Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
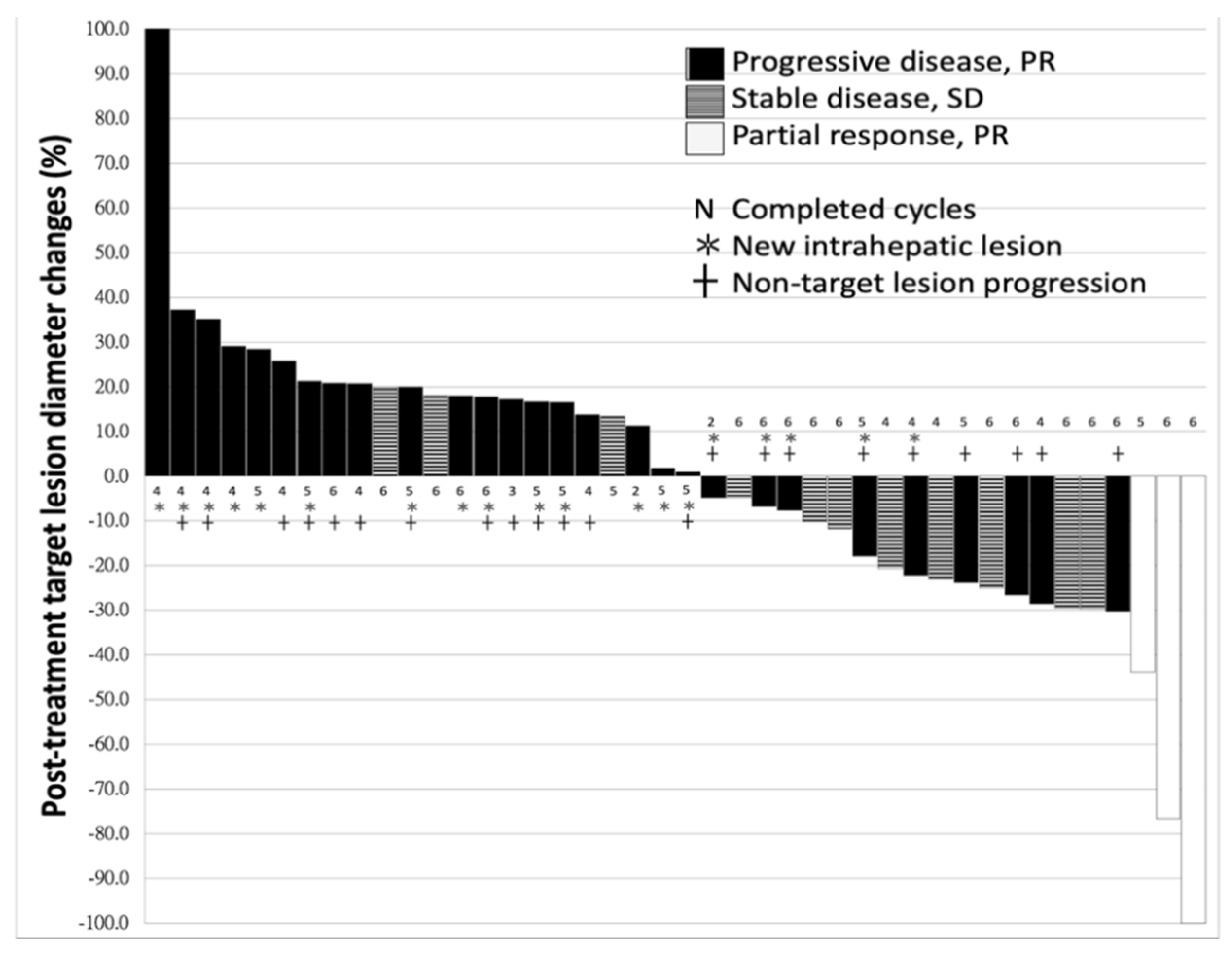
2.1. Characteristics of Patients and Response to Nivolumab
2.2. Difference between the Patients With or Without Disease-Control
2.3. Univariate and Multivariate Logistic Regression
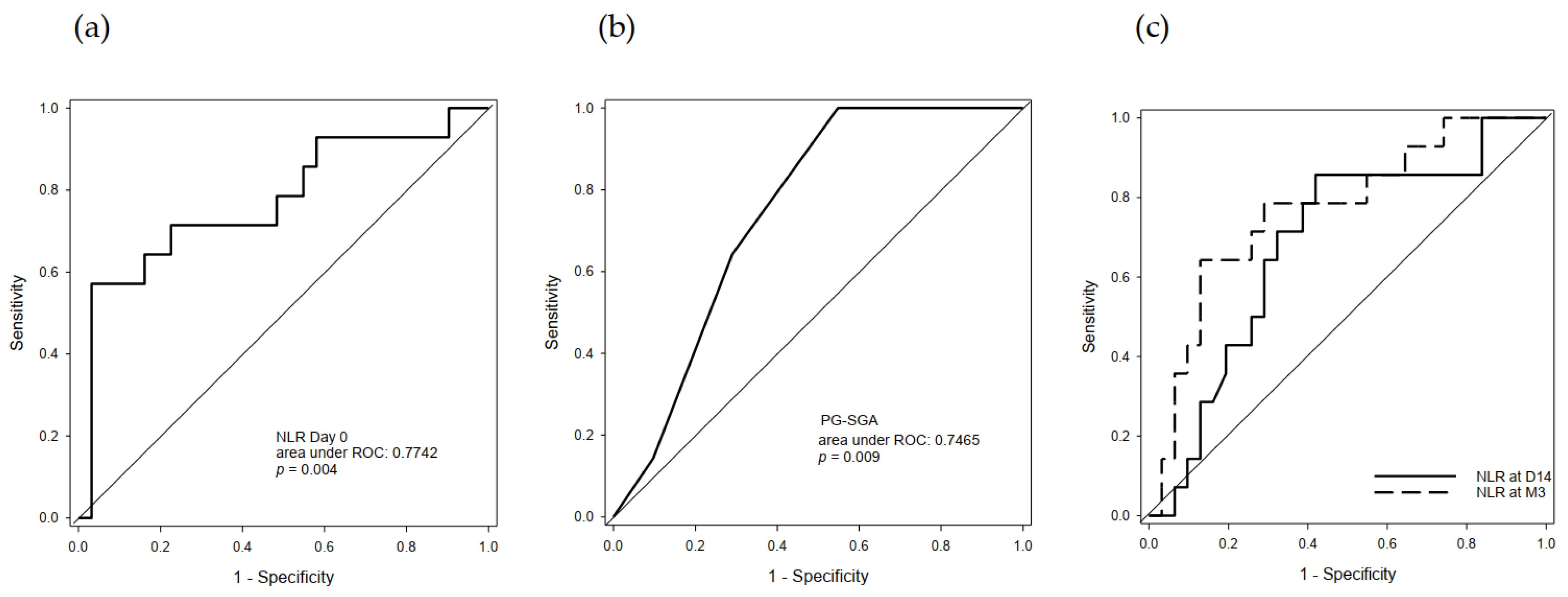
2.4. Predictive Value of Serum NLR and PG-SGA
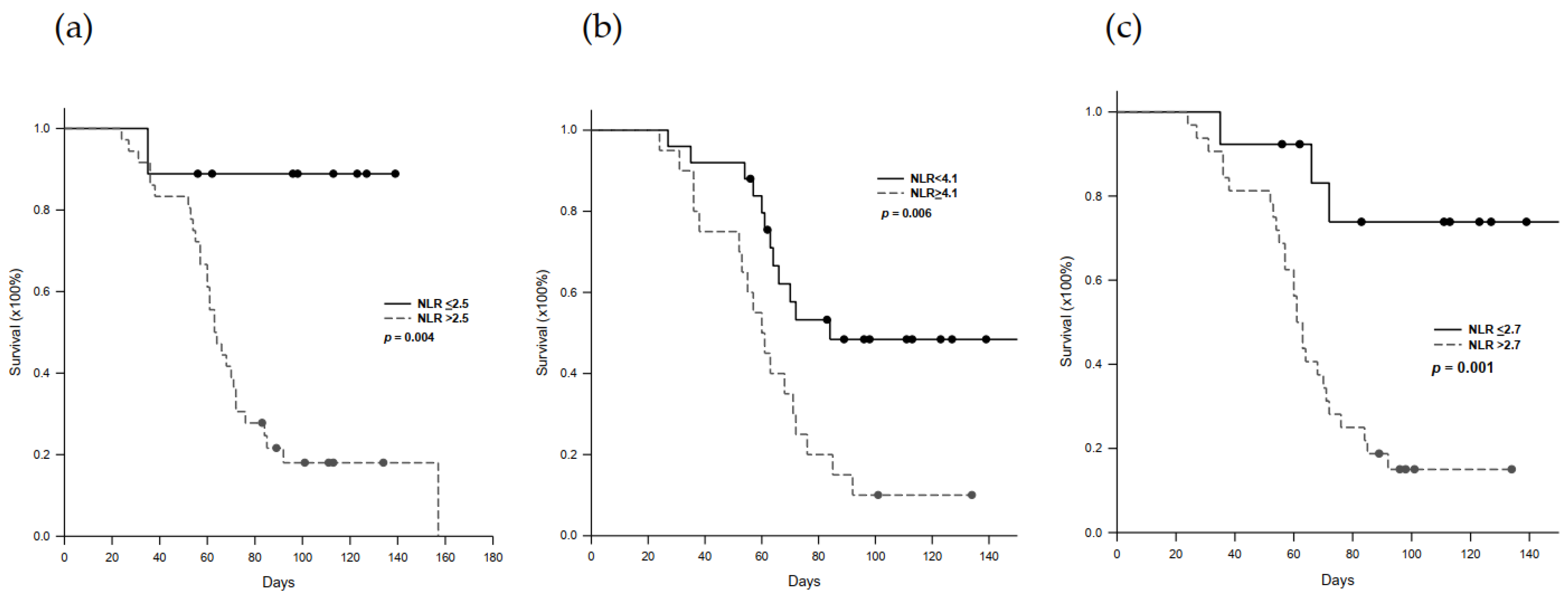
2.5. Progression-Free Survival in Nivolumab-Treated Patients
2.6. Immune-Related Adverse Effect (irAE) Profiles
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Nivolumab Administration
4.3. Assessment of Responses to Treatment
4.4. Clinical Profiles
4.5. Neutrophil-to-Lymphocyte Ratio
4.6. Clinical Benefits of Treatment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tinkle, C.L.; Haas-Kagan, D. Hepatocellular carcinoma: Natural history, current management, and emerging tool. Biol. Target Ther. 2012, 6, 207–219. [Google Scholar]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; YKang, O.-K.; Chen, Z. Effi cacy and safety of sorafenib in patients in the Asia-Pacifi c region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.-H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2017, 32, 317–326. [Google Scholar] [CrossRef]
- McDermott, D.; Haanen, J.; Chen, T.-T.; Lorigan, P.; O’Day, S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann. Oncol. 2013, 24, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [Green Version]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, P.A. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, T.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Kang, Y.-K.; Hou, M.-M.; Numata, K.; Yeo, W.; Chopra, A.; Ikeda, M.; et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J. Hepatol. 2019, 71, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Lavoie, J.-M.; Black, P.C.; Eigl, B.J. Predictive Biomarkers for Checkpoint Blockade in Urothelial Cancer: A Systematic Review. J. Urol. 2019, 202, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, D.E.; Banerji, S. Biomarkers of Immune Checkpoint Inhibitor Efficacy in Cancer. Curr. Oncol. 2020, 27, 106–114. [Google Scholar] [CrossRef]
- Bustamante-Alvarez, J.G.; Owen, D.H. Biomarkers for Immunotherapy. Thorac. Surg. Clin. 2020, 30, 207–214. [Google Scholar] [CrossRef]
- Fumet, J.-D.; Truntzer, C.; Yarchoan, M.; Ghiringhelli, F. Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts. Eur. J. Cancer 2020, 131, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bellesoeur, A.; Torossian, N.; Amigorena, S.; Romano, E. Advances in theranostic biomarkers for tumor immunotherapy. Curr. Opin. Chem. Biol. 2020, 56, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, H.; Sun, B.; Karin, M. The immunobiology of hepatocellular carcinoma in humans and mice: Basic concepts and therapeutic implications. J. Hepatol. 2020, 72, 167–182. [Google Scholar] [CrossRef]
- Jung, M.R.; Park, Y.K.; Jeong, O.; Seon, J.W.; Ryu, S.Y.; Kim, D.Y.; Kim, Y.J. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J. Surg. Oncol. 2011, 104, 504–510. [Google Scholar] [CrossRef]
- Van Berckelaer, C.; Van Geyt, M.; Linders, S. A high neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are associated with a worse outcome in inflammatory breast cancer. Breast 2020, 53, 212–220. [Google Scholar] [CrossRef]
- Mazaki, J.; Katsumata, K.; Kasahara, K.; Tago, T.; Wada, T.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Nagakawa, Y.; Tsuchida, A. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: A propensity score analysis. BMC Cancer 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Wu, J.N.; Lv, X.D. The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS ONE 2020, 15, e0237947. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Lee, J.-C.; Cheng, C.-H.; Wu, T.-H.; Wang, Y.-C.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection. J. Hepato-Biliary-Pancreat. Sci. 2017, 24, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.-K.; Chen, D.; Li, S.-Q.; Fu, S.-J.; Peng, B.-G.; Liang, L.-J. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer 2014, 14, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.-D.; Shi, X.-J.; Chen, Y.-G.; Wang, C.-L.; Ma, Q.; Lv, G.-Y. Elevated Preoperative Neutrophil-Lymphocyte Ratio Is Associated with Poor Prognosis in Hepatocellular Carcinoma Patients Treated with Liver Transplantation: A Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Bai, Y.; Zhou, F.; Li, W.; Gao, G.; Su, C.; Ren, S.; Chen, X.; Zhou, C. Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer 2019, 130, 76–83. [Google Scholar] [CrossRef]
- Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. [Google Scholar] [CrossRef]
- Suzuki, K.; Terakawa, T.; Furukawa, J.; Harada, K.; Hinata, N.; Nakano, Y.; Fujisawa, M. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int. J. Clin. Oncol. 2020, 25, 135–144. [Google Scholar] [CrossRef]
- Yasumatsu, R.; Wakasaki, T.; Hashimoto, K.; Nakashima, K.; Manako, T.; Taura, M.; Matsuo, M.; Nakagawa, T. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck 2019, 41, 2610–2618. [Google Scholar] [CrossRef]
- Jeyakumar, G.; Kim, S.; Bumma, N.; Landry, C.; Silski, C.; Suisham, S.; Dickow, B.; Heath, E.; Fontana, J.; Vaishampayan, U. Neutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J. Immunother. Cancer 2017, 5, 82. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, R.; Matthews, K.; Williams, V. Using Global Criteria to Detect Malnutrition: Application in Disease States. Nutr. Clin. Pract. 2019, 35, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Chao, Y.; Chen, M.-H.; Lan, K.-H.; Lee, C.-J.; Lee, I.-C.; Chen, S.-C.; Hou, M.-C.; Huang, Y.-H. Predictors of Response and Survival in Immune Checkpoint Inhibitor-Treated Unresectable Hepatocellular Carcinoma. Cancers 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharmapuri, S.; Özbek, U.; Lin, J.; Sung, M.; Schwartz, M.; Branch, A.D.; Ang, C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti–PD-1 therapy. Cancer Med. 2020, 9, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Chen, Q.; Zhang, B.; Liu, J.; Liu, S.; Mo, X.; Li, M.; Chen, Z.; Chen, L.; et al. Predicting hyperprogressive disease in patients with advanced hepatocellular carcinoma treated with anti-programmed cell death 1 therapy. EClinicalMedicine 2020, 31, 100673. [Google Scholar] [CrossRef] [PubMed]
- Kwee, S.A.; Tiirikainen, M. Beta-catenin activation and immunotherapy resistance in hepatocellular carcinoma: Mechanisms and biomarkers. Hepatoma Res. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Li, Y.; Ren, H.P. 2010 guideline for the management of hepatocellular carcinoma recommended by the American Association for the Study of Liver Diseases. Zhonghua Gan Zang Bing Za Zhi 2011, 19, 249–250. [Google Scholar]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [Green Version]
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharm. 1989, 24, 148–154. [Google Scholar] [CrossRef]
| Factors | n = 45 (100%) |
|---|---|
| Baseline conditions | |
| Age (years) | 61.8 ± 9.6 |
| Gender (Male) | 41 (91.1%) |
| CTP score | 5.3 ± 0.6 |
| ALBI score | −2.49 ± 0.39 |
| Thrombocytopenia | 11 (24.4%) |
| EV | 7 (15.6%) |
| Ascites | 10 (22.2%) |
| Cirrhosis | 41 (91.1%) |
| ECOG-PS (0/1/2) | 35/9/1 |
| Viral hepatitis | 38 (84.4%) |
| Alcohol consumption | 8 (17.8%) |
| PG-SGA score | 3.8 ± 2.9 |
| Tumor-associated factors | |
| Maximum tumor diameter (cm) | 7.2 ± 4.2 |
| Total tumor volume (cm3) | 619.0 ± 831.1 |
| Alpha-fetoprotein (ng/mL) | 82,584.2 ± 499,364.2 |
| T stage (2/3/4) | 13/12/20 (28.9/26.7/44.4%) |
| N stage (1) | 10 (22.2%) |
| M stage (1) | 22 (48.9%) |
| PVT | 19 (42.2%) |
| Serum NLR | 4.0 ± 2.2 |
| Medical treatment | |
| Anti-viral agent | 22 (48.9%) |
| Previous history of hepatectomy | 19 (42.2%) |
| Previous Sorafenib | 45 (100.0%) |
| Period between diagnosis and ICI (months) | 37.0 ± 32.5 |
| Early drop-out of ICI treatment | 11 (24.4%) |
| Response to ICI (PR/SD/PD) | 3/11/31 (6.7/24.4/68.9%) |
| Factors | PR + SD (n = 14) | PD (n = 31) | p-Value |
|---|---|---|---|
| Age (years) | 65.2 ± 10.2 | 60.3 ± 9.1 | 0.117 |
| Gender (Male) | 14 (100.0%) | 27 (87.1%) | 0.159 |
| WBC (×109/L) | 6.1 ± 2.0 | 5.7 ± 2.2 | 0.571 |
| Platelet(×103/μL) | 168.6 ± 76.3 | 193.4 ± 149.3 | 0.558 |
| INR | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.163 |
| NLR | 2.9 ± 1.3 | 4.4 ± 2.3 | 0.028 |
| PLR | 123.7 ± 65.4 | 190.7 ± 118.6 | 0.185 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 0.9 ± 0.6 | 0.734 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.9 ± 0.5 | 0.184 |
| AST (U/L) | 62.3 ± 30.5 | 82.7 ± 54.7 | 0.200 |
| ALT (U/L) | 48.1 ± 29.2 | 63.5 ± 49.9 | 0.291 |
| Albumin (g/dL) | 4.0 ± 0.4 | 3.7 ± 0.4 | 0.059 |
| CTP class (A/B) | 14/0 (100.0/0.0%) | 29/2 (93.5/6.5%) | 0.331 |
| ALBI score | −2.7 ± 0.3 | −2.4 ± 0.4 | 0.042 |
| EV | 3 (21.4%) | 4 (12.9%) | 0.465 |
| Ascites | 2 (14.3%) | 8 (25.8%) | 0.389 |
| Cirrhosis | 11 (78.6%) | 30 (96.8%) | 0.047 |
| ECOG-PS (0/1/2) | 12/2/0 (85.7/14.3/0.0%) | 23/7/1 (74.2/22.6/0.3%) | 0.623 |
| Viral hepatitis (Yes) | 12 (85.7%) | 26 (83%) | 0.874 |
| Alcohol use (Yes) | 3 (21.4%) | 5 (16.1%) | 0.667 |
| PG-SGA score | 2.3 ± 0.7 | 4.7 ± 3.2 | 0.003 |
| AFP (ng/mL) | 3540.9 ± 5481.5 | 118281.2 ± 601217.5 | 0.482 |
| Max. tumor diameter (cm) | 5.6 ± 3.7 | 8.0 ± 4.3 | 0.091 |
| Total tumor volume (cm3) | 397.2 ± 659.3 | 719.1 ± 889.6 | 0.233 |
| T (2/3/4) | 6/5/3 (42.9/35.7/21.4%) | 7/7/17 (22.6/22.6/54.8%) | 0.110 |
| N (1) | 2 (11.1%) | 8 (27.3%) | 0.389 |
| M (1) | 7 (50.0%) | 15 (48.4%) | 0.920 |
| PVT | 4 (28.6%) | 15 (48.4%) | 0.213 |
| Anti-viral agent | 8 (57.1%) | 14 (45.2%) | 0.457 |
| Period between diagnosis and IC (months) | 35.4 ± 43.2 | 37.8 ± 27.1 | 0.822 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Serum NLR | 2.07 | 1.10–3.90 | 0.025 | 2.04 | 1.10–3.80 | 0.025 |
| Max. tumor size | 1.17 | 0.97–1.40 | 0.099 | |||
| ALBI score | 6.11 | 1.01–37.23 | 0.050 | |||
| Cirrhosis | 8.18 | 0.77–87.20 | 0.082 | |||
| PG-SGA score | 2.02 | 1.08–3.80 | 0.029 | 2.30 | 1.04–5.09 | 0.039 |
| Category | Total (n = 45) | Patients, N (%) a Grade 1–2 | Grade 3–4 c | Grade 5 b | Weeks to Onset Median (Range) |
|---|---|---|---|---|---|
| Any | 29 (64.4) | 17 (37.7) | 11 (24.4) | 1 (2.2) | |
| Skin | 13 (28.9) | 13 (28.9) | 0 (0.0) | 0 (0.0) | 3.1 (0.6–7.6) |
| Rash | 6 (13.3) | 6 (13.3) | 0 (0.0) | 0 (0.0) | |
| Pruritus | 9 (20.0) | 9 (20.0) | 0 (0.0) | 0 (0.0) | |
| Pneumonitis | 4 (8.9) | 1 (2.2) | 3 (6.7) | 0 (0.0) | 8.3 (2.0–12.0) |
| Endocrine | 1 (2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 6.0 (NA) |
| Thyroditis/hypothyroidism | 1 (2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | |
| Hypophysitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Gastrointestinal | 7 (15.6) | 6 (13.3) | 1 (2.2) | 0 (0.0) | 7.6 (0.6–8.9) |
| Mucositis | 2 (4.4) | 2(4.4) | 0 (0.0) | 0 (0.0) | |
| Esophagitis | 1 (2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) | |
| Diarrhea/colitis | 5 (11.1) | 4 (8.9) | 1 (2.2) | 0 (0.0) | |
| Hepatobiliary | 11 (24.4) | 2(4.4) | 9 (20.0) | 0 (0.0) | 4.6 (1.0–14.6) |
| Hepatitis | 9 (20.0) | 2(4.4) | 6 (13.3) | 1 (2.2) | |
| Cholangitis | 4 (8.9) | 0 (0.0) | 4 (8.9) | 0 (0.0) | |
| Others | 12 (26.7) | 15 (33.3) | 0 (0.0) | 0 (0.0) | 4.3 (1.0–10.3) |
| Fatigue | 7 (15.6) | 6 (13.3) | 1 (2.2) | 0 (0.0) | |
| Anorexia | 7 (15.6) | 7 (15.6) | 0 (0.0) | 0 (0.0) | |
| Polyarthritis | 1(2.2) | 1 (2.2) | 0 (0.0) | 0 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-C.; Lee, J.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma. Cancers 2021, 13, 1607. https://doi.org/10.3390/cancers13071607
Hung H-C, Lee J-C, Wang Y-C, Cheng C-H, Wu T-H, Lee C-F, Wu T-J, Chou H-S, Chan K-M, Lee W-C. Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma. Cancers. 2021; 13(7):1607. https://doi.org/10.3390/cancers13071607
Chicago/Turabian StyleHung, Hao-Chien, Jin-Chiao Lee, Yu-Chao Wang, Chih-Hsien Cheng, Tsung-Han Wu, Chen-Fang Lee, Ting-Jung Wu, Hong-Shiue Chou, Kun-Ming Chan, and Wei-Chen Lee. 2021. "Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma" Cancers 13, no. 7: 1607. https://doi.org/10.3390/cancers13071607






