Treatment Patterns and Outcomes in a Nationwide Cohort of Older and Younger Veterans with Waldenström Macroglobulinemia, 2006–2019
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort
2.2. Patient and Disease Characteristics
Treatments and Patterns
2.3. Clinical Outcomes
2.4. Treatment Pattern Analyses
2.5. Outcome Analyses
Subgroup and Sensitivity Analyses
3. Results
3.1. Patient Characteristics
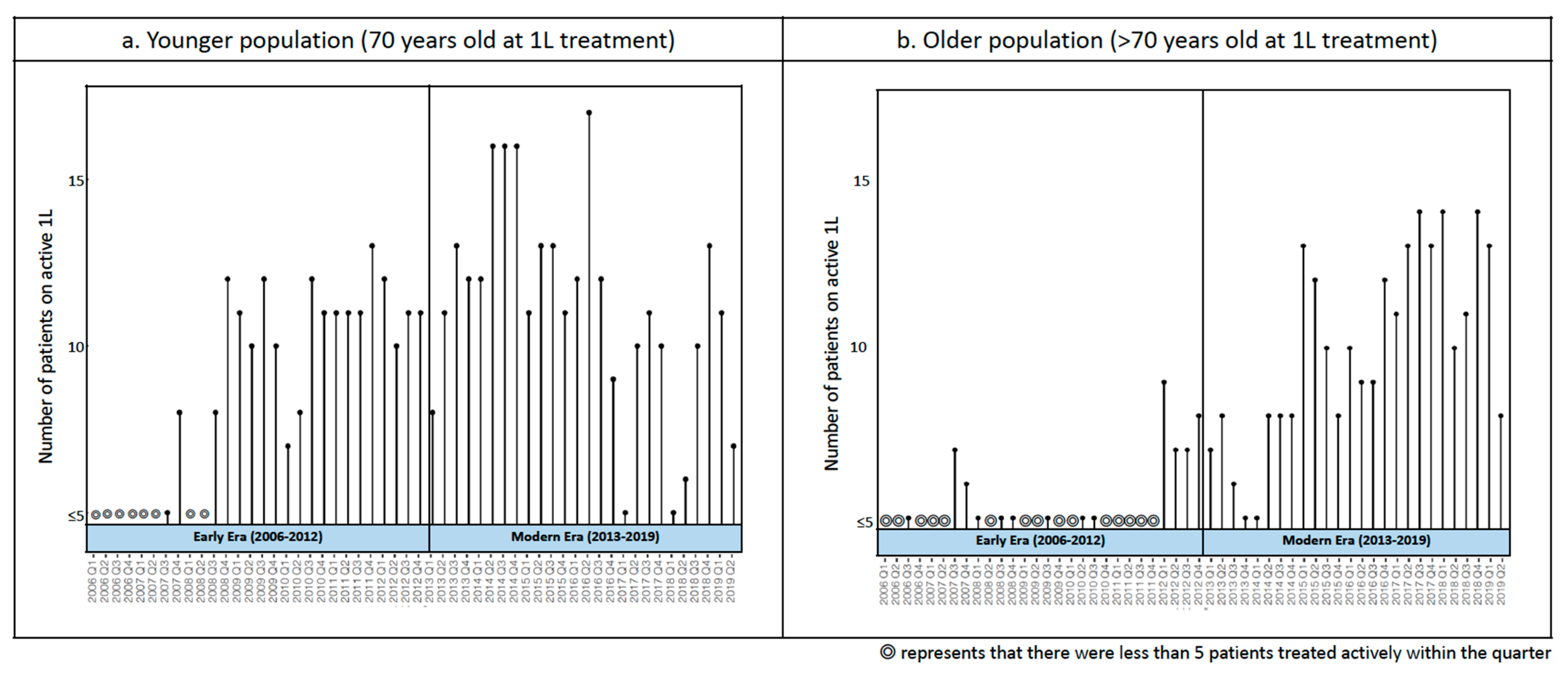
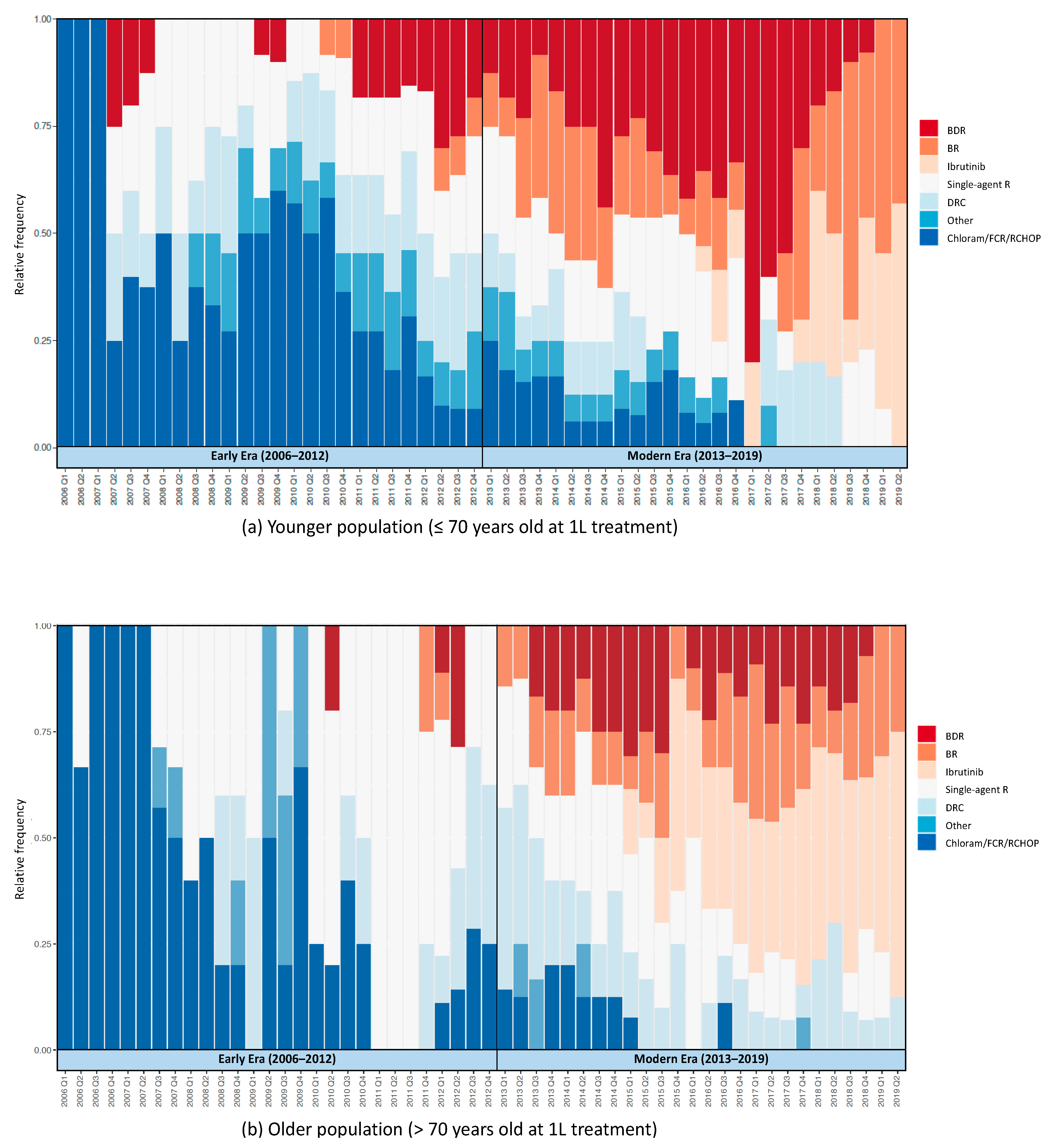
Treatment Patterns
3.2. Treatment Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Groves, F.D.; Travis, L.B.; Devesa, S.S.; Ries, L.A.; Fraumeni, J.F., Jr. Waldenström’s macroglobulinemia: Incidence patterns in the United States, 1988–1994. Cancer 1998, 82, 1078–1081. [Google Scholar] [CrossRef]
- Fonseca, R.; Hayman, S. Waldenström macroglobulinaemia. Br. J. Haematol. 2007, 138, 700–720. [Google Scholar] [CrossRef]
- Kyle, R.A.; Larson, D.R.; McPhail, E.D.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Kapoor, P.; Cerhan, J.R.; Rajkumar, S.V. Fifty-year incidence of Waldenström macroglobulinemia in Olmsted County, Minnesota, from 1961 through 2010: A population-based study with complete case capture and hematopathologic review. Mayo Clin. Proc. 2018, 93, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Olszewski, A.J.; Kanan, S.; Meid, K.; Hunter, Z.R.; Treon, S.P. Overall survival and competing risks of death in patients with Waldenström macroglobulinaemia: An analysis of the Surveillance, Epidemiology and End Results database. Br. J. Haematol. 2015, 169, 81–89. [Google Scholar] [CrossRef]
- Leblond, V.; Kastritis, E.; Advani, R.; Ansell, S.M.; Buske, C.; Castillo, J.J.; García-Sanz, R.; Gert Mz Kimby, E.; Kyriakou, C. Treatment recommendations from the Eighth International Workshop on Waldenström’s Macroglobulinemia. Blood 2016, 128, 1321–1328. [Google Scholar] [CrossRef]
- Treon, S.P.; Gertz, M.A.; Dimopoulos, M.; Anagnostopoulos, A.; Blade, J.; Branagan, A.R.; Garcia-Sanz, R.; Johnson, S.; Kimby, E.; Leblond, V.; et al. Update on treatment recommendations from the Third International Workshop on Waldenstrom’s macroglobulinemia. Blood 2006, 107, 3442–3446. [Google Scholar] [CrossRef]
- Treon, S.P.; Xu, L.; Guerrera, M.L.; Jimenez, C.; Hunter, Z.R.; Liu, X.; Demos, M.; Gustine, J.; Chan, G.; Munshi, M.; et al. Genomic landscape of Waldenström macroglobulinemia and its impact on treatment strategies. J. Clin. Oncol. 2020, 38, 1198–1208. [Google Scholar] [CrossRef]
- Rummel, M.J.; Niederle, N.; Maschmeyer, G.; Banat, G.A.; von Grünhagen, U.; Losem, C.; Kofahl-Krause, D.; Heil, G.; Welslau, M.; Balser, C.; et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013, 381, 1203–1210. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E.; Owen, R.G.; Kyle, R.A.; Landgren, O.; Morra, E.; Leleu, X.; García-Sanz, R.; Munshi, N.; Anderson, K.C.; et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood 2014, 124, 1404–1411. [Google Scholar] [CrossRef]
- Treon, S.P.; Ioakimidis, L.; Soumerai, J.D.; Patterson, C.J.; Sheehy, P.; Nelson, M.; Willen, M.; Matous, J.; Mattern, J., 2nd; Diener, J.G.; et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J. Clin. Oncol. 2009, 27, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Xie, W.; Padmanabhan, S.; Badros, A.; Rourke, M.; Leduc, R.; Chuma, S.; Kunsman, J.; Warren, D.; Poon, T.; et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenström Macroglobulinemia. Am. J. Hematol. 2010, 85, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; García-Sanz, R.; Gavriatopoulou, M.; Morel, P.; Kyrtsonis, M.-C.; Michalis, E.; Kartasis, Z.; Leleu, X.; Palladini, G.; Tedeschi, A.; et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): Long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood 2013, 122, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- IMBRUVICA®. Drugs@FDA: FDA-Approved Drugs. Published January 29, 2015. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=205552 (accessed on 13 September 2020).
- Klabunde, C.N.; Potosky, A.L.; Legler, J.M.; Warren, J.L. Development of a comorbidity index using physician claims data. J. Clin. Epidemiol. 2000, 53, 1258–1267. [Google Scholar] [CrossRef]
- Center for Drug Evaluation, Research. Clinical Trial Endpoints for the Approval of Non-Small Cell Lung Cancer. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-non-small-cell-lung-cancer-drugs-and-biologics (accessed on 14 September 2020).
- Wagner, A.K.; Soumerai, S.B.; Zhang, F.; Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002, 27, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2016, 46, 348–355. [Google Scholar]
- Liang, K.-Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Buske, C.; Leblond, V.; Dimopoulos, M.; Kimby, E.; Jäger, U.; Dreyling, M. Waldenström’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi155–vi159. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Anagnostopoulos, A.; Kyrtsonis, M.C.; Zervas, K.; Tsatalas, C.; Kokkinis, G.; Repoussis, P.; Symeonidis, A.; Delimpasi, S.; Katodritou, E.; et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J. Clin. Oncol. 2007, 25, 3344–3349. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Zervas, C.; Zomas, A.; Kiamouris, C.; Viniou, N.A.; Grigoraki, V.; Karkantaris, C.; Mitsouli, C.; Gika, D.; Christakis, J.; et al. Treatment of Waldenström’s macroglobulinemia with rituximab. J. Clin. Oncol. 2002, 20, 2327–2333. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Cummins, S.; Gasparrini, A. The use of controls in interrupted time series studies of public health interventions. Int. J. Epidemiol. 2018, 47, 2082–2093. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Tedeschi, A.; Trotman, J.; Trotman, J.; García-Sanz, R.; Macdonald, D.; Leblond, V.; Mahe, B.; Herbaux, C.; Tam, C.; et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N. Engl. J. Med. 2018, 378, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Treon, S.P.; Alexanian, R.; Barlogie, B.; Björkholm, M.; Dhodapkar, M.; Lister, T.A.; Merlini, G.; Morel, P.; Stone, M.; et al. Prognostic markers and criteria to initiate therapy in Waldenstrom’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003, 30, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Ferrari, A.; Frustaci, A.M.; Ferretti, V.V.; Rizzi, R.; Motta, M.; Piazza, F.; Merli, M.; Benevolo, G.; Visco, C.; et al. Younger patients with Waldenström macroglobulinemia exhibit low risk profile and excellent outcomes in the era of immunotherapy and targeted therapies. Am. J. Hematol. 2020, 95, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Sadullah, S.; Kastritis, E.; Tedeschi, A.; García-Sanz, R.; Bolkun, L.; Leleu, X.; Willenbacher, W.; Hájek, R.; Minnema, M.C.; et al. Treatment and outcome patterns in European patients with Waldenström’s macroglobulinaemia: A large, observational, retrospective chart review. Lancet Haematol. 2018, 5, e299–e309. [Google Scholar] [CrossRef]
- Zheng, Y.-H.; Xu, L.; Cao, C.; Feng, J.; Tang, H.; Shu, M.; Gao, G.; Chen, X. Rituximab-based combination therapy in patients with Waldenström macroglobulinemia: A systematic review and meta-analysis. Onco Targets Ther. 2019, 12, 2751–2766. [Google Scholar] [CrossRef] [PubMed]
- Brandefors, L.; Melin, B.; Lindh, J.; Lundqvist, K.; Kimby, E. Prognostic factors and primary treatment for Waldenström macroglobulinemia-a Swedish Lymphoma Registry study. Br. J. Haematol. 2018, 183, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Paludo, J.; Abeykoon, J.P.; Shreders, A.; Ansell, S.M.; Kumar, S.; Ailawadhi, S.; King, R.L.; Koehler, A.B.; Reeder, C.B.; Buadi, F.K.; et al. Bendamustine and rituximab (BR) versus dexamethasone, rituximab, and cyclophosphamide (DRC) in patients with Waldenström macroglobulinemia. Ann. Hematol. 2018, 97, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Leblond, V.; Dimopoulos, M.A.; Kimby, E.; Staber, P.; Kersten, M.J.; Tedeschi, A.; Buske, C. ESMO Guidelines Committee. Waldenström’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv41–iv50. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Xu, L.; Hunter, Z. MYD88 Mutations and response to ibrutinib in Waldenström’s macroglobulinemia. N. Engl. J. Med. 2015, 373, 584–586. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Waldenstrom’s Macroglobulinemia/Lymphoplasmacytic Lymphoma, Version 2.2016. 2016. Available online: http://www.nccn.org/ (accessed on 8 December 2020).
- Hawley, S.; Ali, M.S.; Berencsi, K.; Judge, A.; Prieto-Alhambra, D. Sample size and power considerations for ordinary least squares interrupted time series analysis: A simulation study. Clin. Epidemiol. 2019, 11, 197–205. [Google Scholar] [CrossRef]



| Characteristics | Younger Population (<70 Years of Age at 1 L Treatment) (n = 166) | Older Population (>70 Years of Age at 1 L Treatment) (n = 152) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2006–2019 (n = 166) | 2006–2012 (n = 75) | 2013–2019 (n = 91) | p-Value | 2006–2019 (n = 152) | 2006–2012 (n = 63) | 2013–2019 (n = 89) | p-Value | |
| Age at 1 L | ||||||||
| Mean (SD) | 62.8 (6.0) | 61.4 (6.3) | 64.0 (5.5) | 0.01 | 77.7 (5.7) | 78.6 (5.5) | 77.0 (5.7) | 0.07 |
| Age at diagnosis | ||||||||
| Mean (SD) | 62.4 (6.0) | 61.0 (6.3) | 63.2 (5.6) | 0.02 | 77.0 (5.8) | 78.3 (5.7) | 76.1 (5.8) | 0.02 |
| >70 years of age no. (%) | – | – | – | 145 (95.4) | 61 (96.8) | 84 (94.4) | 0.75 | |
| Male sex, no. (%) | 162 (97.5) | 73 (97.3) | 89 (97.8) | 1.0 | 150 (98.7) | 62 (98.4) | 88 (98.9) | 1.0 |
| Race, no. (%) | ||||||||
| Non-Hispanic White | 134 (80.7) | 62 (82.7) | 72 (79.1) | 0.69 | 137 (90.1) | 56 (88.9) | 81 (91.0) | 0.93 |
| Black | 25 (15.1) | 10 (13.3) | 15 (16.5) | 10 (6.6) | 5 (7.9) | 5 (5.6) | ||
| Other | 7 (4.2) | <5 | <5 | 5 (3.3) | <5 | <5 | ||
| Residential community, no. (%) | ||||||||
| Rural/Suburban | 34 (20.5) | 15 (20.0) | 19 (20.9) | 0.92 | 32 (21.1) | 17 (27.0) | 15 (16.9) | 0.05 |
| Metropolitan | 132 (79.5) | 60 (80.0) | 72 (79.1) | 120 (78.9) | 46 (73.0) | 74 (83.1) | ||
| Residential geographic region, no. (%) | ||||||||
| Midwest | 45 (27.1) | 20 (26.7) | 25 (27.5) | 0.97 | 42 (27.6) | 16 (25.4) | 26 (29.2) | 0.67 |
| Northeast | 28 (16.9) | 12 (16.0) | 16 (17.6) | 24 (15.8) | 12 (19.0) | 12 (13.5) | ||
| South | 56 (33.7) | 25 (33.3) | 31 (34.1) | 41 (27.0) | 19 (30.2) | 22 (24.7) | ||
| West | 37 (22.3) | 18 (24.0) | 19 (20.9) | 41 (27.0) | 14 (22.2) | 27 (30.3) | ||
| BMI ≥ 35 kg/m2, no. (%) | 12 (7.2) | 5 (6.7) | 7 (7.7) | 1.0 | 11 (7.2) | <5 | 9 (10.1) | 0.19 |
| NCI index at 1 L, no. (%) | ||||||||
| 0 | 77 (46.4) | 36 (48.0) | 41 (45.1) | 0.64 | 56 (36.8) | 28 (31.5) | 28 (31.5) | 0.17 |
| 1 | 42 (25.3) | 18 (24.0) | 24 (26.4) | 42 (27.6) | 21 (23.6) | 21 (23.6) | ||
| ≥2 | 42 (25.3) | 17 (23.9) | 25 (27.8) | 59 (38.8) | 20 (31.7) | 39 (43.8) | ||
| Not available, no. (%) | 5 (3.0) | <5 | <5 | <5 | 0 (0) | <5 | ||
| Laboratory values within a year prior to 1 L | ||||||||
| Hemoglobin, g/dL | ||||||||
| Median (range) | 10.8 (5.8–17.3) | 11.2 (6.8–17.3) | 10.6 (5.8–16.2) | 0.28 | 9.9 (5.9–15.1) | 9.7 (6.9–13.9) | 10.1 (5.9–15.1) | 0.88 |
| Below LRL, no. (%) | 142 (85.5) | 63 (84.0) | 79 (86.8) | 0.79 | 137 (90.1) | 58 (92.1) | 79 (88.8) | 0.61 |
| Platelet count, 109/L | ||||||||
| Median (range) | 219.5 (26.5–866.3) | 226.2 (26.5–866.3) | 215.7 (33.1–451.5) | 0.68 | 191.2 (11.1–586.7) | 192.4 (11.1–586.7) | 186.6 (26.5–503.0) | 1.0 |
| Below LRL, no. (%) | 38 (22.9) | 18 (24.0) | 20 (22.0) | 0.90 | 61 (33.6) | 23 (36.5) | 28 (31.5) | 0.67 |
| IgM, mg/dL | ||||||||
| Median (range) | 3617 (16–9270) | 3740 (229–8200) | 3587 (16–9270) | 0.50 | 3085.0 (10–9944) | 3932 (10–9944) | 2556 (84–9740) | 0.08 |
| Above URL, no. (%) | 136/141 (96.4) | 62/64 (96.9) | 74/77 (96.1) | 1.0 | 123/125 (98.4) | 48 (76.2) | 75 (84.3) | 1.0 |
| Not available, no. (%) | 25 (15.1) | 11 (14.7) | 14 (15.4) | 1.0 | 27 (17.8) | 14 (22.2) | 13 (14.6) | 0.32 |
| MYD88, no. (%) | ||||||||
| Tested | 21 (12.7) | 0 (0) | 21 (23.1) | < 0.01 | 19 (12.5) | 0 (0) | 19 (21.3) | < 0.01 |
| Wild type | 6 (28.6) | – | 6 (28.6) | < 5 | – | < 5 | ||
| Mutation | 10 (47.6) | – | 10 (47.6) | 16 (84.2) | – | 16 (84.2) | ||
| Results not available | 5 (23.8) | – | 5 (23.8) | < 5 | – | < 5 | ||
| Hepatitis C Virus, no. (%) | ||||||||
| Tested | 40 (24.1) | 13 (17.3) | 27 (29.7) | 0.1 | 21 (13.8) | 8 (12.7) | 13 (14.6) | 0.92 |
| Positive | 4 (10.0) | <5 | <5 | 0 (0) | 0 (0) | 0 (0) | ||
| Negative | 17 (42.5) | 6 (46.2) | 11 (40.7) | 14 (66.7) | <5 | 10 (76.9) | ||
| Results not available | 19 (47.5) | <5 | 15 (55.6) | 7 (33.3) | <5 | <5 | ||
| Time from diagnosis to 1 L | ||||||||
| Median, months (range) | 1.3 (0.0–99.4) | 1.2 (0.0–52.3) | 1.4 (0.1–99.4) | 0.42 | 1.2 (0.0–113.0) | 0.7 (0.0–65.7) | 1.6 (0.0–113.0) | <0.01 |
| ≥3 months, no. (%) | 111 (66.9) | 50 (66.7) | 61 (67.0) | 0.20 | 105 (69.1) | 47 (74.6) | 58 (65.2) | 0.34 |
| 1 L Treatment, no. (%) | ||||||||
| BR | 24 (14.5) | <5 | 22 (24.2) | <0.01 | 16 (10.5) | <5 | 15 (16.9) | <0.01 |
| BDR | 31 (18.7) | 8 (10.7) | 23 (25.3) | 17 (11.2) | <5 | 14 (15.7) | ||
| Ibrutinib +/− R | 8 (4.8) | 0 (0) | 8 (8.8) | 17 (11.2) | 0 (0) | 17 (19.1) | ||
| Single-agent R | 46 (27.7) | 23 (30.7) | 23 (25.3) | 52 (34.2) | 27 (42.9) | 25 (28.1) | ||
| DRC | 19 (11.4) | 11 (14.7) | 8 (8.8) | 19 (12.5) | 8 (12.7) | 11 (12.4) | ||
| Chloram/FCR/R-CHOP | 31 (18.7) | 26 (34.7) | 5 (5.5) | 23 (15.1) | 20 (31.7) | <5 | ||
| Other | 7 (4.2) | 5 (6.7) | <5 | 8 (5.3) | <5 | <5 | ||
| Duration of 1 L | ||||||||
| Median, months (range) | 3.5 (0.0–41.5) | 3.6 (0.0–39.7) | 3.1 (0.0–41.5) | 0.31 | 1.9 (0.0–35.9) | 1.8 (0.0–23.4) | 2.3 (0.0–35.9) | 0.27 |
| Regimen Category | Treatment Regimen | Number of Patients Treated |
|---|---|---|
| BR | Bendamustine monotherapy | <5 |
| Bendamustine and rituximab | 39 | |
| BDR | Bortezomib monotherapy | <5 |
| Bortezomib and dexamethasone | 6 | |
| Bortezomib and rituximab | 5 | |
| Bortezomib, dexamethasone, and rituximab | 35 | |
| Chloram/FCR/R-CHOP | Chlorambucil | 19 |
| Chlorambucil and rituximab | <5 | |
| Cyclophosphamide, vincristine, and prednisone with rituximab | 11 | |
| Cyclophosphamide, doxorubicin, vincristine, and prednisone with rituximab | 6 | |
| Cyclophosphamide, vincristine, and prednisone | <5 | |
| Fludarabine, cyclophosphamide, and rituximab | <5 | |
| Fludarabine monotherapy | <5 | |
| Fludarabine and rituximab | 11 | |
| DRC | Dexamethasone, cyclophosphamide, and rituximab | 38 |
| Ibrutinib +/− R | Ibrutinib | 23 |
| Ibrutinib and rituximab | <5 | |
| Others | Bortezomib, cyclophosphamide, and dexamethasone | <5 |
| Bortezomib, cyclophosphamide, dexamethasone, and rituximab | <5 | |
| Cladribine and rituximab | <5 | |
| Carfilzomib, dexamethasone, and rituximab | <5 | |
| Cyclophosphamide, melphalan, and rituximab | <5 | |
| Cyclophosphamide monotherapy | <5 | |
| Lenalidomide, bortezomib, and dexamethasone with rituximab | <5 | |
| Thalidomide and dexamethasone | <5 | |
| Thalidomide and rituximab | <5 | |
| Single-agent R | Rituximab and dexamethasone | <5 |
| Rituximab monotherapy | 96 |
| Treatment Regimen | Date of Publication of First Phase 2 Clinical Trial | Date of Initial Prescription in VA | Start Date of Pre-transition Period |
|---|---|---|---|
| BR | NA | August 2010 | August 2010 |
| BDR | June 2009 [12] | June 2007 | June 2009 |
| DRC | June 2007 [20] | June 2007 | June 2007 |
| Single-agent R | May 2002 [21] | January 2006 | January 2006 |
| Treatment Regimen | Coefficient Estimates (95% CI) | ||
|---|---|---|---|
| Pre-Transition Slope | Post-Transition Slope | Change in Slope | |
| BDR | |||
| Younger | 0.58 (0.21 to 0.95) | −0.67 (−0.92 to −0.42) | −1.29 (−1.75 to −0.82) |
| Older | 0.10 (0.02 to 0.18) | −0.41 (−0.59 to −0.22) | −0.51 (−0.70 to −0.32) |
| BR | |||
| Younger | 0.31 (−0.16 to 0.77) | 0.69 (0.46 to 0.92) | 0.38 (−0.11 to 0.88) |
| Older | 0.08 (−0.31 to 0.47) | 0.0 (−0.11 to 0.11) | −0.08 (−0.50 to 0.35) |
| Ibrutinib +/− R | |||
| Younger | – | 0.83 (0.61 to 1.04) | – |
| Older | – | 0.89 (0.69 to 1.10) | – |
| DRC | |||
| Younger | 0.19 (0.09 to 0.28) | 0.01 (−0.16 to 0.19) | −0.17 (−0.38 to 0.03) |
| Older | 0.38 (0.23 to 0.52) | 0.06 (−0.06 to 0.18) | −0.37 (−0.69 to −0.05) |
| Single-agent R | |||
| Younger | 0.10 (−0.04 to 0.23) | −0.27 (−0.49 to −0.05) | −0.37 (−0.65 to −0.08) |
| Older | 0.68 (0.47 to 0.89) | −0.46 (−0.69 to −0.24) | −1.14 (−1.61 to −0.67) |
| Clinical Outcomes | Younger Population (<70 Years of Age at 1 L Treatment) (n = 166) | Older Population (>70 Years of Age at 1 L Treatment) (n = 152) | ||||
|---|---|---|---|---|---|---|
| 2006–2019 (n = 166) | 2006–2012 (n = 75) | 2013–2019 (n = 91) | 2006–2019 (n = 152) | 2006–2012 (n = 63) | 2013–2019 (n = 89) | |
| Overall survival | ||||||
| Median, months (95% CI) | 109.2 (94.3–NA) | 122.4 (100.9–NA) | NA | 68.5 (55.5–102.6) | 55.5 (31.8–92.1) | NA |
| Progression-free survival | ||||||
| Median, months (95% CI) | 52.7 (43.5–94.3) | 52.7 (43.1–97.4) | 52.8 (41.2–NA) | 36.9 (29.3–63.3) | 28.3 (18.5–55.6) | 63.3 (32.0–NA) |
| Best response to treatment, no. (%) | ||||||
| ORR | 117 (75.5) | 54 (75.0) | 63 (75.9) | 97 (69.0) | 37 (63.8) | 60 (72.3) |
| CR or VGPR | 35 (22.6) | 13 (18.1) | 22 (26.5) | 25 (17.7) | 6 (10.3) | 19 (22.9) |
| PR | 59 (38.1) | 27 (36.0) | 32 (35.2) | 42 (29.8) | 14 (22.2) | 28 (31.5) |
| MR | 23 (14.8) | 14 (18.7) | 9 (9.9) | 30 (21.3) | 17 (27.0) | 13 (14.6) |
| SD or PD | 38 (24.5) | 18 (25.0) | 20 (24.1) | 44 (31.2) | 21 (36.2) | 23 (27.7) |
| Not reported | 11 (6.6) | <5 | 8 (8.8) | 11 (7.2) | 5 (7.9) | 6 (6.7) |
| Adverse Events | Younger Population (<70 Years of Age at 1 L Treatment) (n = 166) | Older Population (>70 Years of Age at 1 L Treatment) (n = 152) | ||||
|---|---|---|---|---|---|---|
| 2006–2019 (n = 166) | 2006–2012 (n = 75) | 2013–2019 (n = 91) | 2006–2019 (n = 152) | 2006–2012 (n = 63) | 2013–2019 (n = 89) | |
| Discontinuation due to AE, no. (%) | 16 (9.6) | <5 | 13 (14.3) | 30 (19.7) | 14 (22.2) | 16 (18.0) |
| 1 L discontinued, no./N ( %) | ||||||
| BDR | <5 | 0 (0) | <5 | <5 | <5 | <5 |
| BR | <5 | 0 (0) | <5 | 5/16 (31.3) | <5 | <5 |
| Chloram/FCR/R-CHOP | <5 | <5 | 0 (0) | 5/23 (21.7) | 5/20 (25.0) | 0 (0) |
| DRC | <5 | 0 (0) | <5 | <5 | <5 | <5 |
| Ibrutinib +/- R | <5 | – | <5 | 6/17 (35.3) | – | 6/17 (35.3) |
| Other | <5 | <5 | 0 (0) | <5 | <5 | <5 |
| Single-agent R | <5 | 0 (0) | <5 | 6/52 (11.5) | <5 | <5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, H.-C.; Morreall, D.; Patil, V.; Rasmussen, K.M.; Yong, C.; Li, C.; Passey, D.G.; Burningham, Z.; Sauer, B.C.; Halwani, A.S. Treatment Patterns and Outcomes in a Nationwide Cohort of Older and Younger Veterans with Waldenström Macroglobulinemia, 2006–2019. Cancers 2021, 13, 1708. https://doi.org/10.3390/cancers13071708
Chien H-C, Morreall D, Patil V, Rasmussen KM, Yong C, Li C, Passey DG, Burningham Z, Sauer BC, Halwani AS. Treatment Patterns and Outcomes in a Nationwide Cohort of Older and Younger Veterans with Waldenström Macroglobulinemia, 2006–2019. Cancers. 2021; 13(7):1708. https://doi.org/10.3390/cancers13071708
Chicago/Turabian StyleChien, Hsu-Chih, Deborah Morreall, Vikas Patil, Kelli M. Rasmussen, Christina Yong, Chunyang Li, Deborah G. Passey, Zachary Burningham, Brian C. Sauer, and Ahmad S. Halwani. 2021. "Treatment Patterns and Outcomes in a Nationwide Cohort of Older and Younger Veterans with Waldenström Macroglobulinemia, 2006–2019" Cancers 13, no. 7: 1708. https://doi.org/10.3390/cancers13071708
APA StyleChien, H.-C., Morreall, D., Patil, V., Rasmussen, K. M., Yong, C., Li, C., Passey, D. G., Burningham, Z., Sauer, B. C., & Halwani, A. S. (2021). Treatment Patterns and Outcomes in a Nationwide Cohort of Older and Younger Veterans with Waldenström Macroglobulinemia, 2006–2019. Cancers, 13(7), 1708. https://doi.org/10.3390/cancers13071708






