Fibroblast Growth Factor Receptor 2 Isoforms Detected via Novel RNA ISH as Predictive Biomarkers for Progestin Therapy in Atypical Hyperplasia and Low-Grade Endometrial Cancer
Abstract
:Simple Summary
Abstract
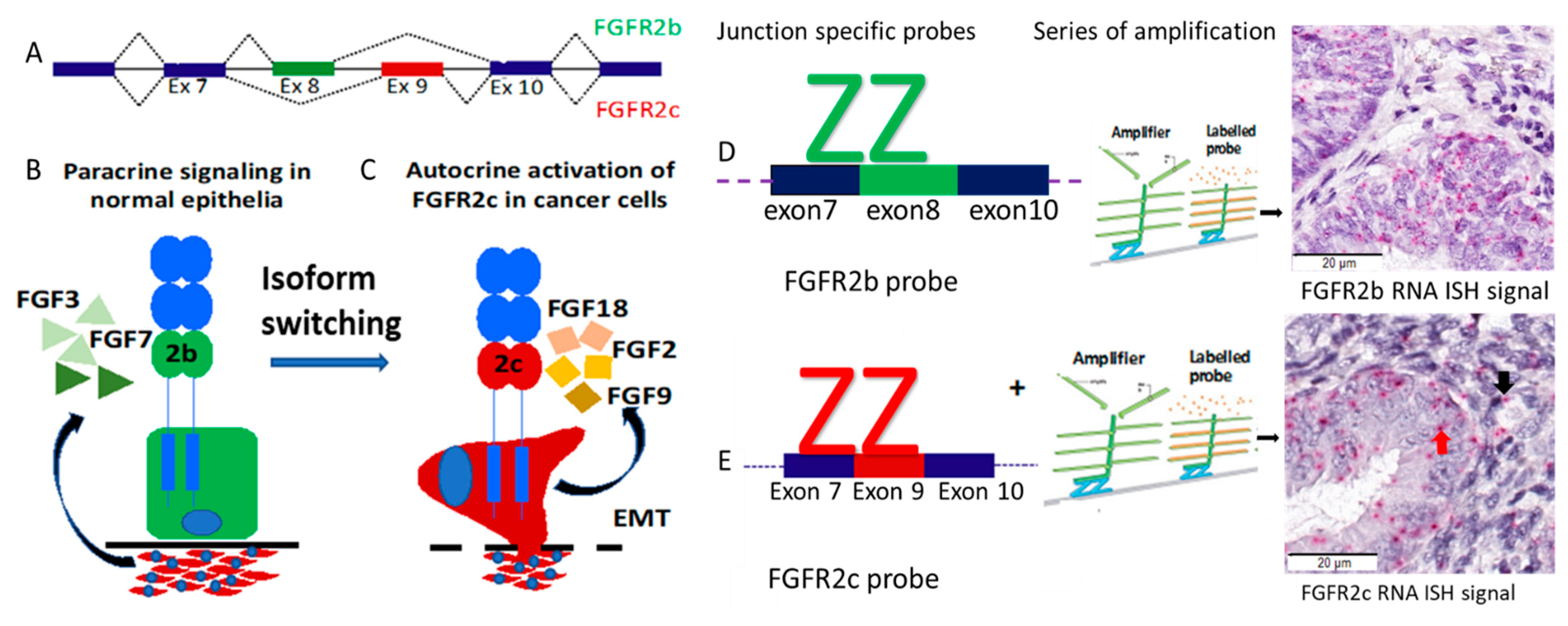
1. Introduction
2. Results
2.1. Patient Cohort Characteristics and Treatment Outcomes
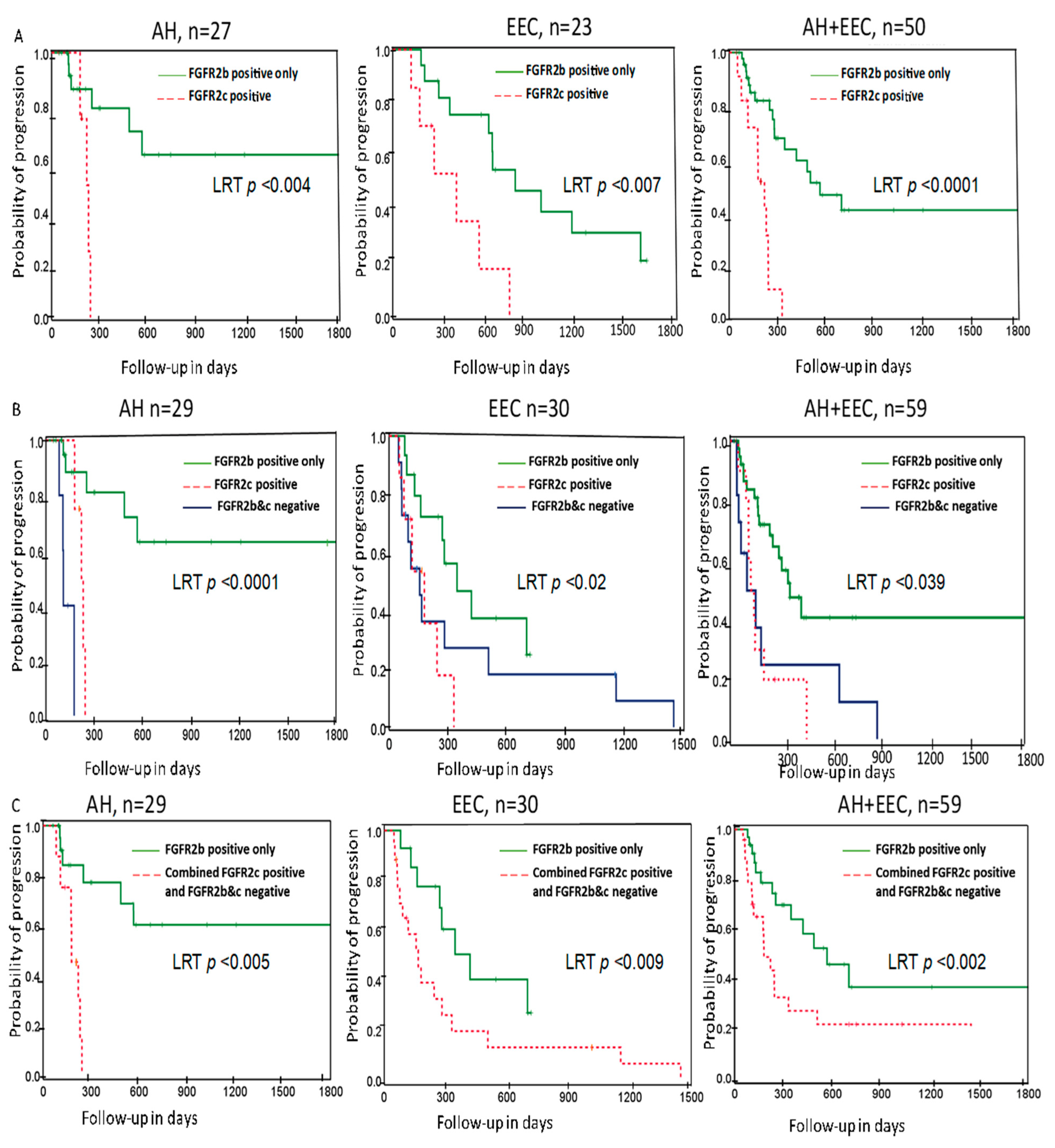
2.2. Association of FGFR2 Isoforms with Treatment Outcome
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Tissue Microarray (TMA) Construction
4.3. Immunohistochemistry Staining
4.4. BaseScope RNA ISH Assay for Detection of FGFR2 Isoforms and Signal Scoring
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beral, V.; Bull, D.; Reeves, G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005, 365, 1543–1551. [Google Scholar]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. Classification of Tumours of Female Reproductive Organ. In WHO Classification of Tumours, 4 ed.; WHO/IARC: Lyon, France, 2014. [Google Scholar]
- Kurman, R.J.; Kaminski, P.F.; Norris, H.J. The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer 1985, 56, 403–412. [Google Scholar] [CrossRef]
- Leitao, M.M.; Han, G.; Lee, L.X.; Abu-Rustum, N.R.; Brown, C.L.; Chi, D.S.; Sonoda, Y.; Levine, D.A.; Gardner, G.J.; Jewell, E.E.; et al. Complex atypical hyperplasia of the uterus: Characteristics and prediction of underlying carcinoma risk. Am. J. Obstet. Gynecol. 2010, 203, 349. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.P.; Huang, Y.; Hou, J.Y.; Tergas, A.I.; Burke, W.M.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. All-cause mortality in young women with endometrial cancer receiving progesterone therapy. Am. J. Obstet. Gynecol. 2017, 217, e661–e669. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, R.S.; Ciccone, M.A.; Nusbaum, D.J.; Khoshchehreh, M.; Purswani, H.; Morocco, E.B.; Smith, M.B.; Matsuzaki, S.; Dancz, C.E.; Ozel, B.; et al. Progestin therapy for obese women with complex atypical hyperplasia: Levonorgestrel-releasing intrauterine device vs systemic therapy. Am. J. Obstet. Gynecol. 2020, 223, e101–e113. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, R.; Ryan, N.A.J.; Barr, C.E.; Derbyshire, A.E.; Wan, Y.L.; Maskell, Z.; Stocking, K.; Pemberton, P.W.; Bolton, J.; McVey, R.J.; et al. Baseline Serum HE4 But Not Tissue HE4 Expression Predicts Response to the Levonorgestrel-Releasing Intrauterine System in Atypical Hyperplasia and Early Stage Endometrial Cancer. Cancers 2020, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Pal, N.; Broaddus, R.R.; Urbauer, D.L.; Balakrishnan, N.; Milbourne, A.; Schmeler, K.M.; Meyer, L.A.; Soliman, P.T.; Lu, K.H.; Ramirez, P.T.; et al. Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia with the Levonorgestrel-Releasing Intrauterine Device. Obstet. Gynecol. 2018, 131, 109–116. [Google Scholar] [CrossRef]
- Maggiore, U.L.R.; Martinelli, F.; Dondi, G.; Bogani, G.; Chiappa, V.; Evangelista, M.T.; Liberale, V.; Ditto, A.; Ferrero, S.; Raspagliesi, F. Efficacy and fertility outcomes of levonorgestrel-releasing intra-uterine system treatment for patients with atypical complex hyperplasia or endometrial cancer: A retrospective study. J. Gynecol. Oncol. 2019, 30, e57. [Google Scholar] [CrossRef]
- Ørbo, A.; Arnes, M.; Vereide, A.B.; Straume, B. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel-impregnated intrauterine system or oral progestogens. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1512–1519. [Google Scholar] [CrossRef]
- Baker, W.D.; Pierce, S.R.; Mills, A.M.; Gehrig, P.A.; Duska, L.R. Nonoperative management of atypical endometrial hyperplasia and grade 1 endometrial cancer with the levonorgestrel intrauterine device in medically ill post-menopausal women. Gynecol. Oncol. 2017, 146, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Chapman-Davis, E. Role of Progesterone in Endometrial Cancer. Semin Reprod. Med. 2010, 28, 081–090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, J.; Hori, M.; Ichigo, S.; Tamaya, T. Expressions of the Fibroblast Growth Factor Family (FGF-1,-2 and-4)mRNA in Endometrial Cancers. Tumour Biol. 1996, 17, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Gartside, M.G.; Dejeza, L.C.; Powell, M.A.; Mallon, M.A.; Davies, H.; Mohammadi, M.; Futreal, P.A.; Stratton, M.R.; Trent, J.M.; et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene 2007, 26, 7158–7162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatius, S.; Velasco, A.; Azueta, A.; Santacana, M.; Pallares, J.; Valls, J.; Dolcet, X.; Prat, J.; Matias-Guiu, X. FGFR2 alterations in endometrial carcinoma. Mod. Pathol. 2011, 24, 1500–1510. [Google Scholar] [CrossRef] [Green Version]
- Dutt, A.; Salvesen, H.B.; Chen, T.H.; Ramos, A.H.; Onofrio, R.C.; Hatton, C.; Nicoletti, R.; Winckler, W.; Grewal, R.; Hanna, M.; et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc. Natl. Acad. Sci. USA 2008, 105, 8713–8717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornitz, D.M.; Xu, J.; Colvin, J.S.; McEwen, D.G.; MacArthur, C.A.; Coulier, F.; Gao, G.; Goldfarb, M. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996, 271, 15292–15297. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, D.; Rosato, B.; Nanni, M.; Magenta, A.; Belleudi, F.; Torrisi, M.R. Expression of the FGFR2 mesenchymal splicing variant in epithelial cells drives epithelial-mesenchymal transition. Oncotarget 2016, 7, 5440–5460. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, D.; Rosato, B.; Nanni, M.; Belleudi, F.; Torrisi, M.R. Expression of the FGFR2c mesenchymal splicing variant in human keratinocytes inhibits differentiation and promotes invasion. Mol. Carcinog. 2018, 57, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Warzecha, C.C.; Sato, T.K.; Nabet, B.; Hogenesch, J.B.; Carstens, R.P. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol. Cell 2009, 33, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Amann, T.; Bataille, F.; Spruss, T.; Dettmer, K.; Wild, P.; Liedtke, C.; Mühlbauer, M.; Kiefer, P.; Oefner, P.J.; Trautwein, C.; et al. Reduced Expression of Fibroblast Growth Factor Receptor 2IIIb in Hepatocellular Carcinoma Induces a More Aggressive Growth. Am. J. Pathol. 2010, 176, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Ricol, D.; Cappellen, D.; El Marjou, A.; Gil-Diez-de-Medina, S.; Girault, J.M.; Yoshida, T.; Ferry, G.; Tucker, G.; Poupon, M.F.; Chopin, D.; et al. Tumour suppressive properties of fibroblast growth factor receptor 2-IIIb in human bladder cancer. Oncogene 1999, 18, 7234–7243. [Google Scholar] [CrossRef] [Green Version]
- Sengal, A.T.; Patch, A.-M.; Snell, C.E.; Smith, D.S.; Leung, S.C.Y.; Talhouk, A.; Williams, E.D.; McAlpine, J.N.; Pollock, P.M. FGFR2c Mesenchymal Isoform Expression Is Associated with Poor Prognosis and Further Refines Risk Stratification within Endometrial Cancer Molecular Subtypes. Clin. Cancer Res. 2020, 26, 4569–4580. [Google Scholar] [CrossRef]
- Penner, K.R.; Dorigo, O.; Aoyama, C.; Ostrzega, N.; Balzer, B.L.; Rao, J.; Walsh, C.S.; Cass, I.; Holschneider, C.H. Predictors of resolution of complex atypical hyperplasia or grade 1 endometrial adenocarcinoma in premenopausal women treated with progestin therapy. Gynecol. Oncol. 2012, 124, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Westin, S.N.; Fellman, B.; Sun, C.C.; Broaddus, R.R.; Woodall, M.L.; Pal, N.; Urbauer, D.L.; Ramondetta, L.M.; Schmeler, K.M.; Soliman, P.T.; et al. Prospective phase II trial of levonorgestrel intrauterine device: Nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am. J. Obstet. Gynecol. 2020, 224, 191-e1–e1. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.L.; Beverley-Stevenson, R.; Carlisle, D.; Clarke, S.; Edmondson, R.J.; Glover, S.; Holland, J.; Hughes, C.; Kitchener, H.C.; Kitson, S.; et al. Working together to shape the endometrial cancer research agenda: The top ten unanswered research questions. Gynecol. Oncol. 2016, 143, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, A.; Kudo, M.; Nakazawa, N.; Onda, M.; Ishiwata, T.; Takeshita, T.; Naito, Z. Expression of keratinocyte growth factor and its receptor in human endometrial cancer in cooperation with steroid hormones. Int. J. Oncol. 2008, 32, 565–574. [Google Scholar] [CrossRef]
- Siegfried, S.; Pekonen, F.; Nyman, T.; Ämmälä, M.; Rutanen, E.-M. Distinct patterns of expression of keratinocyte growth factor and its receptor in endometrial carcinoma. Cancer 1997, 79, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Visco, V.; Carico, E.; Marchese, C.; Torrisi, M.R.; Frati, L.; Vecchione, A.; Muraro, R. Expression of keratinocyte growth factor receptor compared with that of epidermal growth factor receptor and erbB-2 in endometrial adenocarcinoma. Int. J. Oncol. 1999, 15, 431–435. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Toratani, S.; Sato, J.D.; Kan, M.; McKeehan, W.L.; Okamoto, T. Growth inhibition by keratinocyte growth factor receptor of human salivary adenocarcinoma cells through induction of differentiation and apoptosis. Proc. Natl. Acad. Sci. USA 2001, 98, 11336–11340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasumoto, H.; Matsubara, A.; Mutaguchi, K.; Usui, T.; McKeehan, W.L. Restoration of fibroblast growth factor receptor2 suppresses growth and tumorigenicity of malignant human prostate carcinoma PC-3 cells. Prostate 2004, 61, 236–242. [Google Scholar] [CrossRef]
- McNiel, E.A.; Tsichlis, P.N. Analyses of publicly available genomics resources define FGF-2-expressing bladder carcinomas as EMT-prone, proliferative tumors with low mutation rates and high expression of CTLA-4, PD-1 and PD-L1. Signal. Transduct. Target. Ther. 2017, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Sanidas, I.; Polytarchou, C.; Hatziapostolou, M.; Ezell Scott, A.; Kottakis, F.; Hu, L.; Guo, A.; Xie, J.; Comb Michael, J.; Iliopoulos, D.; et al. Phosphoproteomics Screen Reveals Akt Isoform-Specific Signals Linking RNA Processing to Lung Cancer. Mol. Cell 2014, 53, 577–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 6, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Pearson, A.; Sharpe, R.; Lambros, M.; Geyer, F.; Lopez-Garcia, M.A.; Natrajan, R.; Marchio, C.; Iorns, E.; Mackay, A.; et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010, 70, 2085–2094. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, D.C.; Knowles, M.A.; Speirs, V. Mechanisms of FGFR3 actions in endocrine resistant breast cancer. Int. J. Cancer 2012, 130, 2857–2866. [Google Scholar] [CrossRef]
- Byron, S.A.; Gartside, M.G.; Wellens, C.L.; Mallon, M.A.; Keenan, J.B.; Powell, M.A.; Goodfellow, P.J.; Pollock, P.M. Inhibition of activated fibroblast growth factor receptor 2 in endometrial cancer cells induces cell death despite PTEN abrogation. Cancer Res. 2008, 68, 6902–6907. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.-B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.M.; Rosales, M.A.; Paik, D.Y.; Lee, D.S.; Smith, D.A.; Witte, O.N.; Iruela-Arispe, M.L.; Memarzadeh, S. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 2013, 73, 4697–4710. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Torres, S.; Murdock, T.; Matsuno, R.; Beavis, A.L.; Stone, R.L.; Wethington, S.L.; Levinson, K.; Grumbine, F.; Ferriss, J.S.; Tanner, E.J.; et al. The addition of metformin to progestin therapy in the fertility-sparing treatment of women with atypical hyperplasia/endometrial intraepithelial neoplasia or endometrial cancer: Little impact on response and low live-birth rates. Gynecol. Oncol. 2020, 157, 348–356. [Google Scholar] [CrossRef]
- Wheeler, D.T.; Bristow, R.E.; Kurman, R.J. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am. J. Surg. Pathol. 2007, 31, 988–998. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat. Clin. Pract. Urol. 2005, 2, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J. (REDCap) Redc: A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Clinicopathologic Characteristics | Responders | Non-Responders | p-Value a | |||
|---|---|---|---|---|---|---|
| n = 30 | % | n = 39 | % | |||
| Age Category * | <50 | 13 | 43% | 7 | 18% | 0.068 |
| 50–60 | 8 | 27% | 18 | 46% | ||
| ≥60 | 9 | 30% | 14 | 36% | ||
| BMI Category* | <30 | 0 | 0% | 4 | 10% | 0.227 |
| 30–40 | 6 | 20% | 5 | 13% | ||
| ≥40 | 21 | 70% | 28 | 72% | ||
| Unknown b | 3 | 10% | 2 | 5% | ||
| Indication of treatment* | Comorbidities/surgical risk | 12 | 40% | 24 | 62% | 0.36 |
| Patient choice | 3 | 10% | 1 | 3% | ||
| Preserve fertility | 6 | 20% | 4 | 10% | ||
| Symptom control until definite hysterectomy | 8 | 27% | 9 | 23% | ||
| Unknown | 1 | 3% | 1 | 2.6% | ||
| Hysterectomy status | No | 20 | 67% | 11 | 28% | 0.001 |
| Yes | 10 | 33% | 28 | 71% | ||
| Biopsy type during response assessment* | Curette | 19 | 63% | 27 | 69% | 0.466 |
| Hysterectomy | 3 | 10% | 6 | 16% | ||
| Pipelle | 8 | 27% | 6 | 16% | ||
| Pre-treatment diagnosis* | AH | 23 | 77% | 13 | 33% | 0.0001 |
| Endometrioid EC | 7 | 23% | 26 | 67% | ||
| Grade | Not applicable c | 23 | 77% | 13 | 33% | 0.0001 |
| Grade 1 | 5 | 17% | 24 | 62% | ||
| Grade 2 | 2 | 7% | 2 | 5% | ||
| FGFR2 IHC Score | Low | 5 | 17% | 8 | 21% | 0.378 |
| High | 21 | 70% | 26 | 67% | ||
| Missing cores b | 4 | 13% | 5 | 13% | ||
| FGFR2 Isoform status* | FGFR2b+/FGFR2c− | 23 | 77% | 17 | 44% | 0.005 |
| FGFR2b−/FGFR2c− | 2 | 8% | 7 | 18% | ||
| FGFR2b+/FGFR2c+ | 1 | 3% | 9 | 23% | ||
| Unknown | 4 | 13% | 6 | 15% | ||
| Stroma1 PR Score | ≤10% | 11 | 37% | 21 | 54% | 0.049 |
| >10% | 15 | 50% | 13 | 33% | ||
| Missing cores b | 4 | 13 | 5 | 13% | ||
| Epithelial Tumour PR Score | ≤50% | 1 | 3% | 3 | 8% | 0.42 |
| >50% | 28 | 93.4% | 33 | 85% | ||
| Missing cores b | 1 | 3.3% | 3 | 8% | ||
| Concurrent Metformin | LNG-IUD | 23 | 77% | 25 | 64% | 0.403 |
| LNG-IUD + Metformin | 7 | 23% | 13 | 33% | ||
| Unknown b | 0 | 0% | 1 | 3% | ||
| Route of progestin therapy | LNG-IUD only | 25 | 83% | 32 | 82% | 0.93 |
| LNG-IUD + Oral Progestin | 5 | 17% | 7 | 18% | ||
| Variables [Reference] | Univariable Analyses | Multivariable Analyses | |||||
|---|---|---|---|---|---|---|---|
| N Total | HR | 95%CI | LRTP | HR | 95%CI | LRTP | |
| Age in years | 69 | 0.028 | 0.188 | ||||
| Age 50–60 [<50] | 2.67 | 1.163–6.137 | 0.021 | 1.02 | 0.99–2.09 | 0.435 | |
| Age ≥ 60 [<50] | 3.19 | 1.319–7.726 | 0.01 | 1.45 | 0872–2.65 | 0.165 | |
| BMI 30 (Kg/m2) | 64 | 0.001 | 0.052 | ||||
| BMI 30–40 [<30] | 1.32 | 1.15–2.452 | 0.002 | 1.13 | 0.84–1.63 | 0.056 | |
| BMI ≥ 40 [<30] | 1.17 | 1.61–3.444 | 0.001 | 1.37 | 0.87–1.81 | 0.29 | |
| Pre-treatment diagnosis EEC [AH] | 69 | 2.01 | 1.039–3.836 | 0.038 | 1.24 | 0.984 -2.88 | 0.078 |
| Grade at diagnosis | 69 | 0.052 | 0.232 | ||||
| Grade 1 [AH] | 1.98 | 1.023–3.834 | 0.043 | 1.61 | 0.86–3.01 | 0.14 | |
| Grade 2 [AH] | 2.24 | 0.499–10.078 | 0.292 | 1.54 | 0.987–5.3 | 0.097 | |
| FGFR2b+/FGFR2c+ [FGFR2b+/FGFR2c- a] | 50 * | 5.08 | 2.018–12.774 | 0.0001 | 4.50 | 1.92–11.32 | 0.002 |
| FGFR2 protein H-Score High [Low] | 60 | 0.83 | 0.406–1.684 | 0.6 | - | - | - |
| PR tumour expression ≥50% [<50%] | 63 | 0.78 | 0.245–1.367 | 0.121 | - | - | - |
| PR Stromal expression ≥10% [<10%] | 63 | 0.69 | 0.346–1.356 | 0.278 | - | - | - |
| LNG-IUD+ Metformin [LNG-IUD only] | 62 | 0.97 | 0.467–1.772 | 0.78 | - | - | - |
| LNG_IUD +oral progestin [LNG_IUD only] | 68 | 0.61 | 0.268–1.387 | 0.238 | - | - | - |
| Clinicopathologic Characteristics | FGFR2b+ /FGFR2c- | FGFR2b-/ FGFR2c- | FGFR2b+ /FGFR2c+ | Unknown Status | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age in years | <50 | 13 | 28% | 4 | 31% | 3 | 25% | 7 | 41% |
| 50–60 | 15 | 32% | 7 | 54% | 4 | 33% | 6 | 35% | |
| >60 | 19 | 40% | 2 | 15% | 5 | 42% | 4 | 24% | |
| BMI in Kg/m2 | <30 | 3 | 6% | 2 | 15% | 2 | 16.7% | 0 | 0% |
| 30–40 | 6 | 13% | 1 | 8% | 3 | 25% | 6 | 35% | |
| >40 | 33 | 70% | 9 | 69% | 6 | 50% | 10 | 59% | |
| Missing | 5 | 11% | 1 | 8% | 1 | 8% | 1 | 6% | |
| Histologic diagnosis | AH | 27 | 57% | 4 | 31% | 4 | 33% | 5 | 29% |
| Endometrioid EC | 20 | 43% | 9 | 69% | 8 | 67% | 12 | 71% | |
| FGFR2 IHC Score | Low | 9 | 19% | 6 | 46% | 2 | 17% | 0 | 0% |
| High | 36 | 77% | 7 | 54% | 10 | 83% | 4 | 23.5% | |
| missing | 2 | 4% | 0 | 0% | 0 | 0% | 13 | 77% | |
| PR score stroma1 | ≤10 | 23 | 49% | 8 | 62% | 8 | 67% | 3 | 19% |
| >10 | 19 | 40% | 4 | 31% | 4 | 33% | 6 | 36% | |
| Missing | 5 | 11% | 1 | 8% | 0 | 0% | 7 | 44% | |
| Tumour PR Score | ≤50% | 3 | 6% | 2 | 15% | 1 | 8% | 1 | 6% |
| >50% | 41 | 87% | 11 | 85% | 11 | 92% | 10 | 59% | |
| missing | 3 | 6% | 0 | 0% | 0 | 0% | 6 | 35% | |
| Combination treatment | LNG-IUD only | 30 | 71% | 10 | 77% | 7 | 64% | 9 | 56% |
| LNG-IUD + Metformin | 12 | 29% | 3 | 23% | 4 | 36% | 7 | 44% | |
| Missing | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Route of treatment | LNG-IUD only | 40 | 84% | 11 | 85% | 11 | 92% | 14 | 82% |
| LNG-IUD + Oral Progestin | 7 | 16% | 2 | 15% | 1 | 8% | 0 | 0% | |
| Unknown | 0 | 0% | 0 | 0% | 0 | 0% | 3 | 18% | |
| Treatment Outcome | Responders | 23 | 49% | 2 | 15% | 1 | 8% | 4 | 24% |
| Non-responders | 17 | 36% | 7 | 54% | 9 | 75% | 6 | 35% | |
| Excluded from analyses | 7 | 15% | 4 | 31% | 2 | 17% | 7 | 41% | |
| Recurrence | No | 31 | 64% | 9 | 69% | 9 | 75% | 10 | 59% |
| Yes | 9 | 21% | 0 | 0% | 1 | 8% | 0 | 0% | |
| Excluded from analyses | 7 | 15% | 4 | 30.8% | 2 | 17% | 7 | 41% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengal, A.T.; Smith, D.; Rogers, R.; Snell, C.E.; Williams, E.D.; Pollock, P.M. Fibroblast Growth Factor Receptor 2 Isoforms Detected via Novel RNA ISH as Predictive Biomarkers for Progestin Therapy in Atypical Hyperplasia and Low-Grade Endometrial Cancer. Cancers 2021, 13, 1703. https://doi.org/10.3390/cancers13071703
Sengal AT, Smith D, Rogers R, Snell CE, Williams ED, Pollock PM. Fibroblast Growth Factor Receptor 2 Isoforms Detected via Novel RNA ISH as Predictive Biomarkers for Progestin Therapy in Atypical Hyperplasia and Low-Grade Endometrial Cancer. Cancers. 2021; 13(7):1703. https://doi.org/10.3390/cancers13071703
Chicago/Turabian StyleSengal, Asmerom T., Deborah Smith, Rebecca Rogers, Cameron E. Snell, Elizabeth D. Williams, and Pamela M. Pollock. 2021. "Fibroblast Growth Factor Receptor 2 Isoforms Detected via Novel RNA ISH as Predictive Biomarkers for Progestin Therapy in Atypical Hyperplasia and Low-Grade Endometrial Cancer" Cancers 13, no. 7: 1703. https://doi.org/10.3390/cancers13071703






