Multiple Myeloma Patients Undergoing Carfilzomib: Development and Validation of a Risk Score for Cardiovascular Adverse Events Prediction
Abstract
Simple Summary
Abstract
1. Introduction
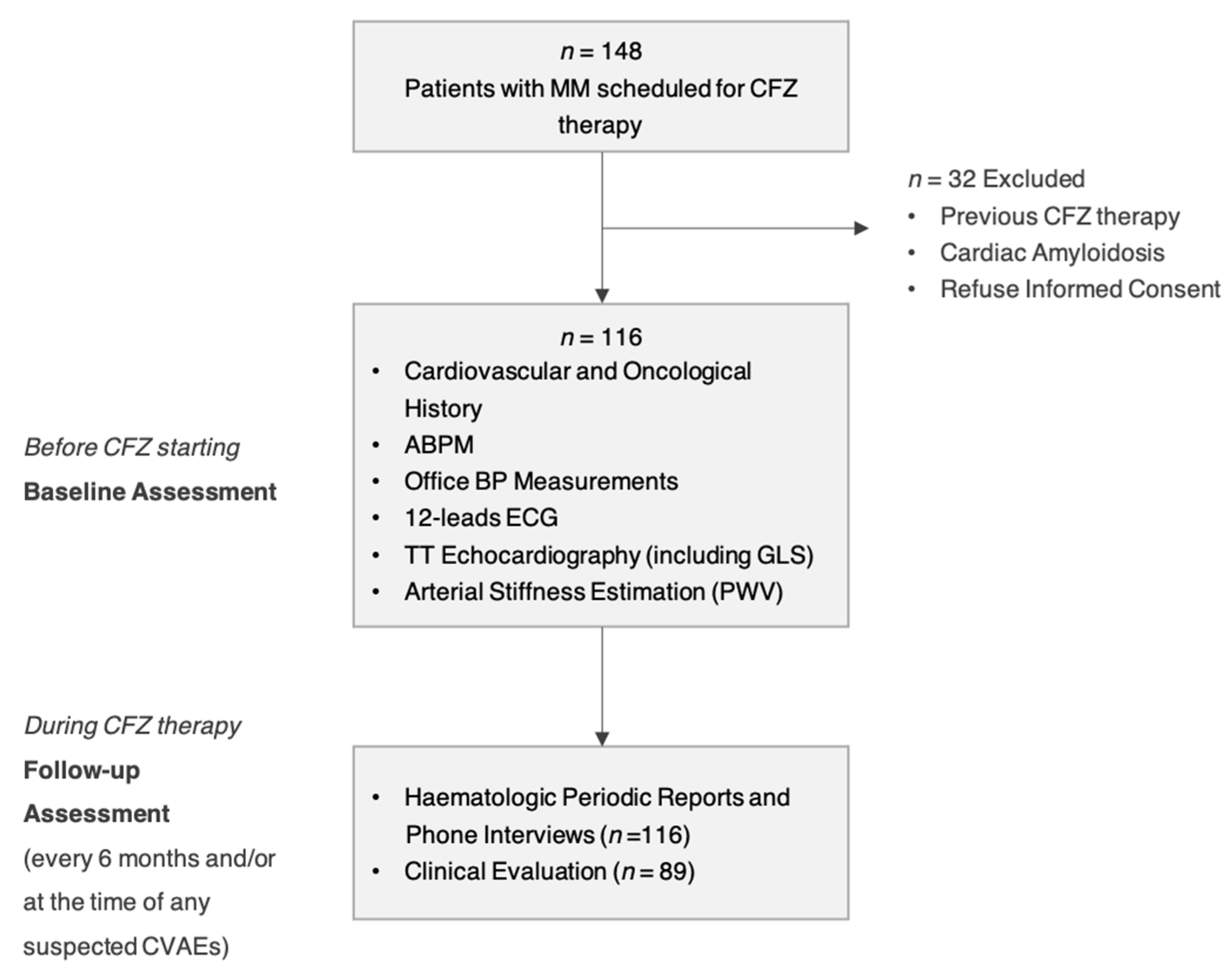
2. Materials and Methods
2.1. Baseline Assessment before Starting CFZ
2.2. Follow-Up Assessments and CVAEs
2.3. Statistical Analysis
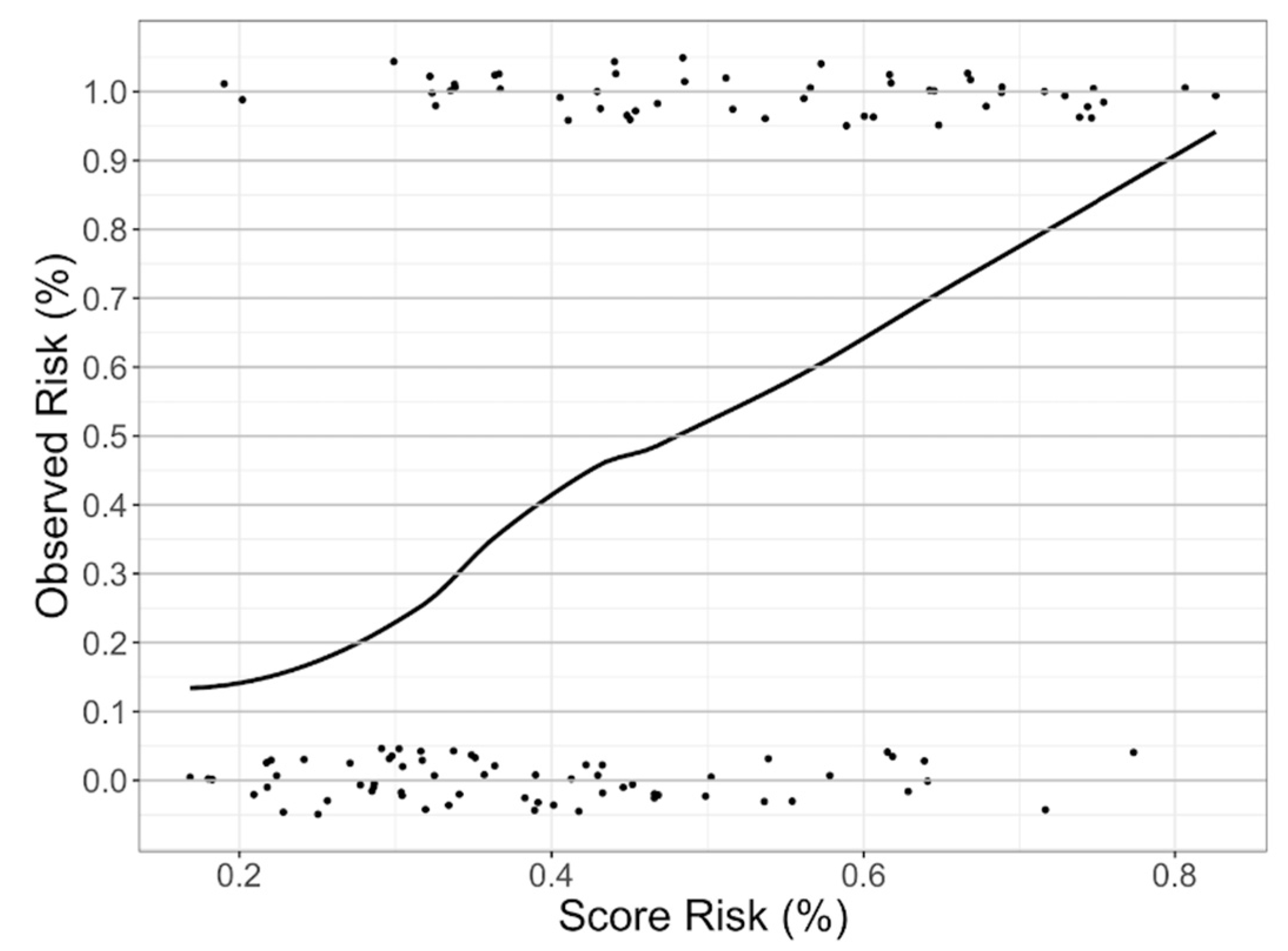
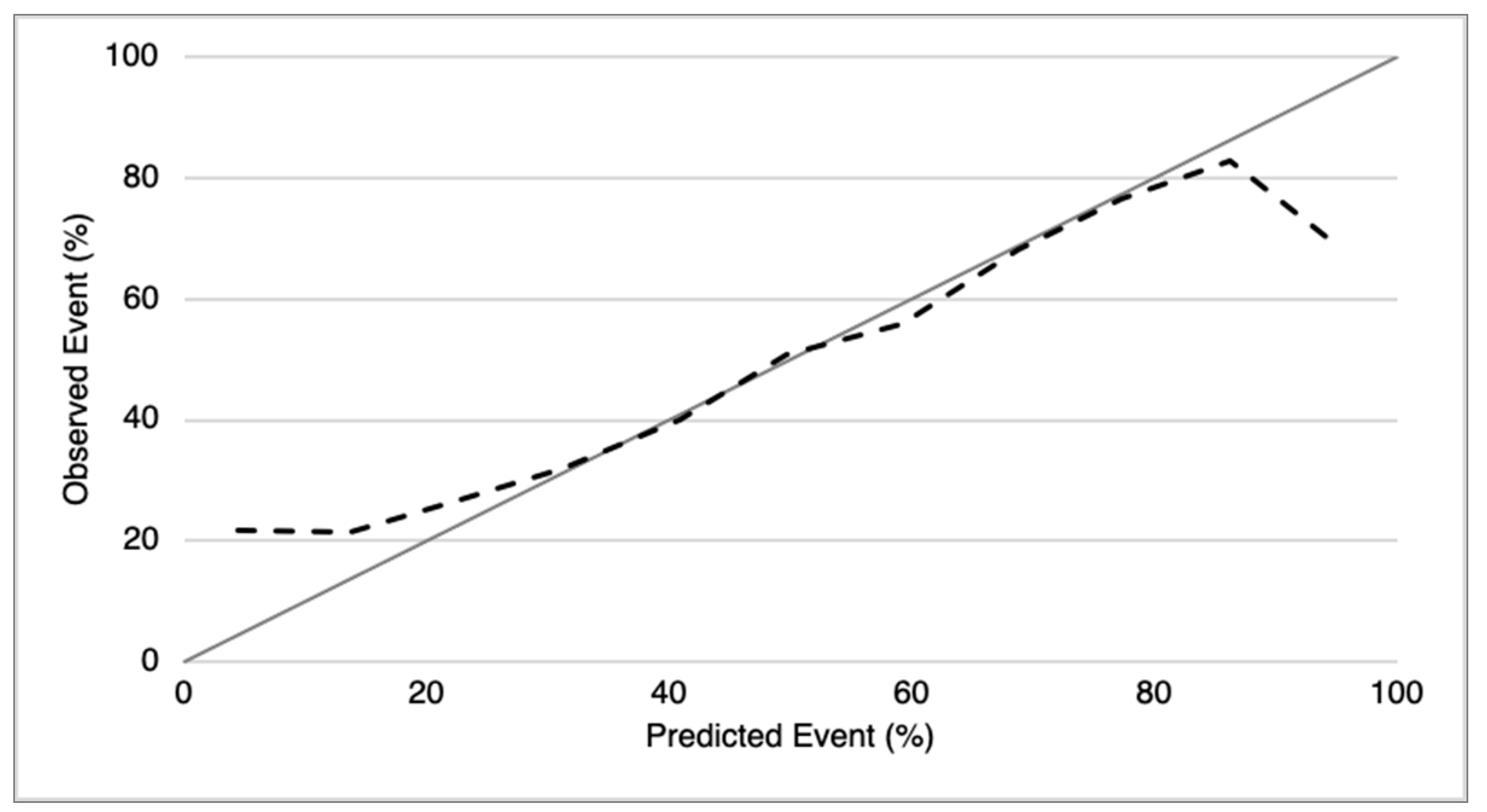
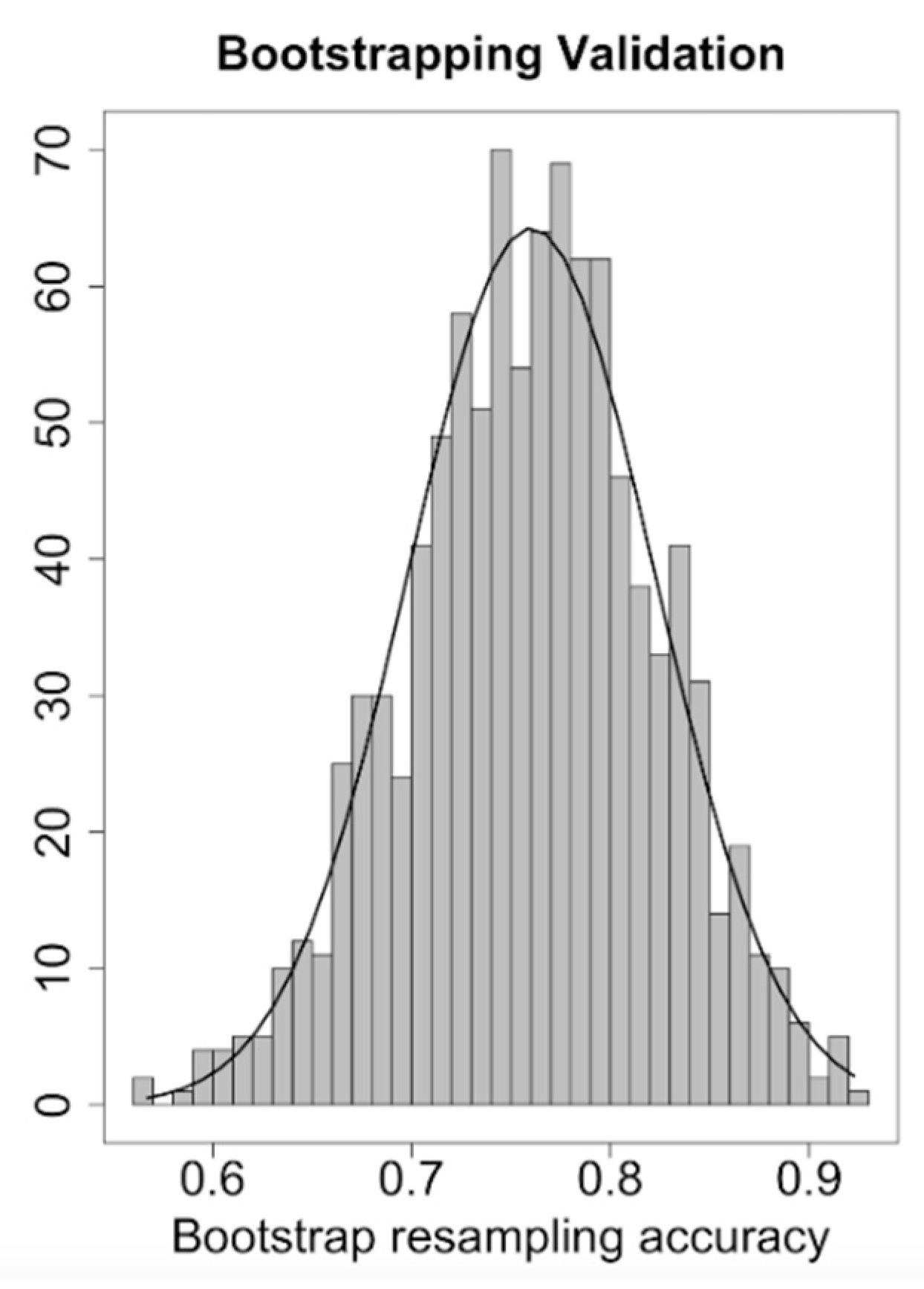
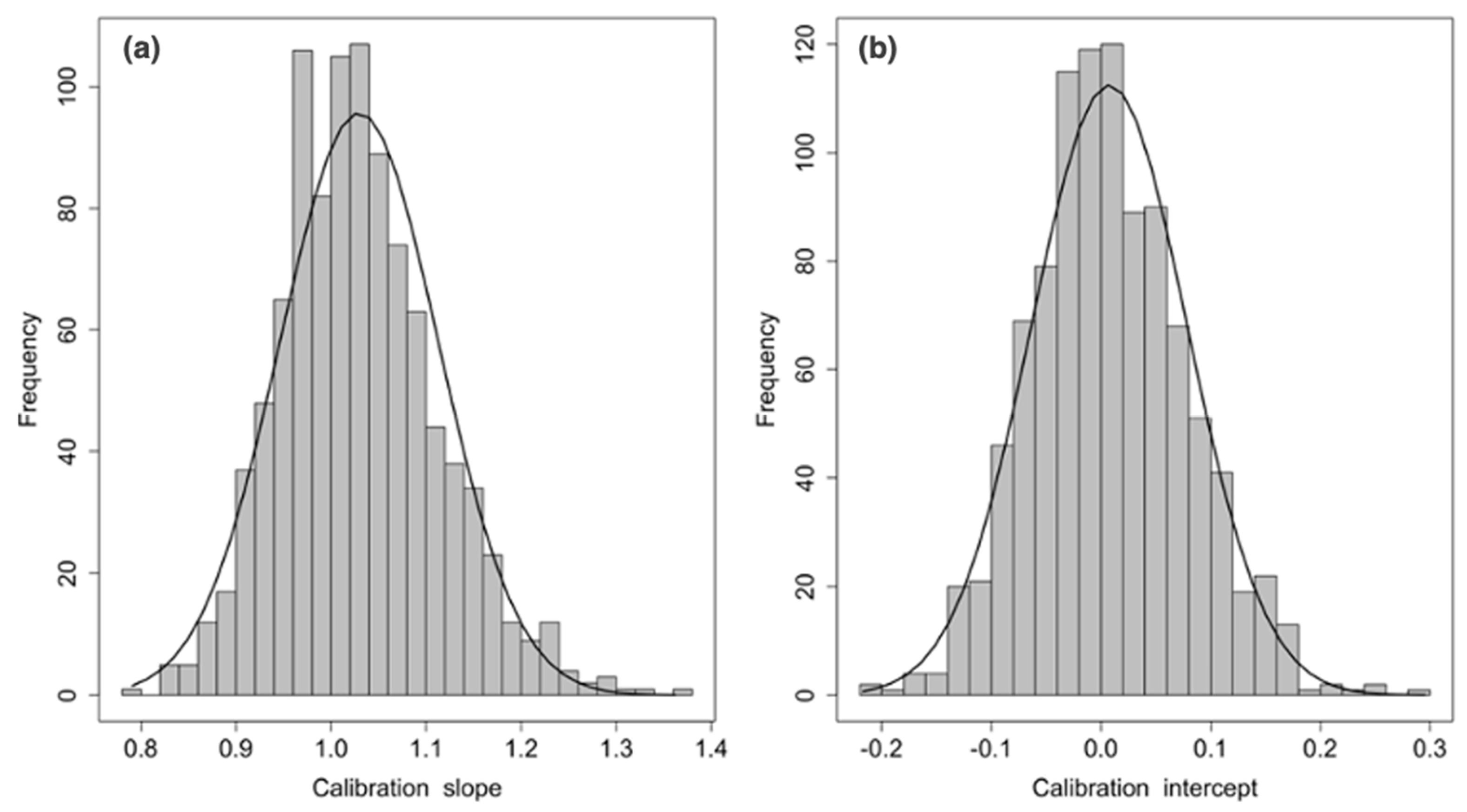
2.4. CFZ CVAEs Risk Score Analysis
3. Results
3.1. Baseline Characteristics and Cardiovascular Risk Factors
3.2. CFZ Therapy Regimen: Timing and Dose
3.3. Rates of CVAEs: All-Types, Major and Hypertension-Related CVAEs
3.4. Comparison between No-CVAEs and CVAEs Group: Baseline CV Parameters
3.5. CFZ CVAEs Risk Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Major Cardiovascular Adverse Events Definitions
Appendix A.2. Arterial Hypertension Cardiovascular Adverse Events Definitions
References
- Siegel, D.S.; Martin, T.; Wang, M.; Vij, R.; Jakubowiak, A.J.; Lonial, S.; Trudel, S.; Kukreti, V.; Bahlis, N.; Alsina, M.; et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012, 120, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef] [PubMed]
- A Dimopoulos, M.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hájek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Hájek, R.; Masszi, T.; Petrucci, M.T.; Palumbo, A.; Rosiñol, L.; Nagler, A.; Yong, K.L.; Oriol, A.; Minarik, J.; Pour, L.; et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia 2017, 31, 107–114. [Google Scholar] [CrossRef]
- Cornell, R.F.; Ky, B.; Weiss, B.M.; Dahm, C.N.; Gupta, D.K.; Du, L.; Carver, J.R.; Cohen, A.D.; Engelhardt, B.G.; Garfall, A.L.; et al. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J. Clin. Oncol. 2019, 37, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Chen-Scarabelli, C.; Corsetti, G.; Pasini, E.; Dioguardi, F.S.; Sahni, G.; Narula, J.; Gavazzoni, M.; Patel, H.; Saravolatz, L.; Knight, R.; et al. Spasmogenic Effects of the Proteasome Inhibitor Carfilzomib on Coronary Resistance, Vascular Tone and Reactivity. EBio Med. 2017, 21, 206–212. [Google Scholar] [CrossRef]
- Waxman, A.J.; Clasen, S.; Hwang, W.-T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-Associated Cardiovascular Adverse Events. JAMA Oncol. 2018, 4, e174519. [Google Scholar] [CrossRef]
- Bringhen, S.; Milan, A.; D’Agostino, M.; Ferri, C.; Wäsch, R.; Gay, F.; LaRocca, A.; Offidani, M.; Zweegman, S.; Terpos, E.; et al. Prevention, monitoring and treatment of cardiovascular adverse events in myeloma patients receiving carfilzomib A consensus paper by the European Myeloma Network and the Italian Society of Arterial Hypertension. J. Intern. Med. 2019, 286, 63–74. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Hear. J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Cancer Therapy Evaluation Program (CTEP): Common Terminology Criteria for Adverse Events (CTCAE) v.5.0. Cancer Ther. Eval. Progr. 2020. Update. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 1 March 2020).
- Moons, K.G.M.; De Groot, J.A.H.; Bouwmeester, W.; Vergouwe, Y.; Mallett, S.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies: The CHARMS Checklist. PLoS Med. 2014, 11, e1001744. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2014, 350, g7594. [Google Scholar] [CrossRef]
- Marshall, A.; Altman, D.G.; Royston, P.; Holder, R.L. Comparison of techniques for handling missing covariate data within prognostic modelling studies: A simulation study. BMC Med. Res. Methodol. 2010, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, M.; Ambler, G.; Seaman, S.R.; Guttmann, O.; Elliott, P.; King, M.; Omar, R.Z. How to develop a more accurate risk prediction model when there are few events. BMJ 2015, 351, h3868. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Miguel, J.S.; Sonneveld, P.; Mateos, M.V.; Zamagni, E.; Avet-Loiseau, H.; Hajek, R.; Dimopoulos, M.A.; Ludwig, H.; Einsele, H.; et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv52–iv61. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the C ardio- O ncology S tudy G roup of the H eart F ailure A ssociation of the E uropean S ociety of C ardiology in collaboration with the I nternational C ardio- O ncology S ociety. Eur. J. Hear. Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.S.; Dolan, E.; Bilo, G. Blood pressure variability: Clinical relevance and application. J. Clin. Hypertens. 2018, 20, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Rhea, I.B.; Uppuluri, S.; Sawada, S.; Schneider, B.P.; Feigenbaum, H. Incremental Prognostic Value of Echocardiographic Strain and Its Association With Mortality in Cancer Patients. J. Am. Soc. Echocardiogr. 2015, 28, 667–673. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Cauwenberghs, N.; Knez, J.; Yang, W.-Y.; Herbots, L.; D’Hooge, J.; Haddad, F.; Thijs, L.; Voigt, J.-U.; Staessen, J.A. Additive Prognostic Value of Left Ventricular Systolic Dysfunction in a Population-Based Cohort. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef]
- Bruno, G.; Bringhen, S.; Maffei, I.; Iannaccone, A.; Crea, T.; Ravera, A.; Astarita, A.; Vallelonga, F.; Salvini, M.; Gay, F.; et al. Cardiovascular Organ Damage and Blood Pressure Levels Predict Adverse Events in Multiple Myeloma Patients Undergoing Carfilzomib Therapy. Cancers 2019, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Atrash, S.; Tullos, A.; Panozzo, S.; Bhutani, M.S.; Van Rhee, F.; Barlogie, B.; Usmani, S.Z. Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J. 2015, 5, e272. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Hajje, D. Case series discussion of cardiac and vascular events following carfilzomib treatment: Possible mechanism, screening, and monitoring. BMC Cancer 2014, 14, 915. [Google Scholar] [CrossRef]
- Danhof, S.; Schreder, M.; Rasche, L.; Strifler, S.; Einsele, H.; Knop, S. ‘Real-life’ experience of preapproval carfilzomib-based therapy in myeloma—Analysis of cardiac toxicity and predisposing factors. Eur. J. Haematol. 2015, 97, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; A Goudevenos, J.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2017, 39, 119–177. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall Population No = 116 |
|---|---|
| General | |
| Age, median (SD), years | 64.53 ± 8.42 |
| Male sex, No. (%) | 65 (56) |
| Individual CV risk factors, No (%) | |
| Tobacco use (prior/current) | 58 (50) |
| Obesity (BMI ≥ 30) | 34 (29.3) |
| Known Arterial Hypertension | 48 (41.4) |
| Diabetes | 12 (10.3) |
| Chronic renal failure (eGFR < 60 mL/m) | 22 (19) |
| Ischemic heart disease | 4 (3.4) |
| Previous episodes of atrial fibrillation | 2 (1.7) |
| Dyslipidaemia | 16 (13.8) |
| Previous stroke | 0 (0) |
| Familiar CV risk factors, No (%) | |
| Ischemic heart disease | 26 (22.4) |
| Stroke | 13 (11.2) |
| Diabetes | 6 (5.2) |
| Arterial Hypertension | 15 (12.9) |
| Anti-hypertensive drugs, No (%) | |
| Beta-blockers | 26 (22.4) |
| ACE-inhibitors/angiotensin receptor blockers | 59 (50.9) |
| Thiazide diuretics/Loop diuretics | 25 (21.6) |
| Aldosterone receptor antagonists | 2 (1.7) |
| Calcium channel blockers | 25 (21.6) |
| Office BP values | |
| SBP, mean (SD), mmHg | 129.4 ± 17.8 |
| DBP, mean (SD), mmHg | 76.9 ± 11.2 |
| ABPM * | |
| Daytime SBP, mean (SD), mmHg | 125.0 ± 13.2 |
| Daytime DBP, mean (SD), mmHg | 75.1 ± 8.9 |
| 24 h SBP, mean (SD), mmHg | 120.9 ± 12.7 |
| 24 h DBP, mean (SD), mmHg | 71.7 ± 8.1 |
| 24 h MBP, mean (SD), mmHg | 88.5 ± 9.1 |
| HMOD, No (%) | |
| LVH | 24 (20.7) |
| GLS value † ≤ 20 % | 24 (20.7) |
| PWV value ‡ ≥ 9 m/s | 31 (26.7) |
| Oncological History | |
| Median MM disease duration, months | 51.1 |
| Carfilzomib line therapy, number | 3 ± 1.6 |
| Previous therapies ˆ, No. (%) | |
| Anthracyclines | 31 (26.7) |
| Alkylating agents | 91 (78.4) |
| Immunomodulating agents | 86 (74.1) |
| Bortezomib | 97 (83.6) |
| CVAE | Patients n = 116 No. (%) * | No. CVAEs | |
|---|---|---|---|
| Grade 1–2 | Grade ≥3 | ||
| Major CVAEs | 17 (14.7) | 12 | 10 |
| ACS (STEMI) | 1 (0.9) | 0 | 1 |
| ACS (NSTEMI) | 3 (2.6) | 0 | 3 |
| Typical Chest pain | 3 (2.6) | 3 | 0 |
| Heart failure | 1 (0.9) | 0 | 1 |
| Dyspnoea post infusion | 4 (3.5) | 2 | 2 |
| Syncope/pre-syncope | 1 (0.9) | 0 | 1 |
| Arrhythmias | 7 (6.0) | 6 | 1 |
| Sudden cardiac death | 1 (0.9) | NA | 1 |
| Hypertension-related CVAEs | 45 (38.7) | 75 | 29 |
| New onset/worsened hypertension | 37 (31.9) | 37 | 0 |
| Masked hypertension | 4 (3.5) | 4 | 0 |
| White coat hypertension | 0 (0) | 0 | 0 |
| Pre-infusion uncontrolled hypertension [infusion limiting] | 11 (9.5) | 6 | 9 |
| Pre-infusion uncontrolled hypertension [not-infusion limiting] | 20 (17.2) | 20 | 10 |
| Post-infusion uncontrolled hypertension | 11 (9.5) | 8 | 6 |
| Symptomatic uncontrolled hypertension | 4 (3.5) | 0 | 4 |
| Hypertensive emergency | 0 (0) | 0 | 0 |
| All-type CVAEs | 52 (44.9) | 87 | 39 |
| Both major and hypertensive CVAEs | 10 (8.6) | 26 | 13 |
| Characteristics | No CVAEs No = 64 (53.4%) | CVAEs No = 52 (46.6%) | p Value |
|---|---|---|---|
| Demographic and clinical | |||
| Age, median (SD), years | 63.34 ± 8.26 | 66.00 ± 8.46 | 0.092 |
| Male sex | 36 (56.3) | 29 (55.8) | 0.959 |
| Individual CV risk factors, No (%) | |||
| Tobacco use (past/current) | 29 (45.3) | 29 (55.8) | 0.263 |
| Known Arterial Hypertension | 25 (39.1) | 23 (44.2) | 0.574 |
| Diabetes | 7 (10.9) | 5 (9.6) | 0.816 |
| Chronic renal failure (eGFR < 60 mL/m) | 15 (23.4) | 7 (13.5) | 0.173 |
| Coronary artery disease | 1 (1.6) | 3 (5.8) | 0.217 |
| Previous Atrial Fibrillation | 2 (3.1) | 0 (0) | 0.501 |
| Dyslipidaemia | 10 (15.6) | 6 (11.5) | 0.526 |
| Previous stroke | 0 (0) | 0 (0) | NA |
| Anti-hypertensive drugs ≥ 3 | 3 (4.7) | 5 (9.6) | 0.298 |
| Office BP values, mean (SD) | |||
| SBP, mmHg | 124.71 ± 17.25 | 135.6 ± 16.87 | 0.002 |
| DBP, mmHg | 74.73 ± 12.39 | 79.35 ± 9.12 | 0.024 |
| HR, bpm | 77.22 ± 13.05 | 76.29 ± 13.49 | 0.720 |
| ABPM * | |||
| Daytime SBP, mean (SD), mmHg | 122.98 ± 13.88 | 127.39 ± 12.15 | 0.008 |
| Daytime DBP, mean (SD), mmHg | 73.95 ± 9.40 | 76.37 ± 8.31 | 0.534 |
| Daytime SD, mean (SD) | 10.81 ± 3.80 | 15.14 ± 11.62 | 0.009 |
| 24 h SBP, mean (SD), mmHg | 119.24 ± 13.48 | 122.94 ± 11.63 | 0.131 |
| 24 h DBP, mean (SD), mmHg | 70.76 ± 8.38 | 72.73 ± 7.73 | 0.208 |
| 24 h MBP, mean (SD), mmHg | 87.69 ± 9.54 | 89.39 ± 8.57 | 0.336 |
| 24 h SD, mean (SD) | 12.43 ± 3.82 | 15.11± 4.64 | 0.002 |
| Night time SD, mean (SD) | 8.45 ± 2.82 | 10.22 ± 4.02 | 0.011 |
| Dipping < 10%, No. (%) | 25 (43.9) | 13 (28.9) | 0.120 |
| Blood pressure variability, No. (%) | 8.10 ± 2.47 | 10.31 ± 4.08 | 0.001 |
| Echocardiography† | |||
| LVMi, mean (SD), g/m2 | 85.30 ± 19.72 | 95.14 ± 21.75 | 0.013 |
| LVEF, mean (SD), %* | 63.03 ± 6.56 | 61.96 ± 7.13 | 0.414 |
| GLS, mean (SD), %* | −22.37 ± 2.56 | −21.3 ± 2.46 | 0.029 |
| Diastolic dysfunction, No. (%) | 1 (1.6) | 0(0) | 0.362 |
| Arterial Stiffness‡ | |||
| PWV value, mean (SD), m/s | 7.41 ± 1.63 | 8.55 ± 1.855 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astarita, A.; Mingrone, G.; Airale, L.; Vallelonga, F.; Covella, M.; Catarinella, C.; Cesareo, M.; Bruno, G.; Leone, D.; Giordana, C.; et al. Multiple Myeloma Patients Undergoing Carfilzomib: Development and Validation of a Risk Score for Cardiovascular Adverse Events Prediction. Cancers 2021, 13, 1631. https://doi.org/10.3390/cancers13071631
Astarita A, Mingrone G, Airale L, Vallelonga F, Covella M, Catarinella C, Cesareo M, Bruno G, Leone D, Giordana C, et al. Multiple Myeloma Patients Undergoing Carfilzomib: Development and Validation of a Risk Score for Cardiovascular Adverse Events Prediction. Cancers. 2021; 13(7):1631. https://doi.org/10.3390/cancers13071631
Chicago/Turabian StyleAstarita, Anna, Giulia Mingrone, Lorenzo Airale, Fabrizio Vallelonga, Michele Covella, Cinzia Catarinella, Marco Cesareo, Giulia Bruno, Dario Leone, Carlo Giordana, and et al. 2021. "Multiple Myeloma Patients Undergoing Carfilzomib: Development and Validation of a Risk Score for Cardiovascular Adverse Events Prediction" Cancers 13, no. 7: 1631. https://doi.org/10.3390/cancers13071631
APA StyleAstarita, A., Mingrone, G., Airale, L., Vallelonga, F., Covella, M., Catarinella, C., Cesareo, M., Bruno, G., Leone, D., Giordana, C., Cetani, G., Salvini, M., Gay, F., Bringhen, S., Rabbia, F., Veglio, F., & Milan, A. (2021). Multiple Myeloma Patients Undergoing Carfilzomib: Development and Validation of a Risk Score for Cardiovascular Adverse Events Prediction. Cancers, 13(7), 1631. https://doi.org/10.3390/cancers13071631








