Prospective Validation of Indocyanine Green Lymphangiography Staging of Breast Cancer-Related Lymphedema
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
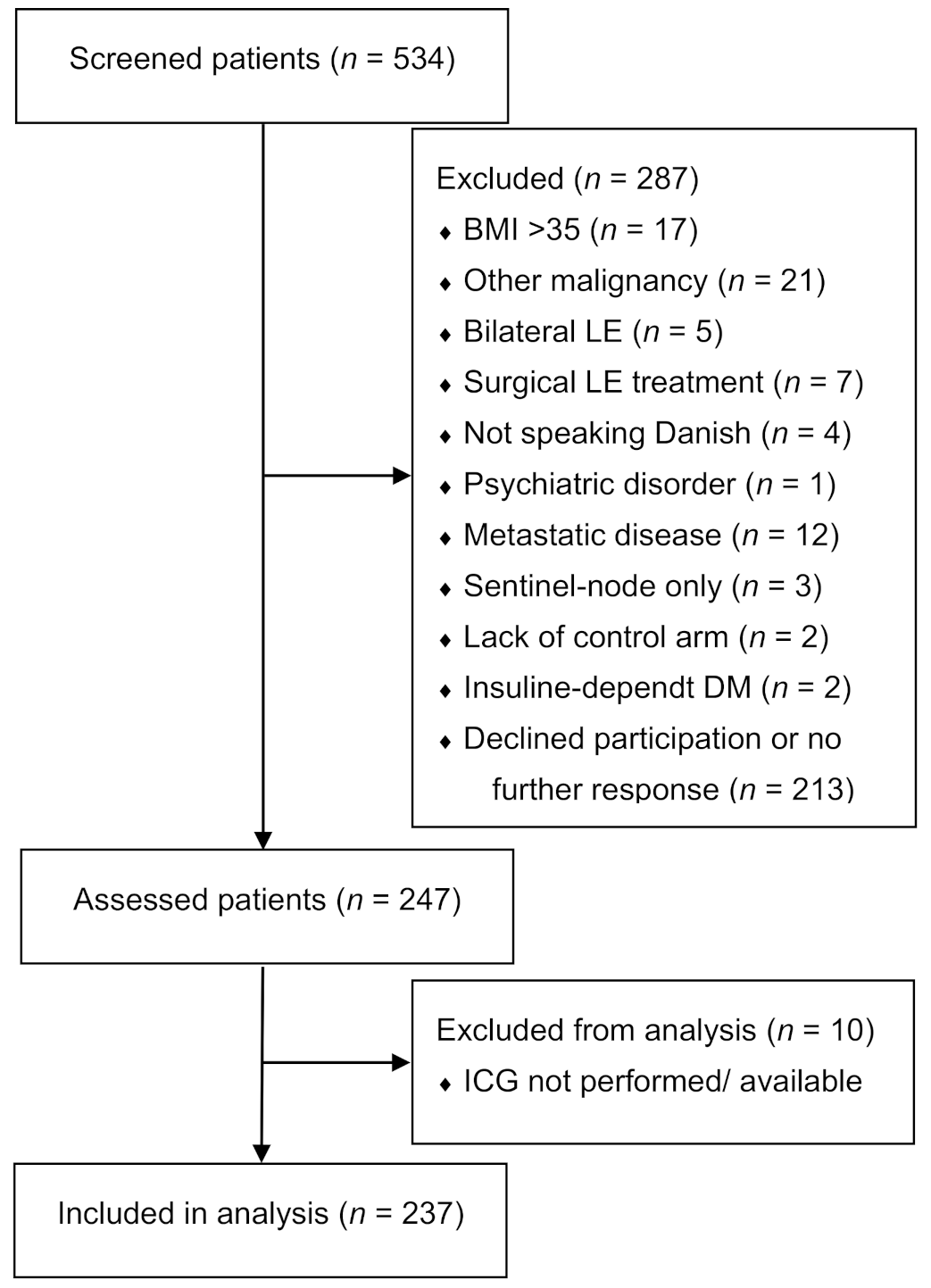
2.1. Participants
- Unilateral arm lymphedema diagnosed clinically by a lymphedema physiotherapist;
- Unilateral arm lymphedema previously treated with completed decongestive therapy by a lymphedema physiotherapist. This treatment consisted of manual lymphatic drainage, skincare, exercise, and bandaging at the lymphedema physiotherapist’s discretion. Following complete decongestive therapy, patients were fitted with a custom-made compression sleeve intended to be worn during the daytime. Patients with lymphedema affecting the hand were additionally fitted with a compression gauntlet. Severe lymphedema cases were also fitted with a night compression sleeve and/or treated by a pneumatic compression device;
- History of loco-regional breast cancer treated with axillary lymph node dissection;
- Recurrence-free and cancer-free for more than one year;
- Lymphedema for more than one year;
- No previous surgery for lymphedema;
- American Society of Anesthesiologists Classification 1 or 2;
- Body mass index (BMI) ≤ 35;
- Able to communicate in oral and written Danish;
- No history of other malignancy apart from breast cancer and non-melanoma skin cancer;
- Healthy contralateral arm for comparison;
- No insulin-dependent diabetes;
- No known hepatitis, HIV, or syphilis infection;
- No primary lymphedema or non-breast cancer-related lymphedema;
- No known allergy to iodine (contraindication for ICG lymphangiography).
2.2. Indocyanine Green Lymphangiography
2.3. Lymphedema Assessment
2.4. Validation Criteria and Standards
2.5. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, A.T.; Suami, H.; Hanasono, M.M.; Womack, V.A.; Wong, F.C.; Chang, E.I. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J. Surg. Oncol. 2017, 115, 84–89. [Google Scholar] [CrossRef]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef]
- Boyages, J.; Koelmeyer, L.A.; Suami, H.; Lam, T.; Ngo, Q.D.; Heydon-White, A.; Czerniec, S.; Munot, S.; Ho-Shon, K.; Mackie, H. The ALERT model of care for the assessment and personalized management of patients with lymphoedema. BJS 2020, 107, 238–247. [Google Scholar] [CrossRef]
- Rosian, K.; Stanak, M. Efficacy and safety assessment of lymphovenous anastomosis in patients with primary and secondary lymphoedema: A systematic review of prospective evidence. Microsurgery 2019, 39, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narushima, M.; Yamamoto, T.; Ogata, F.; Yoshimatsu, H.; Mihara, M.; Koshima, I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J. Reconstr. Microsurg. 2015, 32, 72–79. [Google Scholar] [PubMed]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine Green–Enhanced Lymphography for Upper Extremity Lymphedema. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Aso, K.; Tsukuura, R. Universal ICG lymphography stage for reproducible severity evaluation of extremity lymphedema. J. Plast. Reconstr. Aesthetic Surg. 2021. [Google Scholar] [CrossRef]
- Chen, W.F.; Zhao, H.; Yamamoto, T.; Hara, H.; Ding, J. Indocyanine Green Lymphographic Evidence of Surgical Efficacy Following Microsurgical and Supermicrosurgical Lymphedema Reconstructions. J. Reconstr. Microsurg. 2016, 32, 688–698. [Google Scholar]
- Wiser, I.; Mehrara, B.J.; Coriddi, M.; Kenworthy, E.; Cavalli, M.; Encarnacion, E.; Dayan, J.H. Preoperative Assessment of Upper Extremity Secondary Lymphedema. Cancers 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihara, M.; Hara, H.; Narushima, M.; Todokoro, T.; Iida, T.; Ohtsu, H.; Murai, N.; Koshima, I. Indocyanine green lymphography is superior to lymphoscintigraphy in imaging diagnosis of secondary lymphedema of the lower limbs. J. Vasc. Surg. Venous Lymphat. Disord. 2013, 1, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Garza, R.M.; Ooi, A.S.H.; Falk, J.; Chang, D.W. The Relationship between Clinical and Indocyanine Green Staging in Lymphedema. Lymphat. Res. Biol. 2019, 17, 329–333. [Google Scholar] [CrossRef]
- Suami, H.; Heydon-White, A.; Mackie, H.; Czerniec, S.; Koelmeyer, L.; Boyages, J. A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema. BMC Cancer 2019, 19, 985. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet (Lond. Eng.) 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Christiansen, P.; Ejlertsen, B.; Jensen, M.B.; Mouridsen, H. Danish breast cancer cooperative group. Clin. Epidemiol. 2016, 8, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Yamamoto, N.; Kikuchi, K.; Todokoro, T.; Iida, T.; Koshima, I. Dynamic Indocyanine Green (ICG) lymphography for breast cancer-related arm lymphedema. Ann. Plast. Surg. 2014, 73, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Brorson, H.; Höijer, P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J. Plast. Surg. Hand. Surg. 2012, 46, 410–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 consensus document of the international society of lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Akita, S.; Nakamura, R.; Yamamoto, N.; Tokumoto, H.; Ishigaki, T.; Yamaji, Y.; Sasahara, Y.; Kubota, Y.; Mitsukawa, N.; Satoh, K. Early Detection of Lymphatic Disorder and Treatment for Lymphedema following Breast Cancer. Plast. Reconstr. Surg. 2016, 138, 192e–202e. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Sørensen, J.A. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: A systematic review and meta-analysis. Microsurgery 2017, 38, 576–585. [Google Scholar] [CrossRef]
- Johnson, A.R.; Asban, A.; Granoff, M.D.; Kang, C.O.; Lee, B.T.; Chatterjee, A.; Singhal, D. Is Immediate Lymphatic Reconstruction Cost-effective? Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Furniss, D.; Narushima, M.; Iida, T.; Kikuchi, K.; Ohtsu, H.; Gennaro, P.; Gabriele, G.; Murai, N. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br. J. Surg. 2014, 101, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, A.R.; King, S.W.; Ramsden, A.J.; Furniss, D. Do surgical interventions for limb lymphoedema reduce cellulitis attack frequency? Microsurgery 2017, 37, 348–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Jindan, F.K.; Lin, C.-Y.; Cheng, M.-H. Comparison of Outcomes between Side-to-End and End-to-End Lymphovenous Anastomoses for Early-Grade Extremity Lymphedema. Plast. Reconstr. Surg. 2019, 144, 486–496. [Google Scholar] [CrossRef]
- Hara, H.; Mihara, M.; Seki, Y.; Todokoro, T.; Iida, T.; Koshima, I. Comparison of Indocyanine Green Lymphographic Findings with the Conditions of Collecting Lymphatic Vessels of Limbs in Patients with Lymphedema. Plast. Reconstr. Surg. 2013, 132, 1612–1618. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Hayashi, Y.; Narushima, M.; Yamamoto, T.; Todokoro, T.; Iida, T.; Sawamoto, N.; Araki, J.; Kikuchi, K.; et al. Pathological Steps of Cancer-Related Lymphedema: Histological Changes in the Collecting Lymphatic Vessels after Lymphadenectomy. PLoS ONE 2012, 7, e41126. [Google Scholar] [CrossRef]
- Thomis, S.; Dams, L.; Fourneau, I.; De Vrieze, T.; Nevelsteen, I.; Neven, P.; Gebruers, N.; Devoogdt, N. Correlation Between Clinical Assessment and Lymphofluoroscopy in Patients with Breast Cancer-Related Lymphedema: A Study of Concurrent Validity. Lymphat. Res. Biol. 2020, 18, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Tambour, M.; Holt, M.; Speyer, A.; Christensen, R.; Gram, B. Manual lymphatic drainage adds no further volume reduction to Complete Decongestive Therapy on breast cancer-related lymphoedema: A multicentre, randomised, single-blind trial. Br. J. Cancer 2018, 119, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Bains, S.K.; Peters, A.M.; Zammit, C.; Ryan, N.; Ballinger, J.; Glass, D.M.; Allen, S.; Stanton, A.W.; Mortimer, P.S.; Purushotham, A.D. Global abnormalities in lymphatic function following systemic therapy in patients with breast cancer. Br. J. Surg. 2015, 102, 534–540. [Google Scholar] [CrossRef]
- Pain, S.J.; Purushotham, A.D.; Barber, R.W.; Ballinger, J.R.; Solanki, C.K.; Mortimer, P.S.; Peters, A.M. Variation in lymphatic function may predispose to development of breast cancer-related lymphoedema. Eur. J. Surg. Oncol. 2004, 30, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Guilliod, R.; Fife, C.E.; Maus, E.A.; Smith, L.; Rasmussen, J.C.; Sevick-Muraca, E.M. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed. Opt. Express. 2012, 3, 1256–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnand, K.M.; Glass, D.M.; Mortimer, P.S.; Peters, A.M. Lymphatic Dysfunction in the Apparently Clinically Normal Contralateral Limbs of Patients with Unilateral Lower Limb Swelling. Clin. Nucl. Med. 2012, 37, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Pain, S.J.; Barber, R.W.; Ballinger, J.R.; Solanki, C.K.; Mortimer, P.S.; Purushotham, A.D.; Peters, A.M. Local vascular access of radioprotein injected subcutaneously in healthy subjects and patients with breast cancer-related lymphedema. J. Nucl. Med. 2004, 45, 789–796. [Google Scholar] [PubMed]
- Johnson, A.R.; Granoff, M.D.; Suami, H.; Lee, B.T.; Singhal, D. Real-Time Visualization of the Mascagni-Sappey Pathway Utilizing ICG Lymphography. Cancers 2020, 12, 1195. [Google Scholar] [CrossRef]
- Abbaci, M.; Conversano, A.; De Leeuw, F.; Laplace-Builhé, C.; Mazouni, C. Near-infrared fluorescence imaging for the prevention and management of breast cancer-related lymphedema: A systematic review. Eur. J. Surg. Oncol. 2019, 45, 1778–1786. [Google Scholar] [CrossRef]
- Groenlund, J.H.; Telinius, N.; Skov, S.N.; Hjortdal, V. A Validation Study of Near-Infrared Fluorescence Imaging of Lymphatic Vessels in Humans. Lymphat. Res. Biol. 2017, 15, 227–234. [Google Scholar] [CrossRef]
- Karges, J.R.; Mark, B.E.; Stikeleather, S.J.; Worrell, T.W. Concurrent validity of upper-extremity volume estimates: Comparison of calculated volume derived from girth measurements and water displacement volume. Phys. Ther. 2003, 83, 134–145. [Google Scholar] [CrossRef]
- De Sire, A.; Losco, L.; Cigna, E.; Lippi, L.; Gimigliano, F.; Gennari, A.; Cisari, C.; Chen, H.C.; Fusco, N.; Invernizzi, M. Three-dimensional laser scanning as a reliable and reproducible diagnostic tool in breast cancer related lymphedema rehabilitation: A proof-of-principle study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4476–4485. [Google Scholar]
- Beederman, M.; Garza, R.M.; Agarwal, S.; Chang, D.W. Outcomes for Physiologic Microsurgical Treatment of Secondary Lymphedema Involving the Extremity. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Yang, J.C.-S.; Wu, S.-C.; Chiang, M.-H.; Lin, W.-C.; Hsieh, C.-H. Intraoperative identification and definition of “functional” lymphatic collecting vessels for supermicrosurgical lymphatico-venous anastomosis in treating lymphedema patients. J. Surg. Oncol. 2018, 117, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Fleishman, A.; Tran, B.N.; Shillue, K.; Carroll, B.; Tsai, L.L.; Donohoe, K.J.; James, T.A.; Lee, B.T.; Singhal, D. Developing a Lymphatic Surgery Program. Plast. Reconstr. Surg. 2019, 144, 975e–985e. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-H.; Pappalardo, M.; Lin, C.-Y.C.; Kuo, C.-F.; Lin, C.-Y.C.; Chung, K.C. Validity of the Novel Taiwan Lymphoscintigraphy Staging and Correlation of Cheng Lymphedema Grading for Unilateral Extremity Lymphedema. Ann. Surg. 2018, 268, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Kajita, H.; Oh, A.; Urano, M.; Takemaru, M.; Imanishi, N.; Otaki, M.; Yagi, T.; Aiso, S.; Kishi, K. Photoacoustic lymphangiography. J. Surg. Oncol. 2020, 121, 48–50. [Google Scholar] [CrossRef] [Green Version]




| Stages | MD Anderson Scale | Arm Dermal Backflow Scale |
|---|---|---|
| Findings | ||
| Stage 0 | No dermal backflow | No dermal backflow |
| Stage 1 | Many patent lymphatics and minimal dermal backflow | Splash pattern around the axilla |
| Stage 2 | Moderate number of patent lymphatics and segmental dermal backflow | Stardust limited between olecranon and axilla |
| Stage 3 | Few patent lymphatics with extensive dermal backflow | Stardust distal to olecranon |
| Stage 4 | Dermal backflow involving the hand | Stardust involving the hand |
| Stage 5 | ICG does not move proximally to injection site | Diffuse and stardust pattern involving the entire limb |
| Variables | Data Distribution | All Patients (n = 237) |
|---|---|---|
| Age (years) | Mean ± SD | 59.68 ± 9.94 |
| In relationship (yes) | N (%) | 171 (27.85%) |
| Employed (yes) | N (%) | 140 (59.07%) |
| BMI (kg/m2) | Median (IQR) | 27.44 (7.27) |
| Breast cancer treatment | ||
| Radiation therapy (yes) | N (%) | 223 (94.09%) |
| Chemotherapy (yes) | N (%) | 199 (83.96%) |
| Endocrine therapy (yes) | N (%) | 191 (80.59%) |
| Mastectomy (yes) | N (%) | 122 (51.69%) |
| Post-mastectomy reconstruction (yes) | N (%) | 57 (46.72%) |
| Abdominal free flap (yes) | N (%) | 24 (42.11%) |
| Pedicled back flap (yes) | N (%) | 17 (29.82%) |
| Implant-based reconstruction (yes) | N (%) | 16 (28.07%) |
| Lymph nodes removed (No.) | Median (IQR) | 17 (8) |
| Lymphedema characteristics | ||
| Lymphedema latency (years) | Median (IQR) | 0.71(1.42) |
| Lymphedema duration (years) | Median (IQR) | 4.47(5.50) |
| Lymphedema duration: <2 years | N (%) | 45 (19.99%) |
| Lymphedema duration: 2–3 years | N (%) | 31 (13.08%) |
| Lymphedema duration: 3–4 years | N (%) | 28 (11.81%) |
| Lymphedema duration: 4–5 years | N (%) | 26 (10.97%) |
| Lymphedema duration: 5–6 years | N (%) | 12 (5.06%) |
| Lymphedema duration: 6–7 years | N (%) | 15 (6.33%) |
| Lymphedema duration: 7–8 years | N (%) | 18 (7.59%) |
| Lymphedema duration: 8–9 years | N (%) | 14 (5.91%) |
| Lymphedema duration: 9–10 years | N (%) | 39 (16.46%) |
| Lymphedema duration: >10 years | N (%) | 9 (3.80%) |
| Lymphedema volume (mL) | Mean ± SD | 410.51 ± 326.73 |
| Lymphedema volume (%) | Mean ± SD | 18.77 ± 14.06 |
| Lymphedema in dominant arm (yes) | N (%) | 114 (48.10%) |
| Previous episode of cellulitis (yes) | N (%) | 82 (34.60%) |
| Current lymphedema treatment | ||
| Compression sleeve (yes) | N (%) | 207 (87.34%) |
| Compression gauntlet (yes) | N (%) | 133 (56.12%) |
| Night compression (yes) | N (%) | 72 (30.38%) |
| Pneumatic compression device (yes) | N (%) | 44 (18.57%) |
| Assessment | Agreement (%) | Expected Agreement (%) | Kappa Value | Standard Error |
|---|---|---|---|---|
| MDA | ||||
| Interrater agreement | ||||
| R1–R2 | 93.25 | 27.92 | 0.90 | 0.04 |
| R1–R3 | 91.98 | 28.29 | 0.88 | 0.04 |
| R2–R3 | 87.76 | 28.10 | 0.82 | 0.04 |
| Intrarater agreement | ||||
| R2–R2 a | 88.61 | 28.15 | 0.84 | 0.04 |
| R3–R3 a | 95.78 | 28.94 | 0.94 | 0.04 |
| ADB | ||||
| Interrater agreement | ||||
| R1–R2 | 94.51 | 40.01 | 0.91 | 0.05 |
| R1–R3 | 89.45 | 41.31 | 0.82 | 0.05 |
| R2–R3 | 88.61 | 41.65 | 0.80 | 0.05 |
| Intrarater agreement | ||||
| R2–R2 a | 93.25 | 40.80 | 0.89 | 0.04 |
| R3–R3 a | 93.25 | 43.70 | 0.88 | 0.05 |
| Interscale agreement | ||||
| R1 | 77.22 | 31.54 | 0.66 | 0.04 |
| R2 | 75.95 | 31.28 | 0.65 | 0.04 |
| R3 | 77.22 | 32.56 | 0.66 | 0.04 |
| R2 a | 78.48 | 32.93 | 0.68 | 0.04 |
| R3 a | 77.64 | 33.70 | 0.66 | 0.04 |
| MDA Scale | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Data Distribution | Stage 0 (n = 11) | Stage 1 (n = 14) | Stage 2 (n = 42) | Stage 3 (n = 79) | Stage 4 (n = 85) | Stage 5 (n = 6) | Comparison p-Value a |
| Age (years) | Mean ± SD | 55.55 ± 9.01 | 58.50 ± 8.02 | 55.71 ± 9.74 | 61.85 ± 8.69 | 60.47 ± 10.65 | 57.83 ± 14.70 | <0.001 |
| BMI (kg/m2) | Median (IQR) | 26.40 (6.59) | 32.87 (8.81) | 26.62 (9.03) | 26.57 (6.11) | 28.81 (6.78) | 24.18 (17.98) | n.s |
| Employed (yes) | N (%) | 7 (63.64%) | 9 (64.29%) | 32 (76.19%) | 39 (49.37%) | 51 (60.00%) | 2 (33.33%) | <0.05 |
| Lymph nodes removed (No.) | Median (IQR) | 15 (6) | 17 (9) | 17 (6) | 17 (8) | 18 (8) | 13 (7) | n.s |
| Lymphedema latency (years) | Median (IQR) | 0.44 (1.00) | 0.43 (0.76) | 0.73 (0.83) | 0.75 (1.78) | 0.73 (1.45) | 0.29 (0.55) | n.s |
| Lymphedema duration (years) | Median (IQR) | 3.42 (6.08) | 3.71 (2.25) | 3.68 (5.95) | 4.84 (5.39) | 4.47 (6.48) | 7.21 (5.61) | <0.05 |
| Dominant arm affected (yes) | N (%) | 4 (36.36%) | 10 (71.43%) | 18 (42.86%) | 41 (51.90%) | 37 (43.53%) | 4 (66.67%) | n.s |
| Cellulitis (yes) | N (%) | 1 (9.09%) | 1 (7.14/) | 10 (23.81%) | 37 (46.84%) | 30 (35.29%) | 3 (50.00%) | <0.001 |
| ADB Scale | ||||||||
| Variables | Data Distribution | Stage 0 (n = 11) | Stage 1 (n = 0) | Stage 2 (n = 12) | Stage 3 (n = 123) | Stage 4 (n = 85) | Stage 5 (n = 0) | Comparison p-Value a |
| Age (years) | Mean ± SD | 55.55 ± 9.01 | N/A | 61.42 ± 10.55 | 59.41 ± 9.23 | 60.47 ± 10.65 | N/A | n.s |
| BMI (kg/m2) | Median (IQR) | 26.40 (6.59) | N/A | 33.96 (8.63) | 26.59 (6.86) | 28.81 (6.78) | N/A | n.s |
| Employed (yes) | N (%) | 7 (63.64%) | N/A | 5 (41.67%) | 75 (60.98%) | 51 (60.00%) | N/A | n.s |
| Lymph nodes removed (No.) | Median (IQR) | 15 (6) | N/A | 17(5) | 17(8) | 18(8) | N/A | n.s |
| Lymphedema latency (years) | Median (IQR) | 0.44 (1.00) | N/A | 0.37 (0.52) | 0.79 (1.60) | 0.73 (1.45) | N/A | <0.05 |
| Lymphedema duration (years) | Median (IQR) | 3.42 (6.08) | N/A | 4.00 (6.38) | 4.55 (5.12) | 4.47 (6.48) | N/A | n.s |
| Dominant arm affected (yes) | N (%) | 4 (36.36%) | N/A | 7 (58.33%) | 62 (50.41%) | 37 43.53%) | N/A | n.s |
| Cellulitis (yes) | N (%) | 1 (9.09%) | N/A | 1 (8.33%) | 47 (38.21%) | 30 (35.295) | N/A | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, M.G.; Toyserkani, N.M.; Hansen, F.C.G.; Thomsen, J.B.; Sørensen, J.A. Prospective Validation of Indocyanine Green Lymphangiography Staging of Breast Cancer-Related Lymphedema. Cancers 2021, 13, 1540. https://doi.org/10.3390/cancers13071540
Jørgensen MG, Toyserkani NM, Hansen FCG, Thomsen JB, Sørensen JA. Prospective Validation of Indocyanine Green Lymphangiography Staging of Breast Cancer-Related Lymphedema. Cancers. 2021; 13(7):1540. https://doi.org/10.3390/cancers13071540
Chicago/Turabian StyleJørgensen, Mads Gustaf, Navid Mohamadpour Toyserkani, Frederik Christopher Gulmark Hansen, Jørn Bo Thomsen, and Jens Ahm Sørensen. 2021. "Prospective Validation of Indocyanine Green Lymphangiography Staging of Breast Cancer-Related Lymphedema" Cancers 13, no. 7: 1540. https://doi.org/10.3390/cancers13071540
APA StyleJørgensen, M. G., Toyserkani, N. M., Hansen, F. C. G., Thomsen, J. B., & Sørensen, J. A. (2021). Prospective Validation of Indocyanine Green Lymphangiography Staging of Breast Cancer-Related Lymphedema. Cancers, 13(7), 1540. https://doi.org/10.3390/cancers13071540






