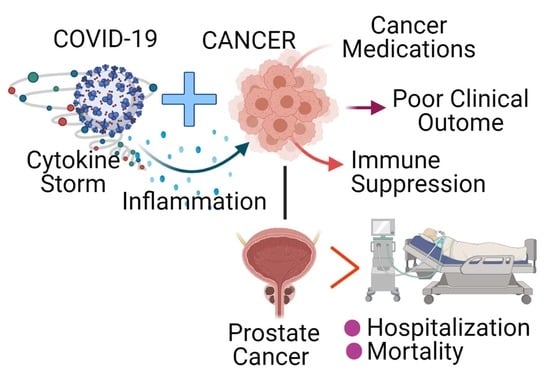
Increased Hospitalization and Mortality from COVID-19 in Prostate Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Variables and Outcome
2.3. Statistical Analysis
3. Results
3.1. Old Age, Gender, and Comorbidities Are Risk Factors for COVID-19
3.2. Prostate Cancer Patients Had a Higher Likelihood of Severe Illness Due to COVID-19
3.3. Prostate Cancer Patients Reported Higher Hospitalization and Mortality Rates Due to COVID-19
3.4. Prostate Cancer Patients Had Higher Hospitalization and Mortality Rates Compared with Breast Cancer Patients Due to COVID-19
3.5. Prostate Cancer Patients Reported Higher Incidence, Hospitalization, and Mortality Rates Due to COVID-19 Across Several Cohorts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; Sengupta, R.; Locke, T.; Zaidi, S.K.; Campbell, K.M.; Carethers, J.M.; Jaffee, E.M.; Wherry, E.J.; Soria, J.C.; D’Souza, G. Priority COVID-19 Vaccination for Patients with Cancer while Vaccine Supply Is Limited. Cancer Discov. 2021, 11, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Berger, N.A.; Xu, R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA Oncol. 2021, 7, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Nair, S.S.; Hammouda, N.; Ratnani, P.; Gharib, Y.; Wagaskar, V.; Mohamed, N.; Lundon, D.; Dovey, Z.; Kyprianou, N.; et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun. Biol. 2020, 3, 374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, W.; Liu, X.S.; Carroll, J.S.; Jänne, O.A.; Keeton, E.K.; Chinnaiyan, A.M.; Pienta, K.J.; Brown, M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol. Cell 2007, 27, 380–392. [Google Scholar] [CrossRef] [Green Version]
- Sahu, B.; Laakso, M.; Ovaska, K.; Mirtti, T.; Lundin, J.; Rannikko, A.; Sankila, A.; Turunen, J.P.; Lundin, M.; Konsti, J.; et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011, 30, 3962–3976. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.; Mani, R.S.; Cao, Q.; Brenner, C.J.; Cao, X.; Wang, X.; Wu, L.; Li, J.; Hu, M.; et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 2010, 17, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011, 30, 2719–2733. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.L.; Massie, C.E.; Ramos-Montoya, A.; Zecchini, V.; Scott, H.E.; Lamb, A.D.; MacArthur, S.; Stark, R.; Warren, A.Y.; Mills, I.G.; et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 2013, 23, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Clinckemalie, L.; Spans, L.; Dubois, V.; Laurent, M.; Helsen, C.; Joniau, S.; Claessens, F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol. Endocrinol. 2013, 27, 2028–2040. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Lin, A.A.; Wojciechowski, S.E.; Hildeman, D.A. Androgens suppress antigen-specific T cell responses and IFN-gamma production during intracranial LCMV infection. J. Neuroimmunol. 2010, 226, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffo, O.; Gasparro, D.; Di Lorenzo, G.; Dalla Volta, A.; Guglielmini, P.; Zucali, P.; Bortolus, R.; Cavo, A.; Ceresoli, G.; Chiari, R.; et al. Incidence and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with metastatic castration-resistant prostate cancer. Eur. J. Cancer 2020, 140, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Buonerba, L.; Ingenito, C.; Crocetto, F.; Buonerba, C.; Libroia, A.; Sciarra, A.; Ragone, G.; Sanseverino, R.; Iaccarino, S.; et al. Clinical Characteristics of Metastatic Prostate Cancer Patients Infected with COVID-19 in South Italy. Oncology 2020, 98, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.V.; Carbone, G.M.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: A population-based study (n = 4532). Ann. Oncol. 2020, 31, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Mou, R.; Jin, X.; Li, W.; Wu, M.; Liu, X.; Liu, Z.; Guo, S.; Li, X.; Jia, Y. Prostate cancer: A risk factor for COVID-19 in males?: A protocol for systematic review and meta analysis. Medicine (Baltimore) 2020, 99, e22591. [Google Scholar] [CrossRef]
- Singh, S.R.; Thanikachalam, K.; Jabbour-Aida, H.; Poisson, L.M.; Khan, G. COVID-19 and Cancer: Lessons Learnt from a Michigan Hotspot. Cancers 2020, 12, 2377. [Google Scholar] [CrossRef]
- Pujadas, E.; Ibeh, N.; Hernandez, M.M.; Waluszko, A.; Sidorenko, T.; Flores, V.; Shiffrin, B.; Chiu, N.; Young-Francois, A.; Nowak, M.D.; et al. Comparison of SARS-CoV-2 detection from nasopharyngeal swab samples by the Roche cobas 6800 SARS-CoV-2 test and a laboratory-developed real-time RT-PCR test. J. Med. Virol. 2020, 92, 1695–1698. [Google Scholar] [CrossRef]
- Risbridger, G.P.; Davis, I.D.; Birrell, S.N.; Tilley, W.D. Breast and prostate cancer: More similar than different. Nat. Rev. Cancer 2010, 10, 205–212. [Google Scholar] [CrossRef]
- Smithson, G.; Couse, J.F.; Lubahn, D.B.; Korach, K.S.; Kincade, P.W. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J. Immunol. 1998, 161, 27–34. [Google Scholar]
- Özdemir, N.; Dizdar, Ö.; Yazıcı, O.; Aksoy, S.; Sener Dede, D.; Budakoğlu, B.; Metan, G.; Alp, A.; Budakoğlu, I.I.; Çakmak Öksüzoğlu, Ö.B.; et al. Clinical features and outcomes of COVID-19 in patients with solid tumors: Turkish National Registry Data. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Ramachandran, P.; Kathirvelu, B.; Chakraborti, A.; Gajendran, M.; Zhahid, U.; Ghanta, S.; Onukogu, I.; Narh, J.T.; Wang, J.C.; Anwer, F. COVID-19 in Cancer Patients From New York City: A Comparative Single Center Retrospective Analysis. Cancer Control 2020. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Rasool, R.U.; Russell, R.M.; Natesan, R.; Asangani, I.A. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. IScience 2021, 24, 102254. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, X.M.; Mannan, R.; Pitchiaya, S.; Zhang, Y.; Wotring, J.W.; Xiao, L.; Robinson, D.R.; Wu, Y.-M.; Tien, J.C.-Y.; et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc. Natl. Acad. Sci. USA 2020. [Google Scholar] [CrossRef]
- Trigunaite, A.; Dimo, J.; Jorgensen, T.N. Suppressive effects of androgens on the immune system. Cell Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef]
- Muehlenbein, M.P.; Cogswell, F.B.; James, M.A.; Koterski, J.; Ludwig, G.V. Testosterone correlates with Venezuelan equine encephalitis virus infection in macaques. Virol. J. 2006, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissick, H.T.; Sanda, M.G.; Dunn, L.K.; Pellegrini, K.L.; On, S.T.; Noel, J.K.; Arredouani, M.S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 9887–9892. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zou, Z.; Deng, Z.; Liang, D.; Zhou, X.; Sun, R.; Lan, K. Male hormones activate EphA2 to facilitate Kaposi’s sarcoma-associated herpesvirus infection: Implications for gender disparity in Kaposi’s sarcoma. PLoS Pathog. 2017, 13, e1006580. [Google Scholar] [CrossRef]
- Klein, S.L. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci. Biobehav. Rev. 2000, 24, 627–638. [Google Scholar] [CrossRef]
- Vom Steeg, L.G.; Klein, S.L. Sex and sex steroids impact influenza pathogenesis across the life course. Semin. Immunopathol. 2019, 41, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Ryan, C.J. Androgen hazards with COVID-19. Endocr. Relat. Cancer 2020, 27, E1–E3. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Renia, L. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Administration USFaD. Potential for False Results with Roche Molecular Systems, Inc. Cobas SARS-CoV-2 & Influenza Test for Use on Cobas Liat System-Letter to Clinical Laboratory Staff, Point-of-Care Facility Staff, and Health Care Providers. 2021. Available online: https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-results-roche-molecular-systems-inc-cobas-sars-cov-2-influenza-test-use-cobas-liat (accessed on 9 January 2021).








| Covariates | COVID − ve (n = 270,055) | COVID + ve (n = 16,554) | p-Value |
|---|---|---|---|
| Median Age (IQR): | 53 (35, 67) | 59 (41, 73) | <0.0001 |
| SEX | <0.0001 | ||
| Male | 121,989 (45.17%) | 8476 (51.2%) | |
| Female | 148,066 (54.83%) | 8078 (48.80%) | |
| Race/Ethnicity: | <0.0001 | ||
| African American | 53,155 (19.68%) | 3763 (22.73%) | |
| White | 96,989 (35.91%) | 4543 (27.44%) | |
| Asian | 18,741 (6.94%) | 978 (5.91%) | |
| Hispanic | 55,592 (20.59%) | 4307 (26.02%) | |
| Others | 25,363 (9.39%) | 2195 (13.26%) | |
| Unknown | 20,215 (7.49%) | 768 (4.64%) | |
| Smoking: | <0.0001 | ||
| Current | 29,126 (10.79%) | 1006 (6.08%) | |
| Never | 171,456 (63.49%) | 11,196 (67.63%) | |
| Not Asked | 6271 (2.32%) | 697 (4.21%) | |
| Quit | 63,202 (23.40%) | 3655 (22.08%) | |
| Cancer Type: | <0.0001 | ||
| Other Cancer | 37,680 (13.95%) | 1524 (9.21%) | |
| Prostate Cancer | 3956 (1.46%) | 228 (1.38%) | |
| No cancer | 228,419 (84.58%) | 14,802 (89.42%) | |
| Genitourinary cancer | 6320 (15.93%) | 350 (2.11%) | |
| Solid cancer | 33,353 (84.07%) | 1253 (7.56%) | |
| Hypertension: | <0.0001 | ||
| No | 203,152 (75.23%) | 11,492 (69.42%) | |
| yes | 66,903 (24.77%) | 5062 (30.58%) | |
| Diabetes: | <0.0001 | ||
| No | 235,991 (87.39%) | 13,499 (81.55%) | |
| Yes | 34,064 (12.61%) | 3055 (18.45%) | |
| Coronary Artery Disease: | <0.0001 | ||
| No | 243,582 (90.20%) | 14,623 (88.34%) | |
| Yes | 26,473 (9.8%) | 1931 (11.66%) | |
| Crohns Disease | <0.0001 | ||
| No | 267,607 (99.09%) | 16,493 (99.63%) | |
| Yes | 2448 (0.91%) | 61 (0.37%) | |
| Ulcerative Colitis | <0.0001 | ||
| No | 267,943 (99.22%) | 16,473 (99.51%) | |
| Yes | 2112(0.78%) | 81 (0.49%) | |
| Acute Respiratory Distress Syndrome | <0.0001 | ||
| No | 270,000 (99.98%) | 16,516 (99.77%) | |
| Yes | 55 (0.02%) | 38 (0.23%) | |
| Chronic Obstructive Pulmonary Disease: | 0.4716 | ||
| No | 261,313 (96.76%) | 16,035 (96.86%) | |
| Yes | 8742 (3.24%) | 519 (3.14%) | |
| Obesity: | <0.0001 | ||
| No | 250,001 (92.57%) | 15,163 (91.6%) | |
| Yes | 20,054 (7.43%) | 1391 (8.4%) | |
| Asthma: | <0.0001 | ||
| No | 251,098 (92.98%) | 15,569 (94.05%) | |
| Yes | 18,957 (7.02%) | 985 (5.95%) |
| Factors | Prostate Cancer | Solid Cancer | p-Value | Total COVID-19-Positive Cases |
|---|---|---|---|---|
| Hospitalization | 148 (64.91%) | 651 (47.34%) | <0.0001 | 7729 |
| ICU | 29 (12.71%) | 138 (10.03%) | 0.2194 | 1684 |
| Intubation | 16 (7.01%) | 79 (5.74%) | 0.4512 | 1121 |
| Death | 48 (21.05%) | 218 (15.85%) | 0.0547 | 1990 |
| Total | 228 | 1375 |
| Factors | Prostate Cancer | Solid Cancer | p-Value |
|---|---|---|---|
| African American | 47 (31.75%) | 174 (26.73%) | 0.0045 |
| White | 38 (25.67%) | 218 (33.49%) | 0.4665 |
| Asian | 3 (0.20%) | 27 (4.15%) | 1.00 |
| Hispanic | 43 (29.05%) | 172 (26.42%) | 0.0003 |
| Others | 15 (10.13%) | 53 (8.14%) | 0.0106 |
| Unknown | 2 (1.35%) | 7 (1.08%) | 0.5658 |
| Total | 148 | 651 |
| Factors | Prostate Cancer | Non-Prostate Genitourinary Cancer | p-Value |
|---|---|---|---|
| Hospitalization | 148 (64.91%) | 66 (56.33%) | 0.0479 |
| ICU | 29 (12.71%) | 17 (11.97%) | 0.7485 |
| Intubation | 16 (7.01%) | 7 (4.92%) | 0.6452 |
| Death | 48 (21.05%) | 15 (17.6%) | 0.0421 |
| Factors | Prostate Cancer | Breast Cancer | p-Value |
|---|---|---|---|
| Hospitalization | 148 (64.91%) | 115 (42.75%) | <0.0001 |
| ICU | 29 (12.71%) | 13 (4.83%) | 0.0016 |
| Intubation | 16 (7.01%) | 8 (2.97%) | 0.0361 |
| Death | 48 (21.05%) | 29 (10.78%) | 0.0016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakravarty, D.; Ratnani, P.; Sobotka, S.; Lundon, D.; Wiklund, P.; Nair, S.S.; Tewari, A.K. Increased Hospitalization and Mortality from COVID-19 in Prostate Cancer Patients. Cancers 2021, 13, 1630. https://doi.org/10.3390/cancers13071630
Chakravarty D, Ratnani P, Sobotka S, Lundon D, Wiklund P, Nair SS, Tewari AK. Increased Hospitalization and Mortality from COVID-19 in Prostate Cancer Patients. Cancers. 2021; 13(7):1630. https://doi.org/10.3390/cancers13071630
Chicago/Turabian StyleChakravarty, Dimple, Parita Ratnani, Stanislaw Sobotka, Dara Lundon, Peter Wiklund, Sujit S. Nair, and Ashutosh K. Tewari. 2021. "Increased Hospitalization and Mortality from COVID-19 in Prostate Cancer Patients" Cancers 13, no. 7: 1630. https://doi.org/10.3390/cancers13071630