Flavonoids Alleviate Peripheral Neuropathy Induced by Anticancer Drugs
Abstract
:Simple Summary
Abstract
1. The Burden of CIPN and the Hope of Flavonoids
2. Materials and Methods
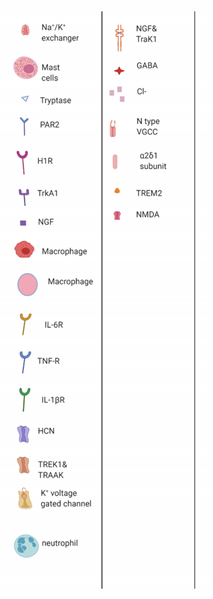
3. Flavonoids Counter the Effects of Anticancer Drugs at the Peripheral Nociceptor
3.1. General Effects of Anticancer Drugs and Flavonoids
3.2. Effects of Anticancer Drugs
3.2.1. Ion Channel Activation
3.2.2. Release of Proinflammatory Mediators
3.3. Flavonoids Counteract the Effects of Anticancer Drugs
3.3.1. Icariin, Trimethoxy- and Dimethoxyflavones
3.3.2. Quercetin
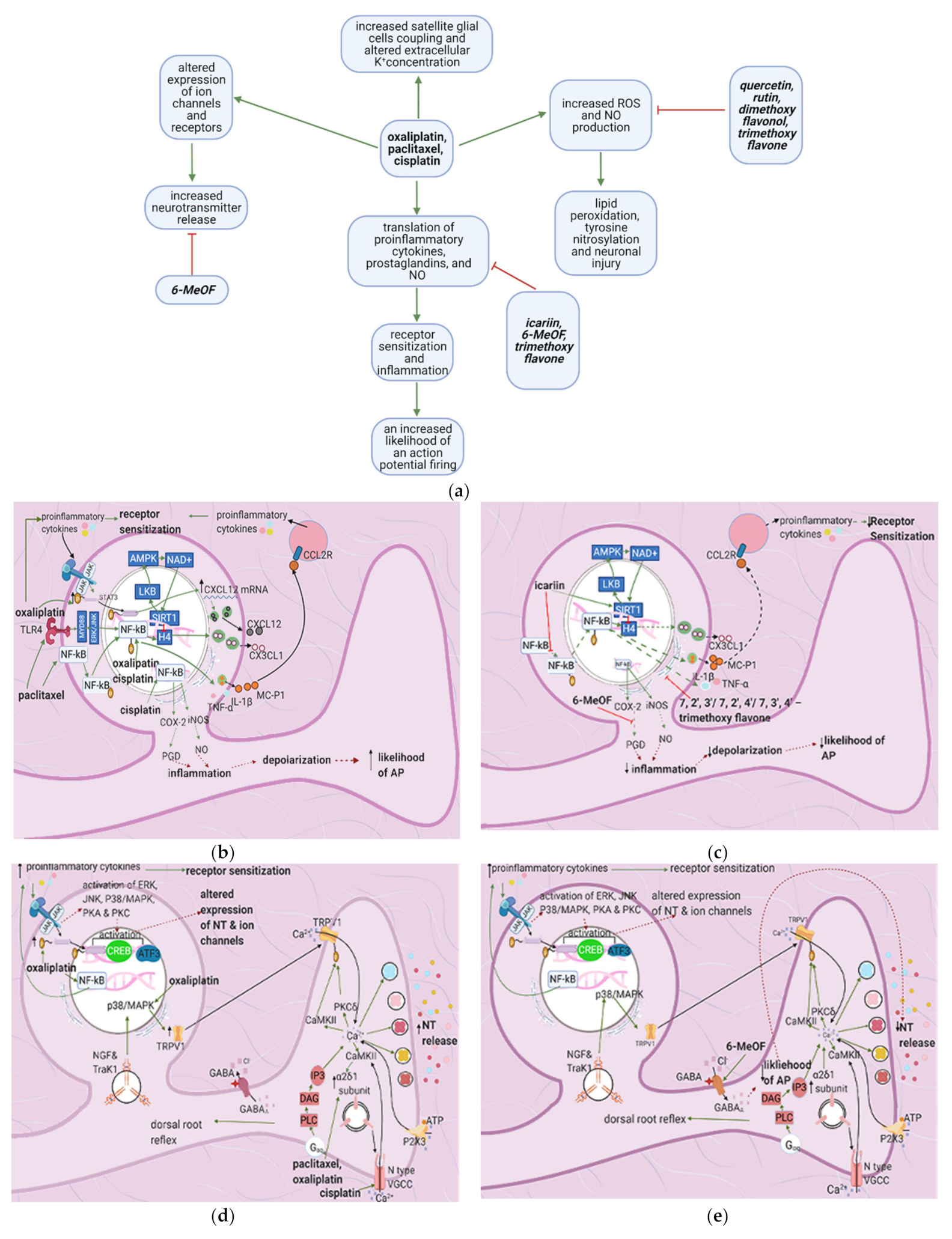
4. Flavonoids Counter the Effects of Anticancer Drugs at the Dorsal Root Ganglion
4.1. General Effects of Anticancer Drugs and Flavonoids
4.2. Effects of Anticancer Drugs
4.2.1. Upregulation of the NF-κB Pathway
4.2.2. Increase in Intracellular Ca2+
4.2.3. Increased GABA Release
4.2.4. Enhanced Activation of Satellite Glial Cells
4.2.5. Increased Oxidative Stress
4.3. Flavonoids Counteract the Effects of Anticancer Drugs
4.3.1. Icariin
4.3.2. Quercetin, Rutin, and Trimethoxy and Dimethoxy Flavones
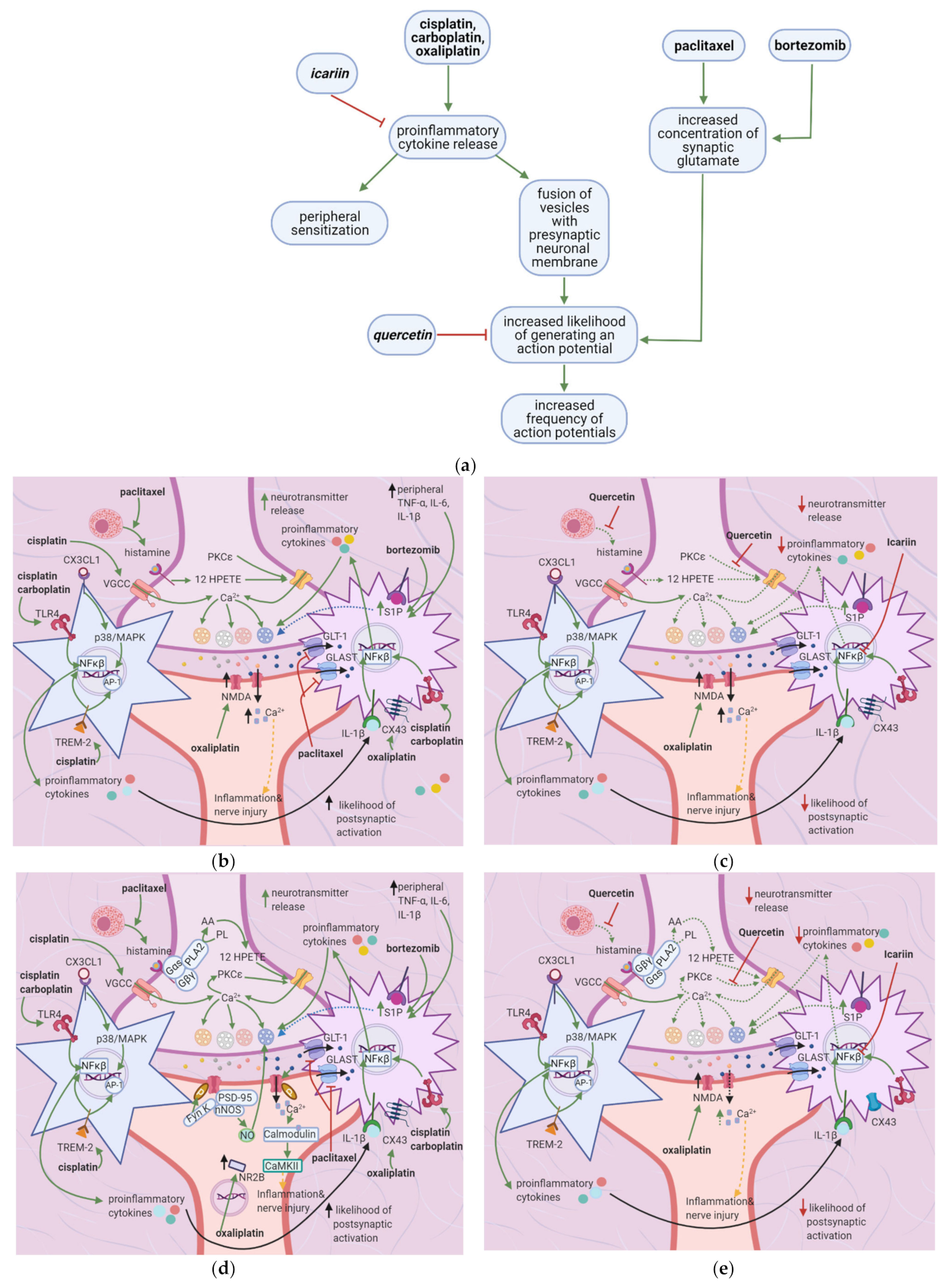
5. Flavonoids Counter the Effects of Anticancer Drugs at the Spinal Cord Dorsal Horn
5.1. General Effects of Anticancer Drugs and Flavonoids
5.2. Effects of Anticancer Drugs
5.3. Flavonoids Counter the Effects of Anticancer Drugs
Quercetin and Icariin
6. Flavonoids Counter the Effects of Anticancer Drugs on Astrocytes and Microglial Cells at the Spinal Cord Dorsal Horn
6.1. General Effects of Anticancer Drugs and Flavonoids
6.2. Effects of Anticancer Drugs
6.2.1. Upregulation of the S1P Pathway
6.2.2. Upregulation of the TLR4 Pathway
6.2.3. Increased Expression of Cx-43
6.2.4. Downregulation of Glutamate Uptake Receptors and Increased GFAP
6.2.5. Enhanced TREM2/DAP12 Signalling
6.2.6. Increased CX3CL1 Expression
6.3. The Flavonoids Icariin and Astragli Radix Counter the Effects of Anticancer Drugs
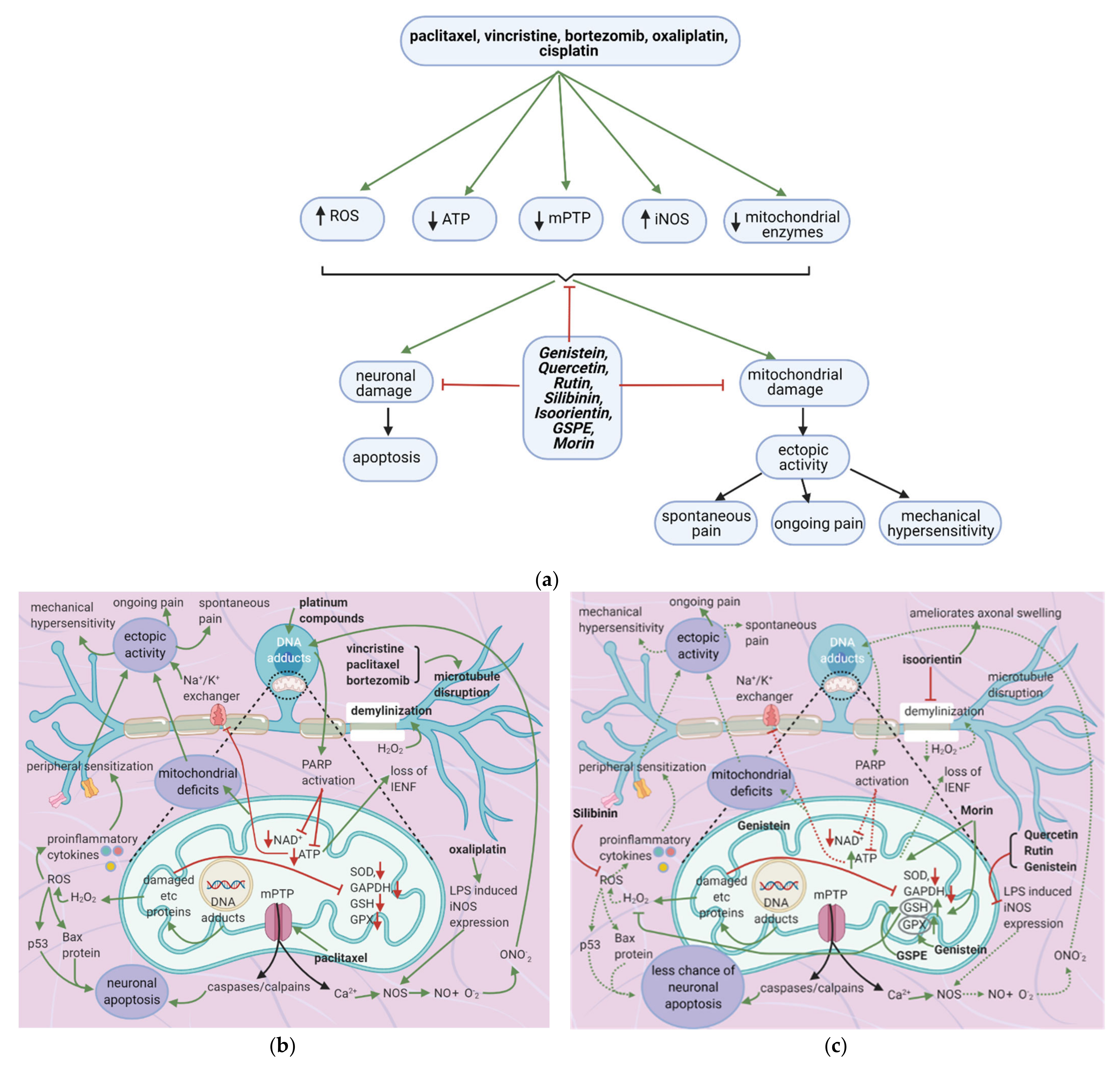
7. Flavonoids Counter Neuronal Injury Induced by Anticancer Drugs
7.1. General Effects of Anticancer Drugs and Flavonoids
7.2. Effects of Anticancer Drugs
7.2.1. Mitochondrial Damage
7.2.2. Neuronal Damage
7.2.3. Enhanced iNOS Expression
7.3. Flavonoids Counter the Effects of Anticancer Drugs
8. Flavonoids: Promise, Applications, and Side Effects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| GSPE | Grape Seed Proanthocyanidins |
| CCI | Chronic Constriction Injury |
| CIPN | Chemotherapy Induced Peripheral Neuropathy |
| SNI | Sciatic Nerve Injury |
| SNL | Sciatic Nerve Ligation |
| PNL | Partial Nerve Ligation |
| LPS | Lipopolysaccharide |
| iNOS | Inducible Nitric Oxide Synthase |
| MnSOD | Manganese Superoxide Dismutase |
| mPTP | Mitochondrial Permeability Transition Pore |
| GAPDH | Glyceraldehyde 3 phosphate Dehydrogenase |
| GSH | Glutathione |
| GPX | Glutathione Peroxidase |
| TLR | Toll Like Receptor |
| DRG | Dorsal Root Ganglion |
| MAP | Mitogen Activated Protein |
| GFAP | Glial Fibrillary Acidic Protein |
| S1P | Sphingosine-1-phosphate |
| HPETE | 5-hydroperoxyeicosatetraenoic acid |
| VGCC | Voltage Gated Calcium Channels |
| SGC | Satellite Glial Cells |
| ICW | Intracellular Calcium Waves |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| RCS | Reactive Carbon Species |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| SIRT1 | Sirtuin 1 |
| NT | Neurotransmitter |
| PAR | Protease Activated Receptor |
| NGF | Nerve Growth factor |
| LKB-1 | serine–threonine liver kinase B1 |

References
- Bubalo, J. Chemotherapy-Induced Peripheral Neuropathy. J. Hematol. Oncol. Pharm. 2014, 4. Available online: http://jhoponline.com/jhop-issue-archive/2014-issues/september-vol-4-no-3/16203-chemotherapy-induced-peripheral-neuropathy (accessed on 25 March 2021).
- American Cancer Society. What Is Peripheral Neuropathy? Available online: https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/peripheral-neuropathy/what-is-peripherial-neuropathy.html (accessed on 4 January 2021).
- Sałat, K. Chemotherapy-induced peripheral neuropathy: Part 1—current state of knowledge and perspectives for pharmacotherapy. Pharmacol. Rep. 2020, 72, 486–507. [Google Scholar] [CrossRef]
- Han, Y.; Smith, M.T.P. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013, 4, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, J.G.; Makker, P.G.; Tonkin, R.S.; Abdulla, M.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur. J. Cancer 2017, 73, 22–29. [Google Scholar] [CrossRef]
- Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [Green Version]
- Boland, E.G.; Selvarajah, D.; Hunter, M.; Ezaydi, Y.; Tesfaye, S.; Ahmedzai, S.H.; Snowden, J.A.; Wilkinson, I.D. Central Pain Processing in Chronic Chemotherapy-Induced Peripheral Neuropathy: A Functional Magnetic Resonance Imaging Study. PLoS ONE 2014, 9, e96474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Thornton, L.M.; Carson, W.E.; Shapiro, C.L.; Farrar, W.B.; Andersen, B.L. Delayed emotional recovery after taxane-based chemotherapy. Cancer 2008, 113, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.-T.; Wu, L.-M.; Lin, P.-C.; Juan, C.-H.; Huang, Y.-Y.; Chou, P.-L.; Chen, J.-L. Emotional distress and quality of life during folinic acid, fluorouracil, and oxaliplatin in colorectal cancer patients with and without chemotherapy-induced peripheral neuropathy: A cross-sectional study. Medicine 2020, 99, e19029. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Wang, D.; Matsushita, K.; Windham, B.G.; Selvin, E. Peripheral Neuropathy and All-Cause and Cardiovascular Mortality in U.S. Adults: A Prospective Cohort Study. Ann. Intern. Med. 2021, 174, 167–174. [Google Scholar] [CrossRef]
- American Cancer Society. Managing Peripheral Neuropathy. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/peripheral-neuropathy/managing-peripheral-neuropathy.html (accessed on 10 January 2021).
- Majithia, N.; Loprinzi, C.L.; Smith, T.J. New Practical Approaches to Chemotherapy-Induced Neuropathic Pain: Prevention, Assessment, and Treatment. Oncology 2016, 30, 1020–1029. [Google Scholar]
- Selvy, M.; Pereira, B.; Kerckhove, N.; Gonneau, C.; Feydel, G.; Pétorin, C.; Vimal-Baguet, A.; Melnikov, S.; Kullab, S.; Hebbar, M.; et al. Long-Term Prevalence of Sensory Chemotherapy-Induced Peripheral Neuropathy for 5 Years after Adjuvant FOLFOX Chemotherapy to Treat Colorectal Cancer: A Multicenter Cross-Sectional Study. J. Clin. Med. 2020, 9, 2400. [Google Scholar] [CrossRef]
- Uddin, S.; Al Mamun, A.; Rahman, A.; Kabir, T.; Alkahtani, S.; Alanazi, I.S.; Perveen, A.; Ashraf, G.M.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Exploring the Promise of Flavonoids to Combat Neuropathic Pain: From Molecular Mechanisms to Therapeutic Implications. Front. Neurosci. 2020, 14, 478. [Google Scholar] [CrossRef]
- Shahid, M.; Subhan, F.; Ahmad, N.; Sewell, R.D. The flavonoid 6-methoxyflavone allays cisplatin-induced neuropathic allodynia and hypoalgesia. Biomed. Pharmacother. 2017, 95, 1725–1733. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Líšková, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Smejkal, K.; Biringer, K.; Petras, M.; Blahutova, D.; et al. Implications of flavonoids as potential modulators of cancer neovascularity. J. Cancer Res. Clin. Oncol. 2020, 146, 3079–3096. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef]
- Basu, P.; Basu, A. In Vitro and In Vivo Effects of Flavonoids on Peripheral Neuropathic Pain. Molecules 2020, 25, 1171. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Qiu, H. Flavones and Flavonols: Phytochemistry and Biochemistry. Nat. Prod. 2013, 1821–1847. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Popa, D.-S.; Rusu, M.E. Isoflavones: Vegetable Sources, Biological Activity, and Analytical Methods for Their Assessment. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytotherapy Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliga, M.S.; Saxena, A.; Kaur, K.; Kalekhan, F.; Chacko, A.; Venkatesh, P.; Fayad, R. Polyphenols in the Prevention of Ulcerative Colitis: Past, Present and Future. In Polyphenols in Human Health and Disease, Ronald Ross Watson; Preedy, V.R., Zibadi, S., Eds.; Academic Press: New York, NY, USA, 2014; Volume 1, pp. 655–663. [Google Scholar]
- Meregalli, C.; Marjanovic, I.; Scali, C.; Monza, L.; Spinoni, N.; Galliani, C.; Brivio, R.; Chiorazzi, A.; Ballarini, E.; Rodriguez-Menendez, V.; et al. High-dose intravenous immunoglobulins reduce nerve macrophage infiltration and the severity of bortezomib-induced peripheral neurotoxicity in rats. J. Neuroinflamm. 2018, 15, 232. [Google Scholar] [CrossRef]
- Peters, C.M.; Jimenez-Andrade, J.M.; Jonas, B.M.; Sevcik, M.A.; Koewler, N.J.; Ghilardi, J.R.; Wong, G.Y.; Mantyh, P.W. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp. Neurol. 2007, 203, 42–54. [Google Scholar] [CrossRef]
- Nilius, B.; Gudermann, T.; Jahn, R.; Lill, R.; Offermanns, S.; Petersen, O.H. The TRPA1 Channel in Inflammatory and Neuropathic Pain and Migraine. In Reviews of Physiology, Biochemistry and Pharmacology; Nilius, B.; Gudermann, T.; Jahn, R.; Lill, R.; Offermanns, S.; Petersen, O.H. Springer International Publishing: Cham, Switzerland, 2014; Volume 167, pp. 1–43. [Google Scholar] [CrossRef]
- Materazzi, S.; Fusi, C.; Benemei, S.; Pedretti, P.; Patacchini, R.; Nilius, B.; Prenen, J.; Creminon, C.; Geppetti, P.; Nassini, R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflüger’s Archiv Die Gesammte Physiol. Menschen Tiere 2012, 463, 561–569. [Google Scholar] [CrossRef]
- Old, E.A.; Nadkarni, S.; Grist, J.; Gentry, C.; Bevan, S.; Kim, K.-W.; Mogg, A.J.; Perretti, M.; Malcangio, M. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J. Clin. Investig. 2014, 124, 2023–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yang, C.; Wang, Z. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011, 193, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.-R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors Are Interleukin-1 Sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef] [Green Version]
- Jin, X. Acute p38-Mediated Modulation of Tetrodotoxin-Resistant Sodium Channels in Mouse Sensory Neurons by Tumor Necrosis Factor. J. Neurosci. 2006, 26, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Oprée, A.; Kress, M. Involvement of the Proinflammatory Cytokines Tumor Necrosis Factor-α, IL-1β, and IL-6 But Not IL-8 in the Development of Heat Hyperalgesia: Effects on Heat-Evoked Calcitonin Gene-Related Peptide Release from Rat Skin. J. Neurosci. 2000, 20, 6289–6293. [Google Scholar] [CrossRef]
- Chukyo, A.; Chiba, T.; Kambe, T.; Yamamoto, K.; Kawakami, K.; Taguchi, K.; Abe, K. Oxaliplatin-induced changes in expression of transient receptor potential channels in the dorsal root ganglion as a neuropathic mechanism for cold hypersensitivity. Neuropeptides 2018, 67, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Descoeur, J.; Pereira, V.; Pizzoccaro, A.; Francois, A.; Ling, B.; Maffre, V.; Couette, B.; Busserolles, J.; Courteix, C.; Noel, J.; et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011, 3, 266–278. [Google Scholar] [CrossRef]
- Gao, W.; Zan, Y.; Wang, Z.-J.J.; Hu, X.-Y.; Huang, F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol. Sin. 2016, 37, 1166–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, Y.; Zhang, J.; Chen, L.; Duan, S.; Tang, J.; Xu, W.; Li, A. Icariin, a flavonoid with anti-cancer effects, alleviated paclitaxel-induced neuropathic pain in a SIRT1-dependent manner. Mol. Pain 2018, 14. [Google Scholar] [CrossRef]
- Nadipelly, J.; Sayeli, V.; Kadhirvelu, P.; Shanmugasundaram, J.; Cheriyan, B.V.; Subramanian, V. Effect of certain trimethoxy flavones on paclitaxel—induced peripheral neuropathy in mice. Integr. Med. Res. 2018, 7, 159–167. [Google Scholar] [CrossRef]
- Sayeli, V.; Nadipelly, J.; Kadhirvelu, P.; Cheriyan, B.V.; Shanmugasundaram, J.; Subramanian, V. Effect of flavonol and its dimethoxy derivatives on paclitaxel-induced peripheral neuropathy in mice. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 525–535. [Google Scholar] [CrossRef]
- Youk, J.; Kim, Y.-S.; Lim, J.-A.; Shin, D.-Y.; Koh, Y.; Lee, S.-T.; Kim, I. Depletion of nerve growth factor in chemotherapy-induced peripheral neuropathy associated with hematologic malignancies. PLoS ONE 2017, 12, e0183491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Huang, Z.-Z.; Ling, Y.-Z.; Wei, J.-Y.; Cui, Y.; Zhang, X.-Z.; Zhu, H.-Q.; Xin, W.-J. Up-regulation of CX3CL1 via Nuclear Factor-κB–dependent Histone Acetylation Is Involved in Paclitaxel-induced Peripheral Neuropathy. Anesthesiology 2015, 122, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Illias, A.M.; Gist, A.C.; Zhang, H.; Kosturakis, A.K.; Dougherty, P.M. Chemokine CCL2 and its receptor CCR2 in the dorsal root ganglion contribute to oxaliplatin-induced mechanical hypersensitivity. Pain 2018, 159, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Subhan, F.; Shahid, M.; Wadood, A.; Shahbaz, N.; Farooq, U.; Ayaz, M.; Raziq, N. 6-Methoxyflavanone abates cisplatin-induced neuropathic pain apropos anti-inflammatory mechanisms: A behavioral and molecular simulation study. Eur. J. Pharmacol. 2020, 872, 172972. [Google Scholar] [CrossRef]
- Leo, M.; Schmitt, L.-I.; Erkel, M.; Melnikova, M.; Thomale, J.; Hagenacker, T. Cisplatin-induced neuropathic pain is mediated by upregulation of N-type voltage-gated calcium channels in dorsal root ganglion neurons. Exp. Neurol. 2017, 288, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Schmitt, L.-I.; Jastrow, H.; Thomale, J.; Kleinschnitz, C.; Hagenacker, T. Cisplatin alters the function and expression of N-type voltage-gated calcium channels in the absence of morphological damage of sensory neurons. Mol. Pain 2017, 13. [Google Scholar] [CrossRef]
- Xiao, W.; Boroujerdi, A.; Bennett, G.J.; Luo, Z.D. Chemotherapy-evoked painful peripheral neuropathy: Analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience 2007, 144, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Tsuboi, M.; Kambe, T.; Abe, K.; Nakatani, Y.; Kawakami, K.; Utsunomiya, I.; Taguchi, K. Oxaliplatin administration increases expression of the voltage-dependent calcium channel α2δ-1 subunit in the rat spinal cord. J. Pharmacol. Sci. 2016, 130, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef]
- Willis, W.D., Jr. Dorsal root potentials and dorsal root reflexes: A double-edged sword. Exp. Brain Res. 1999, 124, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, R.; Cherkas, P.S.; Hanani, M. Peripheral inflammation upregulates P2X receptor expression in satellite glial cells of mouse trigeminal ganglia: A calcium imaging study. Neuropharmacology 2011, 61, 739–746. [Google Scholar] [CrossRef]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.C.; Wong, D.V.T.; Lima-Júnior, R.C.P.; Ribeiro, R.D.A.; Vale, M.L. The Antioxidant Effects of the Flavonoids Rutin and Quercetin Inhibit Oxaliplatin-Induced Chronic Painful Peripheral Neuropathy. Mol. Pain 2013, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Inoue, M.; Hald, A.; Xie, W.; Ueda, H. Inhibition of Paclitaxel-Induced A-Fiber Hypersensitization by Gabapentin. J. Pharmacol. Exp. Ther. 2006, 318, 735–740. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Li, H.; Liu, Z.-L.; Li, Q.; Qiu, H.-W.; Zeng, L.-J.; Yang, W.; Zhang, X.-Z.; Li, Z.-Y. Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Mol. Pain 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.-S.; Tao, R.; Zhang, J.; Liu, L.; Jiang, Y.-H.; Ma, S.-H.; Song, L.-X.; Xia, L.-J. Upregulation of CX3CL1 mediated by NF-κB activation in dorsal root ganglion contributes to peripheral sensitization and chronic pain induced by oxaliplatin administration. Mol. Pain 2017, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, H.; Zhang, H.; Kosturakis, A.K.; Jawad, A.B.; Dougherty, P.M. Toll-Like Receptor 4 Signaling Contributes to Paclitaxel-Induced Peripheral Neuropathy. J. Pain 2014, 15, 712–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philipps, G.R.; Chiemprasert, T. Limited Hydrolysis of tRNA by Phosphodiesterase. Hoppe-Seyler´s Zeitschrift Physiol. Chem. 1975, 356, 1097–1104. [Google Scholar] [CrossRef]
- Warwick, R.; Hanani, M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur. J. Pain 2012, 17, 571–580. [Google Scholar] [CrossRef]
- Schmitt, L.-I.; Leo, M.; Kutritz, A.; Kleinschnitz, C.; Hagenacker, T. Activation and functional modulation of satellite glial cells by oxaliplatin lead to hyperexcitability of sensory neurons in vitro. Mol. Cell. Neurosci. 2020, 105, 103499. [Google Scholar] [CrossRef]
- Park, H.J.; Stokes, J.A.; Corr, M.; Yaksh, T.L. Toll-like receptor signaling regulates cisplatin-induced mechanical allodynia in mice. Cancer Chemother. Pharmacol. 2013, 73, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.-Y.; Robinson, C.R.; Zhang, H.; Dougherty, P.M. Spinal Astrocyte Gap Junctions Contribute to Oxaliplatin-Induced Mechanical Hypersensitivity. J. Pain 2013, 14, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yoon, S.-Y.; Zhang, H.; Dougherty, P.M. Evidence That Spinal Astrocytes but Not Microglia Contribute to the Pathogenesis of Paclitaxel-Induced Painful Neuropathy. J. Pain 2012, 13, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Stockstill, K.; Doyle, T.M.; Yan, X.; Chen, Z.; Janes, K.; Little, J.W.; Braden, K.; Lauro, F.; Giancotti, L.A.; Harada, C.M.; et al. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J. Exp. Med. 2018, 215, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-Y.; Zhou, Y.; Cui, W.-Q.; Hu, X.-M.; Du, L.-X.; Mi, W.-L.; Chu, Y.-X.; Wu, G.-C.; Wang, Y.-Q.; Mao-Ying, Q.-L. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain, Behav. Immun. 2018, 68, 132–145. [Google Scholar] [CrossRef]
- Konishi, H.; Kiyama, H. Microglial TREM2/DAP12 Signaling: A Double-Edged Sword in Neural Diseases. Front. Cell. Neurosci. 2018, 12, 206. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-Z.; Li, D.; Ou-Yang, H.-D.; Liu, C.-C.; Liu, X.-G.; Ma, C.; Wei, J.-Y.; Liu, Y.; Xin, W.-J. Cerebrospinal Fluid Oxaliplatin Contributes to the Acute Pain Induced by Systemic Administration of Oxaliplatin. Anesthesiology 2016, 124, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Li, Y.; Liu, X.; Gong, D.; Zheng, W. Notch activation enhances microglial CX3CR1/P38 MAPK pathway in rats model of vincristine-induced peripheral neuropathy. Neurosci. Lett. 2020, 715, 134624. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, L.D.C.; Pacini, A.; Micheli, L.; Femia, A.P.; Maresca, M.; Zanardelli, M.; Vannacci, A.; Gallo, E.; Bilia, A.R.; Caderni, G.; et al. Astragali radix: Could it be an adjuvant for oxaliplatin-induced neuropathy? Sci. Rep. 2017, 7, srep42021. [Google Scholar] [CrossRef] [Green Version]
- Cata, J.; Weng, H.-R.; Chen, J.-H.; Dougherty, P. Altered discharges of spinal wide dynamic range neurons and down-regulation of glutamate transporter expression in rats with paclitaxel-induced hyperalgesia. Neuroscience 2006, 138, 329–338. [Google Scholar] [CrossRef]
- Weng, H.-R.; Aravindan, N.; Cata, J.P.; Chen, J.-H.; Shaw, A.D.; Dougherty, P.M. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci. Lett. 2005, 386, 18–22. [Google Scholar] [CrossRef]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free. Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef] [Green Version]
- Komirishetty, P.; Areti, A.; Sistla, R.; Kumar, A. Morin Mitigates Chronic Constriction Injury (CCI)-Induced Peripheral Neuropathy by Inhibiting Oxidative Stress Induced PARP Over-Activation and Neuroinflammation. Neurochem. Res. 2016, 41, 2029–2042. [Google Scholar] [CrossRef]
- Kaur, G.; Bedi, O.; Sharma, N.; Singh, S.; Deshmukh, R.; Kumar, P. Anti-hyperalgesic and anti-nociceptive potentials of standardized grape seed proanthocyanidin extract against CCI-induced neuropathic pain in rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, N.; Zhu, C.; Ma, L.; Yang, J.; Du, J.; Zhang, W.; Sun, T.; Niu, J.; Yu, J. Antinociceptive effect of isoorientin against neuropathic pain induced by the chronic constriction injury of the sciatic nerve in mice. Int. Immunopharmacol. 2019, 75, 105753. [Google Scholar] [CrossRef]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Sacerdote, P.; Trovato, A.E.; Colleoni, M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: Anti-inflammatory and antioxidant activity. J. Neurochem. 2008, 107, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. Targeting Microtubules for Cancer Chemotherapy. Curr. Med. Chem. Agents 2005, 5, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Staff, N.P.; Podratz, J.L.; Grassner, L.; Bader, M.; Paz, J.; Knight, A.M.; Loprinzi, C.L.; Trushina, E.; Windebank, A.J. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. NeuroToxicology 2013, 39, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Fukada, I.; Ito, Y.; Kobayashi, K.; Shibayama, T.; Takahashi, S.; Horii, R.; Akiyama, F.; Iwase, T.; Ohno, S. The early onset of peripheral neuropathy might be a robust predictor for time to treatment failure in patients with metastatic breast cancer receiving chemotherapy containing paclitaxel. PLoS ONE 2017, 12, e0184322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, E.S.; Windebank, A.J. Cisplatin-Induced Apoptosis of DRG Neurons Involves Bax Redistribution and Cytochrome cRelease But Not fas Receptor Signaling. Neurobiol. Dis. 2002, 9, 220–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ta, L.E.; Schmelzer, J.D.; Bieber, A.J.; Loprinzi, C.L.; Sieck, G.C.; Brederson, J.D.; Low, P.A.; Windebank, A.J. A Novel and Selective Poly (ADP-Ribose) Polymerase Inhibitor Ameliorates Chemotherapy-Induced Painful Neuropathy. PLoS ONE 2013, 8, e54161. [Google Scholar] [CrossRef] [Green Version]
- Jaggi, A.S.; Singh, N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012, 291, 1–9. [Google Scholar] [CrossRef]
- Canta, A.; Pozzi, E.; Carozzi, V.A. Mitochondrial Dysfunction in Chemotherapy-Induced Peripheral Neuropathy (CIPN). Toxics 2015, 3, 198–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-Induced Neuropathy: Oxidative Stress as Pathological Mechanism. Protective Effect of Silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janes, K.; Doyle, T.; Bryant, L.; Esposito, E.; Cuzzocrea, S.; Ryerse, J.; Bennett, G.J.; Salvemini, D. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain 2013, 154, 2432–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flatters, S.J.; Bennett, G.J. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain 2006, 122, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, S.G.; Kleckner, I.R.; Barton, D.; Mustian, K.; O’Mara, A.; Germain, D.S.; Cavaletti, G.; Danhauer, S.C.; Hershman, D.L.; Hohmann, A.G.; et al. The National Cancer Institute Clinical Trials Planning Meeting for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. J. Natl. Cancer Inst. 2019, 111, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, I.R.; Zhang, J.; Touroutoglou, A.; Chanes, L.; Xia, C.; Simmons, W.K.; Quigley, K.S.; Dickerson, B.C.; Barrett, L.F. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 2017, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Egert, S.; Rimbach, G. Which Sources of Flavonoids: Complex Diets or Dietary Supplements? Adv. Nutr. 2011, 2, 8–14. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [CrossRef]
- Anghileri, L.J.; Thouvenot, P. Natural Polyphenols-Iron Interaction: Its Biological Importance. Biol. Trace Element Res. 2000, 73, 251–258. [Google Scholar] [CrossRef]
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Heal. Perspect. 2002, 110, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Milerová, J.; Čerovská, J.; Zamrazil, V.; Bilek, R.; Lapčík, O.; Hampl, R. Actual levels of soy phytoestrogens in children correlate with thyroid laboratory parameters. Clin. Chem. Lab. Med. 2006, 44, 171–174. [Google Scholar] [CrossRef]
- Walle, U.K.; Walle, T. Induction of Human UDP-Glucuronosyltransferase UGT1A1 by Flavonoids—Structural Requirements. Drug Metab. Dispos. 2002, 30, 564–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miniscalco, A.; Lundahl, J.; Regårdh, C.G.; Edgar, B.; Eriksson, U.G. Inhibition of dihydropyridine metabolism in rat and human liver microsomes by flavonoids found in grapefruit juice. J. Pharmacol. Exp. Ther. 1992, 261, 1195–1199. [Google Scholar]
- Cermak, R.; Wein, S.; Wolffram, S.; Langguth, P. Effects of the flavonol quercetin on the bioavailability of simvastatin in pigs. Eur. J. Pharm. Sci. 2009, 38, 519–524. [Google Scholar] [CrossRef] [PubMed]
- De Wet, H.; McIntosh, D.B.; Conseil, G.; Baubichon-Cortay, H.; Krell, T.; Jault, J.-M.; Daskiewicz, J.-B.; Barron, D.; Di Pietro, A. Sequence requirements of the ATP-binding site within the C-terminal nucleotide-binding domain of mouse P-glycoprotein: Structure-activity relationships for flavonoid binding. Biochemistry 2001, 40, 10382–10391. [Google Scholar] [CrossRef] [PubMed]
- Hooijberg, J.H.; Broxterman, H.J.; Heijn, M.; Fles, D.; Lankelma, J.; Pinedo, H.M. Modulation by (iso)flavonoids of the ATPase activity of the multidrug resistance protein. FEBS Lett. 1997, 413, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Cermak, R. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2007, 4, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietaryflavonoids: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 2019, 120, 18357–18369. [Google Scholar] [CrossRef]
- Wong, M.-Y.; Chiu, G.N. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 834–840. [Google Scholar] [CrossRef]
- Mongiovi, J.M.; Zirpoli, G.R.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; Unger, J.M.; Moore, H.C.F.; Stewart, J.A.; Isaacs, C.; Hobday, T.J.; et al. Associations between self-reported diet during treatment and chemotherapy-induced peripheral neuropathy in a cooperative group trial (S0221). Breast Cancer Res. 2018, 20, 146. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Hershman, D.L.; Shi, Z.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Kushi, L.H. BMI, Lifestyle Factors and Taxane-Induced Neuropathy in Breast Cancer Patients: The Pathways Study. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [Green Version]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef]
- Bonhof, C.S.; Van De Poll-Franse, L.V.; Vissers, P.A.; Wasowicz, D.K.; Wegdam, J.A.; Révész, D.; Vreugdenhil, G.; Mols, F.; Poll-Franse, L.V. Anxiety and depression mediate the association between chemotherapy-induced peripheral neuropathy and fatigue: Results from the population-based PROFILES registry. Psycho-Oncology 2019, 28, 1926–1933. [Google Scholar] [CrossRef] [Green Version]
- Chtourou, Y.; Gargouri, B.; Kebieche, M.; Fetoui, H. Naringin Abrogates Cisplatin-Induced Cognitive Deficits and Cholinergic Dysfunction Through the Down-Regulation of AChE Expression and iNOS Signaling Pathways in Hippocampus of Aged Rats. J. Mol. Neurosci. 2015, 56, 349–362. [Google Scholar] [CrossRef]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; De Sousa, D.P. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxidative Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Velde, M.E.; Kaspers, G.J.L.; Abbink, F.C.H.; Twisk, J.W.R.; Van Der Sluis, I.M.; Bos, C.V.D.; Heuvel-Eibrink, M.M.V.D.; Segers, H.; Chantrain, C.; Bosch, J.V.D.W.T.; et al. Vincristine-Induced Peripheral Neuropathy in Pediatric Oncology: A Randomized Controlled Trial Comparing Push Injections with One-Hour Infusions (The VINCA Trial). Cancers 2020, 12, 3745. [Google Scholar] [CrossRef] [PubMed]






| Mechanism of CIPNP | Neuropathic Pain Model | Mode of Administration/Concentration | Animal Model | Reference |
|---|---|---|---|---|
| Increased macrophage infiltration | Paclitaxel and bortezomib induced | (in vitro) IV bortezomib 0.2 mg/kg, 3 times a week for 8 weeks (in vitro) 2 doses of 18 mg/kg paclitaxel given 3 days apart | Female Wistar rats Adult Male Sprague Dawley Rats | [30,31] |
| Sensitization of TRPA1 via increased production of ROS, RNS, and RCS. | Oxaliplatin induced Cisplatin induced Paclitaxel induced | Intraperitoneal/3 mg/kg Intravenous/ 2 mg/kg Intraperitoneal/3 times per week for 5 weeks (2 mg/kg) Intraperitoneal/6 mg/kg | Male Dunkin-Hartley guinea pigs, male Sprague-Dawley rats, male C57BL/6 mice, wild-type (Trpa1+/+) or TRPA1-deficient mice (Trpa1–/–) Male C57BL/6 mice, wild-type (Trpa1 +/+), or TRPA1-deficient mice (Trpa1 –/–) | [32,33] |
| Increased expression of FKN, which binds to CX3CR1, increasing ROS production and enhancing trafficking of macrophages to the sciatic nerve. ROS activated TRPA1, evoking pain. | Vincristine induced | Intraperitoneal/0.5 mg/kg for two 5 day cycles | Adult male and female C57BL/6 J mice | [34] |
| Increased production of tryptase, which cleaves PAR2, causing increases in PKC∈ and PKA. PKA sensitizes TRPV1, TRPV4 and TRPA1, whereas PKC ∈ sensitizes TRPV1 and TRPV4. | Paclitaxel induced | Intraperitoneal/ Four doses of 1 mg/kg every two days | Male ICR mice | [35] |
| IL-1β increases TTXR Na+ currents via the p38/MAPK pathway. | Isolated DRG cells | IL-1β (10 ng/mL) applied using multibarrel fast drug delivery system | Male Sprague-Dawley Rats | [36] |
| TNF- α increases TTXR Na+ currents via the p38/MAPK pathway | Isolated DRG cells | (in vitro) recombinant murine TNF-α (50 µg/mL) solution (in vivo) 1ng of TNFα- in 10 µL injected into rat hind paw plantar surface | ICR adult male mice | [37] |
| Application of IL-1β, TNF-α, and IL-6 on peripheral nociceptors dose-dependently led to cGRP release | Incubated skin flaps | (in vitro) murine TNF-α (0.05–500 ng), murine IL-1β (0.02–200 ng), human IL-8 (0.1 ng to 1 μg), mIL-6 (0.02–200 ng) | Male Wistar rats | [38] |
| Increased expression of TRPA1 and TRPV1 in small sized DRG neurons and TRPM8 in medium sized DRG neurons. | Oxaliplatin induced | (in vitro) Intraperitoneal One dose of 6 mg/kg | Male Wistar rats | [39] |
| Increased expression of HCN and decreased expression of TREK1 and TRAAK channels. | Oxaliplatin induced | (in vitro) Intraperitoneal 3 injections (1,3,6 mg/kg) | Male C57BL6J mice | [40] |
| Flavonoid | Neuropathic Pain Model | Animal Model | Flavonoid Concentration | Mechanism-based Intervention | Effect on Neuropathy | Reference |
|---|---|---|---|---|---|---|
| Quercetin | Paclitaxel induced | Adult male Sprague-Dawley rats and Institute of Cancer Research mice | 3, 10 and 30 μmol/L (in vitro) Intragastric administration of 20 mg/kg or 60 mg/kg once per day for 40 days for rats and 12 days for mice (in vivo) | Inhibited degranulation of mast cells, PKC epsilon translocation from the cytoplasm to the cell membrane | Dose-dependent increase of thermal hyperalgesia and mechanical allodynia thresholds | [41] |
| Icariin | Paclitaxel induced | 3 to 4 month old male Sprague Dawley Rats | (in vitro and in vivo) 25, 50,100 mg/kg | Reduction of IL-1 β, TNF-α and IL-6 release from the DRG, astrocytes, and microglia | Decreased mechanical allodynia and spinal neuroinflammation | [42] |
| Trimethoxy flavones | Paclitaxel induced | Adult swiss Albino mice of either sex | (in vitro and in vivo) 25, 50, 100 or 200 mg/kg | Concentration-dependent decrease of IL-1β, TNF-α, and free radicals | Dose-dependent decrease of tactile allodynia, thermal hyperalgesia, and cold allodynia | [43] |
| Dimethoxy flavones | Paclitaxel induced | Male Swiss Albino Mice | (in vitro and in vivo) 25, 50, 100 or 200 mg/kg | Concentration-dependent decrease of IL-1β, TNF-α, and free radicals | Dose-dependent decrease of tactile allodynia, thermal hyperalgesia and cold allodynia | [44] |
| Mechanism of CIPNP | Neuropathic Pain Model | Mode of Administration/ Concentration | Animal | Reference |
|---|---|---|---|---|
| Increased VGCC current density in DRG neurons via CaMKII; Increased VGCC protein levels | Cisplatin induced | 5 mg/kg (in vivo), 0.5 μM and 5 μM (in vitro) | Male and female Wistar rats for in vitro procedures. Male Sprague-Dawley rats for in vivo procedures | [49] |
| Upregulation of VGCC α2δ1 subunit in DRG | Paclitaxel induced | IP/4 mg/kg single injection; 4 mg/kg administered 4 times on alternate days Intravenous/ 4 mg/kg single injection | Male ddY mice | [57] |
| Increased phosphorylation of STAT3; increased levels of CXCL12 mRNA and protein | Oxaliplatin induced | IP/5 injections of 4 mg/kg each, administered on consecutive days | Male Sprague–Dawley rats | [58] |
| Upregulation of p65 mediated CX3CL1 expression in DRG | Oxaliplatin induced | Intraperitoneal/ 5 injections of 4 mg/kg each, administered on consecutive days | Male Sprague-Dawley rats | [59] |
| Downregulation of SIRT1 expression and an increase in histone acetylation; induction of NF-κB(p65) activation and nuclear translocation; upregulation of proinflammatory factors (TNF-α, IL-1b, IL-6 ); Activation of astrocytes | Paclitaxel induced | IP/8 mg/kg per day for 3 consecutive days | Male Sprague Dawley rats | [42,43] |
| increased lipid peroxidation and protein nitrosylation; increased inducible nitric oxide synthase. | Oxaliplatin induced | IV/1 mg/kg dose twice a week (total of nine injections). | Male Swiss mice | [56] |
| Increased TNF-α, IL-1β, DPPH, and NO. | Paclitaxel induced | IP/A single dose (10 mg/kg) | Male Swiss albino mice | [44] |
| Stimulates COX-2 expression | Cisplatin induced | IP/3 mg/kg once a week for four consecutive weeks. | Male Sprague-Dawley rats | [48] |
| TLR4 signaling in the spinal cord dorsal horn and DRG induces and maintains CIPN | Paclitaxel induced | IP/4 injections of 2 mg/kg administered every other day | Male Sprague-Dawley rats | [60] |
| Upregulation of CX3CL1 via NF-κB–dependent H4 acetylation | Paclitaxel induced | IP/3 injections of 8 mg/kg, on 3 alternate days | Male Sprague-Dawley rats | [46] |
| Increased expression of CCL2/CCR2 leading to innate immune response | Oxaliplatin induced | IP/1 injection of 3mg/kg | Male Sprague-Dawley rats | [47] |
| Increased the expression of TRPV1 | Oxaliplatin induced | IP/I injection of 6 mg/kg | Male Wistar rats | [39] |
| Increased VGCC expression mediated by CaMKII | Oxaliplatin induced. | In vitro and in vivo w/ variant conc | Wistar rats | [61] |
| Increased gap-junctional coupling among SGCs; increased GFAP production | Taxol and Oxaliplatin induced | Oxaliplatin–IP/ 2 injections of 4 mg/kg–3 days apart. Taxol- IP/ 2 injections of 18 mg/kg–3 days apart | Balb/c mice | [62] |
| increased ROS, GFAP, and Cx-43 decreased Kir4.1 channels | Oxaliplatin induced | in vitro/ 1 and 10 μM for 2,4 and 24 h. | [63] | |
| Peripheral neuropathic pain associated with increase in ∝2δ1 subunit in spinal cord | Paclitaxel induced Oxaliplatin induced | In vitro/Intraperitoneal 2 mg/kg paclitaxel on 4 alternate days In vitro/ 6 mg/kg oxaliplatin intraperitoneal | Adult male Sprague Dawley Rats Male Wistar rats | [51,52] |
| Increased N-type VGCC density in small DRG neurons | Cisplatin induced | In vitro/0.5 μM and 5 μM incubated for 24 or 48 h. | Male and female Wistar rats | [49] |
| Flavonoid | Neuropathic Pain Model | Animal | Mode of Administration/ Concentration of Flavonoid | Mechanism-Based Intervention | Effect on Neuropathy | Reference |
|---|---|---|---|---|---|---|
| Icariin | Paclitaxel induced | Male Sprague Dawley rats | IG/25, 50, 100 mg/kg. | Activated SIRT1 via histone acetylation; Prevented NF-κB(p65) phosphorylation and nuclear translocation; prevented the production of TNF-α, IL-6, and IL-1β; Suppressed astrocyte activation. | Alleviated mechanical allodynia. (100 mg/kg in the long term) and spinal neuroinflammation. | [42] |
| Rutin and quercetin | Oxaliplatin | Male Swiss mice | IP/ rutin or quercetin (25, 50, and 100 mg/kg) 30 min before every oxaliplatin injection (1 mg/kg). | Decreased Fos expression; Decreased nitrotyrosine and iNos expression, and lipid peroxidation. | Inhibited the decrease in mechanical (a.d) and cold nociceptive threshold. Prevented the shrinkage of dorsal horn neurons | [56] |
| 7, 2′, 3′/7,2′, 4′/–,7,3′,4′/7, 5,4′–trimethoxy flavone | Paclitaxel | Male and female adult Swiss albino mice | SC injection/ 25, 50, 100 and 200 mg/kg. | Inhibition of TNF–α, IL–1β (d.d). Scavenging DPPH; Preventing NO generation (d.d) | Alleviated tactile allodynia, cold allodynia and thermal hyperalgesia in mice | [43] |
| 3′,4′/6,3′/7,2′/7,3′-dimethoxy flavonol | Palcitaxel | Male Swiss albino mice | SC/25, 50, 100, and 200 mg/kg | Decreased TNF-α, IL-1β (d.d); Scavenged DPPH and NO (d.d) | Improved tactile allodynia, cold allodynia and thermal hyperalgesia(d.d) | [44] |
| 6-Methoxyflavone | Cisplatin | Male Sprague-Dawley rats | Intraperitoneal/25, 50 and 75 mg/kg Also conducted in silico and in vitro studies | Inhibits COX-2; Stimulates GABAA channels | Improved static and dynamic allodynia | [48] |
| Mechanism of CIPN | Neuropathic Pain Model | Mode of Administration/Concentration | Animal | Reference |
|---|---|---|---|---|
| Strong TREM2/DAP12 signaling continuously activated microglial cells, which resulted in neuropathic pain. | Cisplatin induced | Intraperitoneal/Accumulated dose of 23 mg/kg delivered in 2 rounds daily for 5 days with a 5 day break between rounds. (in vitro and in vivo) | Adult male mice, 9–10 weeks old | [68] |
| Oxaliplatin upregulates spinal CX3CLI, causing central sensitization and acute CIPN | Oxaliplatin induced | Intraperitoneal/single dose of 4 mg/kg (in vitro and in vivo) | Male Sprague Dawley rats | [70] |
| Increased S1P, S1PR1, and dihydro-S1P due to dysregulated sphingolipid metabolism | Bortezomib induced | (in vitro and in vivo) | Male Sprague Dawley rats, S1pr1 knockout and knockdown mice | [67] |
| Downregulation of GLAST and GLT-1 on astrocyte membranes | Paclitaxel induced | Intraperitoneal/4 injections of 2 mg/kg every other day (in vitro) | Adult Male Sprague Dawley Rats, 8–10 weeks old | [66] |
| Upregulation of CX43 gap junctional proteins in astrocytes in the spinal cord dorsal horn | Oxaliplatin induced | Intraperitoneal/4 injections of 2mg/kg each given every other day. (in vitro and in vivo) | Male Sprague Dawley rats | [65] |
| Flavonoid | Neuropathic Pain Model | Animal | Mode of Administration/ Concentration of Flavonoid | Mechanism-based Intervention | Effect on Neuropathy | Reference |
|---|---|---|---|---|---|---|
| Quercetin | Paclitaxel induced | adult male Sprague-Dawley rats and mice | 3, 10 and 30 μmol/L (in vitro) Intragsteral administration of 20 mg/kg or 60 mg/kg once per day for 40 days for rats and 12 days for mice (in vivo) | Inhibited degranulation of mast cells and membrane translocation of PKC epsilon | Dose dependent increase of thermal hyperalgesia and mechanical allodynia thresholds | [41] |
| Icariin | Paclitaxel induced | Male Sprague Dawley rats | IG/25, 50, 100 mg/kg. | Suppressed GFAP and astrocyte production of TNF-α, IL-1b, and IL-6. | Alleviated mechanical allodynia (100 mg/kg in the long term) and spinal neuroinflammation. | [42] |
| Mechanism of CIPNP | Neuropathic Pain Model | Mode of Administration/ Concentration | Animal | Reference |
|---|---|---|---|---|
| Increased sphingosine metabolism and consequently increased ceramide, DH-S1P, and SIP. Increased TNF-α and IL-1β in blood plasma | Bortezomib induced | Intraperitoneal/total 1 mg/kg over 5 consecutive days (0.2 mg/kg per day) and Intraperitoneal/0.4 mg/kg every other day 3 times a week for 4 weeks | Male Sprague Dawley rats and GFAP-Cre breeder mice | [67] |
| Upregulated TREM 2 ligand and thus increased TREM2/DAP12 complex signaling, leading to the activation of microglial cells | Cisplatin induced | Intraperitoneal/ 23mg/kg spread over 2 rounds of 5 consecutive days with a 5day break | Adult male mice | [68] |
| Increase in the astrocyte-specific gap junctional protein CX43 in the spinal cord, leading to enhanced astrocyte activation. | Oxaliplatin induced | Intraperitoneal/4 injections of 2 mg/kg each, every other day | male Sprague-Dawley rats | [65] |
| Downregulation of GLAST and GLT-1 in the spinal cord dorsal horn led to excessive activation of postsynaptic AMPA and NMDA receptors | Paclitaxel induced | Intraperitoneal/1 mg/kg per day for 4 consecutive days Intraperitoneal/1.0 mg/kg on 4 alternate days, total 4 mg/kgIntraperitoneal/2 mg/kg every other day for total 4 injections | male Sprague–Dawley ratsadult male Sprague–Dawley rats Adult male Sprague-Dawley rats | [66,74,75] |
| TLR4, through MYD 88 and TRIF, plays an integral role in nociceptive signaling. | Cisplatin induced | Intraperitoneal/ Six injections of 2.3 mg/kg given every other day | Wild type C57BL/6 mice; Tlr3–/–, Tlr4–/–, and Myd88–/– mice | [64] |
| TLR4 signaling in the spinal cord dorsal horn and DRG induces and maintains CIPN | Paclitaxel induced | Intraperitoneal/4 injections of 2 mg/kg administered every other day | Male Sprague-Dawley rats | [60] |
| Flavonoid | Neuropathic Pain Model | Animal | Mode of Administration/Concentration of Flavonoid | Mechanism-based Intervention | Effect on Neuropathy | Reference |
|---|---|---|---|---|---|---|
| Icariin | Paclitaxel induced | Male Sprague Dawley rats | IG/25, 50, 100 mg/kg | Suppressed GFAP and astrocyte production of TNF-α, IL-1β, and IL-6. | Alleviated mechanical allodynia (100 mg/kg in the long term) and spinal neuroinflammation. | [42] |
| Astragali radix | Oxaliplatin-induced neurotoxicity | Male Sprague Dawley rats | 50% hydroalcoholic extracts of Astragali radix | Numerical reduction of astrocytes within the dorsal horns (demonstrated by GFAP immunohistochemistry) | Reduction of Oxaliplatin-induced molecular and morphometric alterations in peripheral nerve and dorsal-root ganglia. Decrease in the activation of microglia and astrocytes | [73] |
| Mechanism of CIPNP | Neuropathic Pain Model | Mode of Administration/Concentration | Animal | Reference |
|---|---|---|---|---|
| Downregulation of MnSOD through post translational nitration by peroxynitrite. | Paclitaxel, oxaliplatin, bortezomib | Intraperitoneal; Paclitaxel: 4 doses delivered on alternate days, cumulative dose of 8 mg/kg Oxaliplatin: 10 mg/kg delivered over 5 consecutive days Bortezomib: 1 mg/kg delivered over 5 consecutive days | Male Sprague Dawley rats | [91] |
| Formation of DNA adducts | Cisplatin | 2 microgram/ml for 48 h (in vitro) | Harlan–Sprague–Dawley rats and wild type background mice (C57BL/6J) | [85] |
| Increased permeability of mPTP | Paclitaxel | Intraperitoneal/ 1 ml/kg on 4 alternate days (in vitro and in vivo) | Adult male Sprague–Dawley rats | [92] |
| Increased LPS-induced iNOS expression | Oxaliplatin | i.v./nine injections of 1 mg/kg each given twice a week (in vitro and in vivo) | male Swiss mice | [56] |
| Flavonoid | Neuropathic Pain Model | Animal | Mode of Administration/Concentration of Flavonoid | Mechanism Based Intervention | Effect on Neuropathy | Reference |
|---|---|---|---|---|---|---|
| Genistein | Chronic constriction sciatic nerve injury | C57BL/6J male mice | Subcutaneously/once a day for 11 days at doses of 1,3,7.5,15,30 mg/kg (in vitro and in vivo) | Restoration of mitochondrial GPX levels, and reduction of LPS-induced iNOS production | Reversal of mechanical allodynia and thermal hyperalgesia in a time and dose-dependent manner | [81] |
| Morin | Chronic constriction injury | Male Sprague-Dawley rats | Oral/30 mg/kg for 14 days (in vitro and in vivo) | Reduced PARP overactivation and nitrite levels. Restored ATP and glutathione levels; repaired DNA damage | Reversed mechanical, chemical, and thermal hyperalgesia | [78] |
| Isoorientin | Chronic constriction injury | Adult, male specific pathogen free mice from ICR | Intragastric/7.5, 15 or 30 mg/kg per day | Ameliorated axonal swelling; prevented demyelination | Reduced hyperalgesia and allodynia | [80] |
| Quercetin and rutin | Oxaliplatin induced | male Swiss mice | i.v./nine injections of rutin (25, 50, and 100 mg/kg) or quercetin (25, 50, and 100 mg/kg) given twice a week (in vitro and in vivo) | Decreased LPS-induced iNOS expression | Inhibition of thermal and mechanical hyperalgesia | [56] |
| GSPE | Chronic constriction injury | Wistar rats of either sex | Oral/100 and 200 mg/kg for 14 days (in vitro and in vivo) | Increased SOD and GSH | Attenuation of thermal hyperalgesia and mechanical allodynia | [79] |
| Silibinin | Oxaliplatin | Rat model of painful oxaliplatin-induced neuropathy | Silibinin (100 mg/kg), administered once a day, starting from the first day of oxaliplatin injection until the 20th | Prevention of oxidative damage | Antineuropathic effects | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.; Abdellatif, B.; Zhai, K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Flavonoids Alleviate Peripheral Neuropathy Induced by Anticancer Drugs. Cancers 2021, 13, 1576. https://doi.org/10.3390/cancers13071576
Siddiqui M, Abdellatif B, Zhai K, Liskova A, Kubatka P, Büsselberg D. Flavonoids Alleviate Peripheral Neuropathy Induced by Anticancer Drugs. Cancers. 2021; 13(7):1576. https://doi.org/10.3390/cancers13071576
Chicago/Turabian StyleSiddiqui, Manaal, Basma Abdellatif, Kevin Zhai, Alena Liskova, Peter Kubatka, and Dietrich Büsselberg. 2021. "Flavonoids Alleviate Peripheral Neuropathy Induced by Anticancer Drugs" Cancers 13, no. 7: 1576. https://doi.org/10.3390/cancers13071576
APA StyleSiddiqui, M., Abdellatif, B., Zhai, K., Liskova, A., Kubatka, P., & Büsselberg, D. (2021). Flavonoids Alleviate Peripheral Neuropathy Induced by Anticancer Drugs. Cancers, 13(7), 1576. https://doi.org/10.3390/cancers13071576









