwaiger, M.; Eiber, M. 68 Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jilg, C.A.; Drendel, V.; Rischke, H.C.; Beck, T.I.; Reichel, K.; Krönig, M.; Wetterauer, U.; Schultze-Seemann, W.; Meyer, P.T.; Vach, W. Detection rate of 18F-choline PET/CT and 68Ga-PSMA-HBED-CC PET/CT for prostate cancer lymph node metastases with direct link from PET to histopathology: Dependence on the size of tumor deposits in lymph nodes. J. Nucl. Med. 2019, 60, 971–977. [Google Scholar] [CrossRef] [PubMed]



| Total Number of Patients | n = 335 |
|---|---|
| age at PSMA-PET/CT (years), median (range) | 67 (38–84) |
| WHO grading, n = 326 | |
| 1 | 36 (10.7%) |
| 2 | 85 (25.4%) |
| 3 | 57 (17.0%) |
| 4 | 58 (17.3%) |
| 5 | 90 (26.9%) |
| PSA at initial diagnosis (ng/mL), median (range), n = 331 | 11 (1.2–511) |
| <10 | 153 (45.7%) |
| 10–20 | 84 (25.1%) |
| >20 | 94 (28.1%) |
| risk classification according d’Amico, n = 335 | |
| low risk | 15 (4.5%) |
| intermediate risk | 101 (30.1%) |
| high risk | 219 (65.4%) |
| PSMA-PET/CT tracer | |
| 68Ga-PSMA-11 | 272 (81.2%) |
| 18F-PSMA-1007 | 63 (18.8%) |
| N staging according to PSMA-PET/CT, n = 335 | |
| cN0 | 254 (75.8%) |
| cN1 | 81 (24.2%) |
| M staging according to PSMA-PET/CT, n = 335 | |
| cM0 | 253 (75.5%) |
| cM1a | 18 (5.4%) |
| cM1b | 39 (11.6%) |
| cM1c | 25(7.5%) |
| Total Number of Distant Metastases with PSMA-Uptake | n = 173 |
|---|---|
| extrapelvic nodal metastases | |
| total lesions | 60 (35.1%) |
| abdominal | 42 (70.0%) |
| Thoracic | 15 (25.0%) |
| Cervical | 1 (1.7%) |
| others | 2 (3.3%) |
| bone metastases | |
| total lesions | 103 (60.2%) |
| Pelvic | 43 (41.7%) |
| abdominal/thoracic | 49 (47.6%) |
| Extremities | 8 (7.8%) |
| head/cervical | 3 (2.9%) |
| organ metastases | |
| total lesions | 10 (5.7%) |
| Intrahepatic | 4 (40.0%) |
| Pulmonal | 4 (40.0%) |
| others | 2 (20.0%) |
| Variables | - | Regression Coefficient B | Standard Error | p-Value | Odds Ratio | 95% Confidence Intervals for Odds Ratio | |
|---|---|---|---|---|---|---|---|
| - | - | - | - | - | - | Lower value | Upper value |
| SUVMax | - | 0.026 | 0.009 | 0.004 | 1.026 | 1.006 | 1.046 |
| initial PSA | - | 0.008 | 0.004 | 0.051 | 1.008 | 1.000 | 1.016 |
| WHO 1 | - | - | - | 0.264 | - | - | - |
| - | WHO (1) | −1.374 | 0.819 | 0.093 | 0.253 | 0.054 | 1.344 |
| - | WHO (2) | −0.798 | 0.456 | 0.316 | 0.639 | 0.200 | 1.191 |
| - | WHO (3) | −0.448 | 0.446 | 0.316 | 0.639 | 0.252 | 1.479 |
| - | WHO (4) | −0.510 | 0.383 | 0.183 | 0.601 | 0.288 | 1.292 |
| d’Amico 1 | - | - | - | 0.476 | - | - | - |
| - | d’Amico (1) | −0,332 | 0.064 | 0.800 | 0.717 | 0.054 | 9.094 |
| - | d’Amico (2) | −0.559 | 0.459 | 0.224 | 0.572 | 0.204 | 1.280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koerber, S.A.; Boesch, J.; Kratochwil, C.; Schlampp, I.; Ristau, J.; Winter, E.; Zschaebitz, S.; Hofer, L.; Herfarth, K.; Kopka, K.; et al. Predicting the Risk of Metastases by PSMA-PET/CT—Evaluation of 335 Men with Treatment-Naïve Prostate Carcinoma. Cancers 2021, 13, 1508. https://doi.org/10.3390/cancers13071508
Koerber SA, Boesch J, Kratochwil C, Schlampp I, Ristau J, Winter E, Zschaebitz S, Hofer L, Herfarth K, Kopka K, et al. Predicting the Risk of Metastases by PSMA-PET/CT—Evaluation of 335 Men with Treatment-Naïve Prostate Carcinoma. Cancers. 2021; 13(7):1508. https://doi.org/10.3390/cancers13071508
Chicago/Turabian StyleKoerber, Stefan A., Johannes Boesch, Clemens Kratochwil, Ingmar Schlampp, Jonas Ristau, Erik Winter, Stefanie Zschaebitz, Luisa Hofer, Klaus Herfarth, Klaus Kopka, and et al. 2021. "Predicting the Risk of Metastases by PSMA-PET/CT—Evaluation of 335 Men with Treatment-Naïve Prostate Carcinoma" Cancers 13, no. 7: 1508. https://doi.org/10.3390/cancers13071508