Understanding the Molecular Mechanism of miR-877-3p Could Provide Potential Biomarkers and Therapeutic Targets in Squamous Cell Carcinoma of the Cervix
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
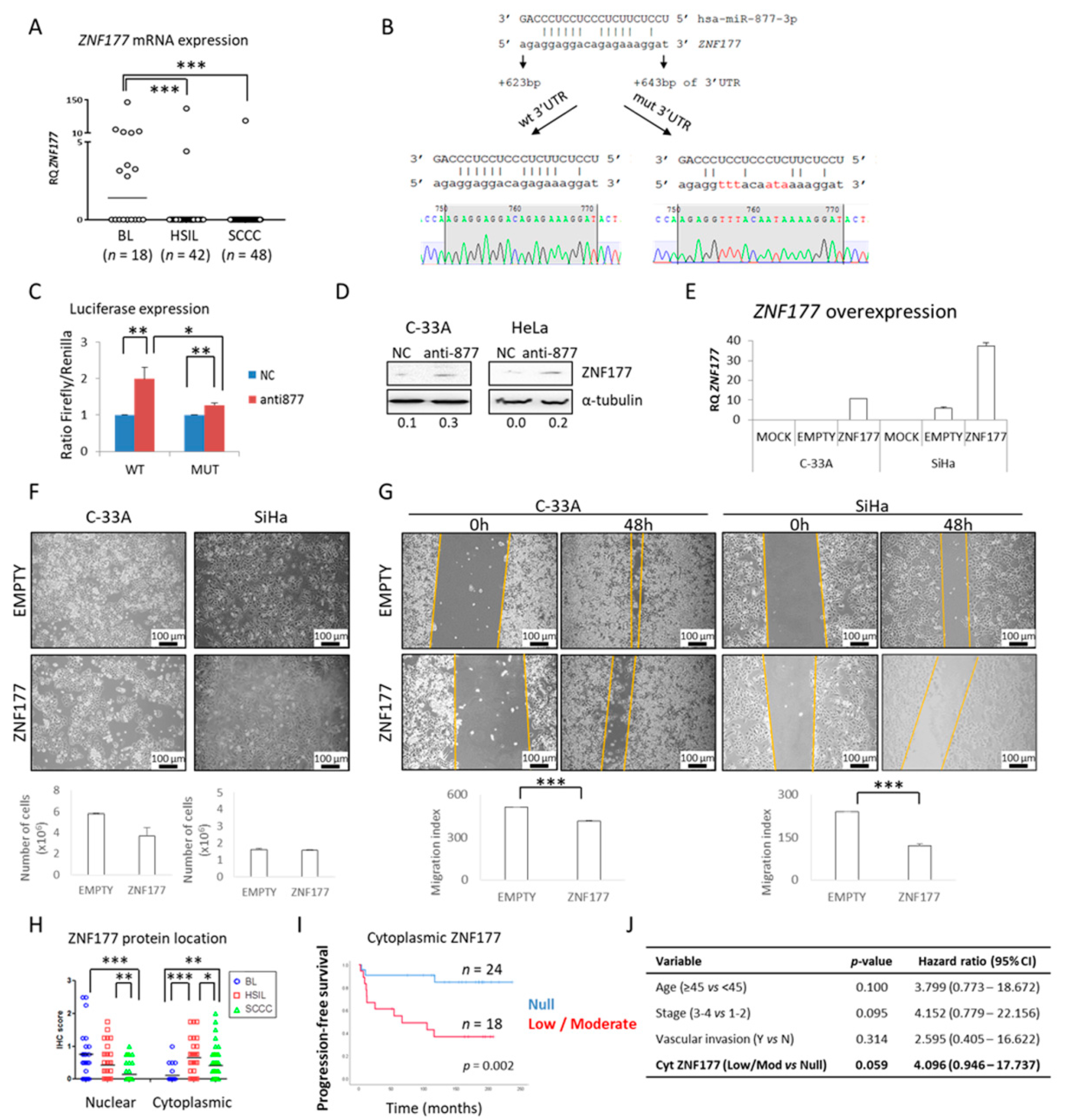
2.1. miR-877-3p is Overexpressed in Cervical Malignancies
2.2. miR-877-3p Silencing Is Not Critical for CC Cell Proliferation
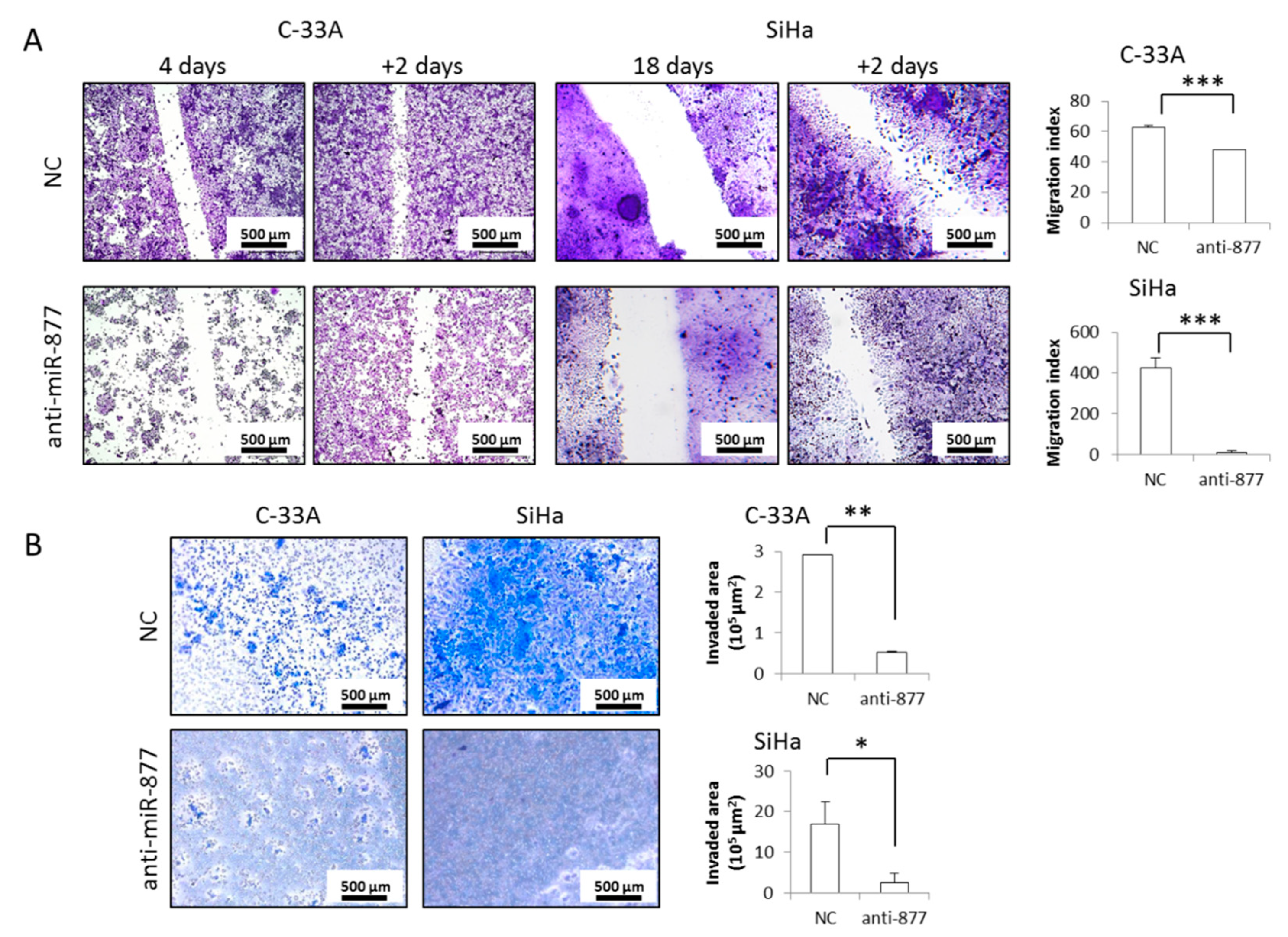
2.3. miR-877-3p Silencing Impairs CC Cell Migration and Invasion
2.4. The ZNF177 Gene Is a Direct Target of miR-877-3p
2.5. miR-877-3p Is Involved in Regulating Cytoskeletal Protein Folding
2.6. miR-877-3p Knockdown Synergizes with Paclitaxel
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Cell Lines
4.3. RNA Extraction and Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.4. miR-877-3p Silencing in CC Cell Lines
4.5. Effects of miR-877-3p Inhibition on CC Cell Survival
4.6. Cell Migration
4.7. Cell Invasion
4.8. Dual-Luciferase Reporter Assay
4.9. ZNF177 Overexpression
4.10. Subcellular Fractionation
4.11. Label-Free Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
4.12. Peptide Identification and Quantification
4.13. Bioinformatic Analysis
4.14. Western Blot
4.15. Immunohistochemistry
4.16. Response to Paclitaxel
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/ (accessed on 29 March 2020).
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Reuschenbach, M.; Wentzensen, N.; Dijkstra, M.G.; Doeberitz, M.V.K.; Arbyn, M. p16INK4a Immunohistochemistry in Cervical Biopsy Specimens. Am. J. Clin. Pathol. 2014, 142, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Albers, A.E.; Qin, J.; Kaufmann, A.M. Prognostic Significance of Overexpressed p16INK4a in Patients with Cervical Cancer: A Meta-Analysis. PLoS ONE 2014, 9, e106384. [Google Scholar] [CrossRef]
- Sun, H.; Shen, K.; Cao, D. Progress in immunocytochemical staining for cervical cancer screening. Cancer Manag. Res. 2019, 11, 1817–1827. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, K.P.; Brebi, P.M.; Roa, J.C.S. Epigenetic alterations in preneoplastic and neoplastic lesions of the cervix. Clin. Epigenetics 2012, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Kocken, M.; Helmerhorst, T.J.; Berkhof, J.; A Louwers, J.; Nobbenhuis, M.A.; Bais, A.G.; Hogewoning, C.J.; Zaal, A.; Verheijen, R.H.; Snijders, P.J.; et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: A long-term multi-cohort study. Lancet Oncol. 2011, 12, 441–450. [Google Scholar] [CrossRef]
- Escobar-Hoyos, L.F.; Yang, J.; Zhu, J.; Cavallo, J.-A.; Zhai, H.; Burke, S.; Koller, A.; I Chen, E.; Shroyer, K.R. Keratin 17 in premalignant and malignant squamous lesions of the cervix: Proteomic discovery and immunohistochemical validation as a diagnostic and prognostic biomarker. Mod. Pathol. 2013, 27, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Yin, A.; Zhang, Q.; Kong, X.; Jia, L.; Yang, Z.; Meng, L.; Li, L.; Wang, X.; Qiao, Y.; Lu, N.; et al. JAM3 methylation status as a biomarker for diagnosis of preneoplastic and neoplastic lesions of the cervix. Oncotarget 2015, 6, 44373–44387. [Google Scholar] [CrossRef] [Green Version]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; André, F.; Baselga, J.; et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef]
- Taneja, P.; Maglic, D.; Kai, F.; Zhu, S.; Kendig, R.D.; Elizabeth, A.F.; Inoue, K. Classical and Novel Prognostic Markers for Breast Cancer and their Clinical Significance. Clin. Med. Insights Oncol. 2010, 4. [Google Scholar] [CrossRef] [Green Version]
- Bernicker, E.H.; Allen, T.C.; Cagle, P.T. Update on emerging biomarkers in lung cancer. J. Thorac. Dis. 2019, 11, S81–S88. [Google Scholar] [CrossRef]
- Calabrese, F.; Lunardi, F.; Pezzuto, F.; Fortarezza, F.; Vuljan, S.E.; Marquette, C.; Hofman, P. Are There New Biomarkers in Tissue and Liquid Biopsies for the Early Detection of Non-Small Cell Lung Cancer? J. Clin. Med. 2019, 8, 414. [Google Scholar] [CrossRef] [Green Version]
- Wick, W.; Weller, M.; Bent, M.V.D.; Sanson, M.; Weiler, M.; Von Deimling, A.; Plass, C.; E Hegi, M.; Platten, M.; Reifenberger, G. MGMT testing—the challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014, 10, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 1453–1486. [Google Scholar] [CrossRef]
- Rivera, A.L.; Pelloski, C.E.; Gilbert, M.R.; Colman, H.; De La Cruz, C.; Sulman, E.P.; Bekele, B.N.; Aldape, K.D. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro-Oncology 2009, 12, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.D.F.S.; Castelletti, C.H.M.; De Lima-Filho, J.L.; Martins, D.B.G.; Teixeira, J.A.C. Putative biomarkers for cervical cancer: SNVs, methylation and expression profiles. Mutat. Res. Mutat. Res. 2017, 773, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Pardini, B.; De Maria, D.; Francavilla, A.; Di Gaetano, C.; Ronco, G.; Naccarati, A. MicroRNAs as markers of progression in cervical cancer: A systematic review. BMC Cancer 2018, 18, 1–17. [Google Scholar] [CrossRef]
- Fiano, V.; Trevisan, M.; Fasanelli, F.; Grasso, C.; Marabese, F.; Bicalho, M.D.G.; De Carvalho, N.S.; Maestri, C.A.; Merletti, F.; Sacerdote, C.; et al. Methylation in host and viral genes as marker of aggressiveness in cervical lesions: Analysis in 543 unscreened women. Gynecol. Oncol. 2018, 151, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, S.; Zhu, J.; Mayr, D.; Kuhn, C.; Schulze, S.; Hofmann, S.; Dannecker, C.; Jeschke, U.; Kost, B.P. Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer. Int. J. Mol. Sci. 2017, 18, 477. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Dua, P.; Agarwal, S.M. A Comprehensive Review of Dysregulated miRNAs Involved in Cervical Cancer. Curr. Genom. 2014, 15, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Lujambio, A.; Lowe, S.W. The microcosmos of cancer. Nat. Cell Biol. 2012, 482, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S. MicroRNAs as Therapeutic Agents: The Future of the Battle against Cancer. Curr. Top. Med. Chem. 2019, 18, 2544–2554. [Google Scholar] [CrossRef]
- Banno, K.; Iida, M.; Yanokura, M.; Kisu, I.; Iwata, T.; Tominaga, E.; Tanaka, K.; Aoki, D. MicroRNA in Cervical Cancer: OncomiRs and Tumor Suppressor miRs in Diagnosis and Treatment. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Movahedi, M.; Rejali, M.; Maleki, F.; Moetamani-Ahmadi, M.; Seifi, S.; Hosseini, Z.; Khazaei, M.; Amerizadeh, F.; Ferns, G.A.; et al. The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J. Cell. Physiol. 2019, 234, 1289–1294. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.-K.; Tuschl, T.; Zheng, Z.-M.; Li, Y.; Hafner, M.; Banerjee, N.S.; Tang, S.; Briskin, D.; Meyers, C.; et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc. Natl. Acad. Sci. USA 2014, 111, 4262–4267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahand, J.S.; Taghizadeh-Boroujeni, S.; Karimzadeh, M.; Borran, S.; Pourhanifeh, M.H.; Moghoofei, M.; Bokharaei-Salim, F.; Karampoor, S.; Jafari, A.; Asemi, Z.; et al. microRNAs: New prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. J. Cell. Physiol. 2019, 234, 17064–17099. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, Y.; Wang, T. A three miRNAs signature predicts survival in cervical cancer using bioinformatics analysis. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Schwarz, J.K.; Lewis, J.S.; Huettner, P.C.; Rader, J.S.; Deasy, J.O.; Grigsby, P.W.; Wang, X. A MicroRNA Expression Signature for Cervical Cancer Prognosis. Cancer Res. 2010, 70, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Villegas-Ruiz, V.; Juárez-Méndez, S.; A Pérez-González, O.; Arreola, H.; Paniagua-García, L.; Parra-Melquiadez, M.; Peralta-Rodríguez, R.; López-Romero, R.; Monroy-García, A.; Mantilla-Morales, A.; et al. Heterogeneity of microRNAs expression in cervical cancer cells: Over-expression of miR-196a. Int. J. Clin. Exp. Pathol. 2014, 7, 1389–1401. [Google Scholar]
- Huang, X.; Qin, J.; Lu, S. Up-regulation of miR-877 induced by paclitaxel inhibits hepatocellular carcinoma cell proliferation though targeting FOXM1. Int. J. Clin. Exp. Pathol. 2015, 8, 1515–1524. [Google Scholar]
- Qi, M.; Huang, X.; Zhou, L.; Zhang, J. Identification of differentially expressed microRNAs in metastatic melanoma using next-generation sequencing technology. Int. J. Mol. Med. 2014, 33, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Hiroki, E.; Akahira, J.-I.; Suzuki, F.; Nagase, S.; Ito, K.; Suzuki, T.; Sasano, H.; Yaegashi, N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2009, 101, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, Z.; Huang, S.; Zhao, Q.; Sun, Z.; Fletcher, C.; Jiang, Y.; Zhang, D. Combined identification of three miRNAs in serum as effective diagnostic biomarkers for HNSCC. EBioMedicine 2019, 50, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Xie, J.; Gao, Z.; Yao, W. MicroRNA-877 inhibits cell proliferation and invasion in non-small cell lung cancer by directly targeting IGF-1R. Exp. Ther. Med. 2019, 18, 1449–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Shi, S.; Chen, Q.; Chen, Z. LncRNA TRG-AS1 promotes glioblastoma cell proliferation by competitively binding with miR-877-5p to regulate SUZ12 expression. Pathol. Res. Pract. 2019, 215, 152476. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Cao, L.; Li, H.; Zou, H.; Li, H.; Pei, H. microRNA-877 inhibits malignant progression of colorectal cancer by directly targeting MTDH and regulating the PTEN/Akt pathway. Cancer Manag. Res. 2019, 11, 2769–2781. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.W.; Song, Y.S.; Lee, H.; Yi, K.; Kim, Y.-B.; Suh, K.W.; Lee, D. MicroRNA Expression Signatures Associated With BRAF-Mutated Versus KRAS-Mutated Colorectal Cancers. Medicine 2016, 95, e3321. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, E.C.; Qu, Y.-L.; Wang, D.-Y.; Qu, W.-W.; Zhang, C.-G.; Wu, T.; Gao, Z.-H. Serum level of co-expressed hub miRNAs as diagnostic and prognostic biomarkers for pancreatic ductal adenocarcinoma. J. Cancer 2018, 9, 3991–3999. [Google Scholar] [CrossRef]
- Yan, T.-H.; Qiu, C.; Sun, J.; Li, W.-H. MiR-877-5p suppresses cell growth, migration and invasion by targeting cyclin dependent kinase 14 and predicts prognosis in hepatocellular carcinoma. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 3038–3046. [Google Scholar]
- Meijer, L.L.; Puik, J.R.; Le Large, T.Y.; Heger, M.; Dijk, F.; Funel, N.; Wurdinger, T.; Garajová, I.; Van Grieken, N.C.; Van De Wiel, M.A.; et al. Unravelling the Diagnostic Dilemma: A MicroRNA Panel of Circulating MiR-16 and MiR-877 as A Diagnostic Classifier for Distal Bile Duct Tumors. Cancers 2019, 11, 1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Hu, G.-Q.; Da Costa, C.; Tang, J.-H.; Li, Q.-R.; Du, L.; Pan, Y.-W.; Lv, S.-Q. Long noncoding RNA UBE2R2-AS1 promotes glioma cell apoptosis via targeting the miR-877-3p/TLR4 axis. OncoTargets Ther. 2019, 12, 3467–3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhu, Y.; Liang, Z.; Wang, X.; Meng, S.; Xu, X.; Xu, X.; Wu, J.; Ji, A.; Hu, Z.; et al. Up-regulation of p16 by miR-877-3p inhibits proliferation of bladder cancer. Oncotarget 2016, 7, 51773–51783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wang, Y.-H.; Yoon, C.; Huang, X.-Y.; Xu, Y.; Xie, J.-W.; Wang, J.-B.; Lin, J.-X.; Chen, Q.-Y.; Cao, L.-L.; et al. Circular RNA circ-RanGAP1 regulates VEGFA expression by targeting miR-877–3p to facilitate gastric cancer invasion and metastasis. Cancer Lett. 2020, 471, 38–48. [Google Scholar] [CrossRef]
- Morale, M.G.; Abjaude, W.D.S.; Silva, A.M.; Villa, L.L.; Boccardo, E. HPV-transformed cells exhibit altered HMGB1-TLR4/MyD88-SARM1 signaling axis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, G.T.; Wang, Y.Q.; Xiang, C.Y.; Zhang, L.; Zhu, S.M.; Pan, F.; Cheng, Y.X. TLR4 promotes the expression of HIF-1α by triggering reactive oxygen species in cervical cancer cells in vitro-implications for therapeutic intervention. Mol. Med. Rep. 2017, 17, 2229–2238. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, Q.; Xie, F.; Li, M.-Q.; Xiao, J.; Sui, L. Toll-like receptor 4 promotes proliferation and apoptosis resistance in human papillomavirus–related cervical cancer cells through the Toll-like receptor 4/nuclear factor-κB pathway. Tumor Biol. 2017, 39, 1010428317710586. [Google Scholar] [CrossRef] [Green Version]
- Daud, I.I.; Scott, M.E.; Ma, Y.; Shiboski, S.; Farhat, S.; Moscicki, A.-B. Association between toll-like receptor expression and human papillomavirus type 16 persistence. Int. J. Cancer 2010, 128, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. Tumours of the uterine cervix. In WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; IARC Press: Lyon, France, 2014; Volume 6, pp. 169–207. [Google Scholar]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Diaz-Lagares, A.; Mendez-Gonzalez, J.; Hervas, D.; Saigi, M.; Pajares, M.J.; Garcia, D.; Crujerias, A.B.; Pio, R.; Montuenga, L.M.; Zulueta, J.; et al. A Novel Epigenetic Signature for Early Diagnosis in Lung Cancer. Clin. Cancer Res. 2016, 22, 3361–3371. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-C.; Lin, C.-Y.; Shih, Y.-L.; Hsieh, C.-B.; Lin, P.-Y.; Guan, S.-B.; Hsieh, M.-S.; Lai, H.-C.; Chen, C.-J.; Lin, Y.-W. Frequent methylation of HOXA9 gene in tumor tissues and plasma samples from human hepatocellular carcinomas. Clin. Chem. Lab. Med. 2014, 52, 1235–1245. [Google Scholar] [CrossRef]
- Yamashita, S.; Tsujino, Y.; Moriguchi, K.; Tatematsu, M.; Ushijima, T. Chemical genomic screening for methylation-silenced genes in gastric cancer cell lines using 5-aza-2′-deoxycytidine treatment and oligonucleotide microarray. Cancer Sci. 2005, 97, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Tsao, C.-M.; Kuo, C.-C.; Yu, M.-H.; Lin, Y.-W.; Yang, C.-Y.; Li, H.-J.; Yan, M.-D.; Wang, T.-J.; Chou, Y.-C.; et al. Quantitative DNA methylation analysis of selected genes in endometrial carcinogenesis. Taiwan. J. Obstet. Gynecol. 2015, 54, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.-H.; Ye, Q.; Zhang, G.-B.; Jiang, J.-Y.; Zhao, H.-Y.; Shao, Y.-F.; Ye, Z.-Q.; Xuan, Z.-X.; Huang, P. Identification of differentially methylated genes as diagnostic and prognostic biomarkers of breast cancer. World J. Surg. Oncol. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ahmad, A.; Zubair, H.; Miree, O.; Singh, S.; Rocconi, R.P.; Scalici, J.; Singh, A.P. MicroRNAs in gynecological cancers: Small molecules with big implications. Cancer Lett. 2017, 407, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, Y.; Zhang, L.; Zhang, T.; Yu, J.; Qin, S.; Gao, Y. Optimal subset of signature miRNAs consisting of 7 miRNAs that can serve as a novel diagnostic and prognostic predictor for the progression of cervical cancer. Oncol. Rep. 2019, 41, 3167–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, J.; Eom, K.; Oh, S.; Kim, S.; Kim, G.; Ahn, S.; Park, K.H.; Chung, D.; Lee, H. microRNA-944 overexpression is a biomarker for poor prognosis of advanced cervical cancer. BMC Cancer 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Fan, G.; Deng, C.; Wu, L. miR-4429 sensitized cervical cancer cells to irradiation by targeting RAD51. J. Cell. Physiol. 2020, 235, 185–193. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, X.; Huo, D.; Zhao, Y.; Zhang, H. MiR-449b-5p regulates cell proliferation, migration and radioresistance in cervical cancer by interacting with the transcription suppressor FOXP1. Eur. J. Pharmacol. 2019, 856, 172399. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, X.; Peng, H. Therapeutic Delivery of miR-29b Enhances Radiosensitivity in Cervical Cancer. Mol. Ther. 2019, 27, 1183–1194. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Choi, C.H.; Choi, J.-J.; Park, Y.-A.; Kim, S.-J.; Hwang, S.Y.; Kim, W.Y.; Kim, T.-J.; Lee, J.-H.; Kim, B.-G.; et al. Altered MicroRNA Expression in Cervical Carcinomas. Clin. Cancer Res. 2008, 14, 2535–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Su, J.; Liu, Z.; Wang, J.; Wang, T. miR-144 reverses cisplatin resistance in cervical cancer via targeting LHX2. J. Cell. Biochem. 2019, 120, 15018–15026. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, P.; Li, Y.; Ye, F.; Wang, F.; Wan, X.; Cheng, X.; Lu, W.; Xie, X. miR-375 is upregulated in acquired paclitaxel resistance in cervical cancer. Br. J. Cancer 2013, 109, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Zhang, S.; Wang, W.; Xu, Y.; Kawuli, A.; Lu, J.; Xiu, X. Long non-coding RNA DSCAM-AS1 contributes to the tumorigenesis of cervical cancer by targeting miR-877-5p/ATXN7L3 axis. Biosci. Rep. 2020, 40, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Ou, J.; Liu, J.; Li, X.; Meng, Y.; Yan, L.; Deng, P.; Sun, B. MicroRNA-877 is downregulated in cervical cancer and directly targets MACC1 to inhibit cell proliferation and invasion. Exp. Ther. Med. 2019, 18, 3650–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Córdova-Rivas, S.; Fraire-Soto, I.; Torres, A.M.-C.; Servín-González, L.S.; Granados-López, A.J.; López-Hernández, Y.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; Castañeda-Delgado, J.E.; Ramírez-Hernández, L.; et al. 5p and 3p Strands of miR-34 Family Members Have Differential Effects in Cell Proliferation, Migration, and Invasion in Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 545. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Yang, H.; Ma, X.; Wu, G. Strand-specific miR-28-3p and miR-28-5p have differential effects on nasopharyngeal cancer cells proliferation, apoptosis, migration and invasion. Cancer Cell Int. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Pandey, A.; Chandra, S.; Nautiyal, R.; Shrivastav, V. Expression of p16INK4a and human papillomavirus 16 with associated risk factors in cervical premalignant and malignant lesions. South Asian J. Cancer 2018, 7, 236–239. [Google Scholar] [CrossRef]
- Volgareva, G.; Zavalishina, L.; Andreeva, Y.; Frank, G.; Krutikova, E.; Golovina, D.; Bliev, A.; Spitkovsky, D.; Ermilova, V.; Kisseljov, F. Protein p16 as a marker of dysplastic and neoplastic alterations in cervical epithelial cells. BMC Cancer 2004, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-W.; Wang, F.; Wei, Q.; Zhao, Y.-F.; Liu, M.; Li, X.; Tang, H. miR-20a promotes migration and invasion by regulating TNKS2 in human cervical cancer cells. FEBS Lett. 2012, 586, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yao, D.; Chen, J.; Ding, N.; Ren, F. MiR-20a Promotes Cervical Cancer Proliferation and Metastasis In Vitro and In Vivo. PLoS ONE 2015, 10, e0120905. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kinoshita, T.; Nohata, N.; Itesako, T.; Yoshino, H.; Enokida, H.; Nakagawa, M.; Shozu, M.; Seki, N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int. J. Oncol. 2013, 42, 1523–1532. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhou, W.; Li, Y.; Gan, Y.; Peng, Y.; Xiao, Q.; Ouyang, C.; Wu, A.; Zhang, S.; Liu, J.; et al. MiR-4524b-5p/WTX/β-catenin axis functions as a regulator of metastasis in cervical cancer. PLoS ONE 2019, 14, e0214822. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Xu, F.; Wang, L.; Sun, G.; Wang, J.; Yang, Y. By downregulating PBX3, miR-526b suppresses the epithelial–mesenchymal transition process in cervical cancer cells. Future Oncol. 2019, 15, 1577–1591. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chu, Z.-P.; Han, H.; Zhang, Y.; Tian, F.; Zhang, J.-Q.; Huang, X.-H. Suppression of miR-93-5p inhibits high-risk HPV-positive cervical cancer progression via targeting of BTG3. Hum. Cell 2019, 32, 160–171. [Google Scholar] [CrossRef]
- Yao, R.; Zheng, H.; Wu, L.; Cai, P. miRNA-641 inhibits the proliferation, migration, and invasion and induces apoptosis of cervical cancer cells by directly targeting ZEB1. OncoTargets Ther. 2018, 11, 8965–8976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Jin, H.; Zheng, Y.; Mao, Y.; Fu, Z.; Li, X.; Dong, L. DANCR-mediated microRNA-665 regulates proliferation and metastasis of cervical cancer through the ERK/SMAD pathway. Cancer Sci. 2018, 110, 913–925. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Deng, Y.; Ao, L.; Song, Y.; Xu, Y.; Wang, C.C.; Choy, K.W.; Chung, K.H.T.; Du, Q.; Sui, Y.; et al. The high-risk HPV oncogene E7 upregulates miR-182 expression through the TGF-β/Smad pathway in cervical cancer. Cancer Lett. 2019, 460, 75–85. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Chen, L.-J. The role of miRNAs in the invasion and metastasis of cervical cancer. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-H.; Chu, T.-Y. Repression of miR-126 and upregulation of adrenomedullin in the stromal endothelium by cancer-stromal cross talks confers angiogenesis of cervical cancer. Oncogene 2013, 33, 3636–3647. [Google Scholar] [CrossRef]
- Santos, J.M.; Da Silva, S.P.; Costa, N.R.; Gil Da Costa, R.M.; Medeiros, R. The Role of MicroRNAs in the Metastatic Process of High-Risk HPV-Induced Cancers. Cancers 2018, 10, 493. [Google Scholar] [CrossRef] [Green Version]
- Dogterom, M.; Koenderink, G.H. Actin–microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 38–54. [Google Scholar] [CrossRef]
- Riggi, N.; Aguet, M.; Stamenkovic, I. Cancer Metastasis: A Reappraisal of Its Underlying Mechanisms and Their Relevance to Treatment. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 117–140. [Google Scholar] [CrossRef]
- Vallin, J.; Grantham, J. The role of the molecular chaperone CCT in protein folding and mediation of cytoskeleton-associated processes: Implications for cancer cell biology. Cell Stress Chaperon 2019, 24, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Boudiaf-Benmammar, C.; Cresteil, T.; Melki, R. The Cytosolic Chaperonin CCT/TRiC and Cancer Cell Proliferation. PLoS ONE 2013, 8, e60895. [Google Scholar] [CrossRef]
- Roh, S.-H.; Kasembeli, M.; Bakthavatsalam, D.; Chiu, W.; Tweardy, D.J. Contribution of the Type II Chaperonin, TRiC/CCT, to Oncogenesis. Int. J. Mol. Sci. 2015, 16, 26706–26720. [Google Scholar] [CrossRef] [Green Version]
- Amit, M.; Weisberg, S.J.; Nadler-Holly, M.; McCormack, E.A.; Feldmesser, E.; Kaganovich, D.; Willison, K.R.; Horovitz, A. Equivalent Mutations in the Eight Subunits of the Chaperonin CCT Produce Dramatically Different Cellular and Gene Expression Phenotypes. J. Mol. Biol. 2010, 401, 532–543. [Google Scholar] [CrossRef]
- Yin, H.; Miao, X.; Wu, Y.; Wei, Y.; Zong, G.; Yang, S.; Chen, X.; Zheng, G.; Zhu, X.; Guo, Y.; et al. The role of the Chaperonin containing t-complex polypeptide 1, subunit 8 (CCT8) in B-cell non-Hodgkin’s lymphoma. Leuk. Res. 2016, 45, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.; Cheng, C.; Cai, J.; He, S.; Wang, H.; Liu, F.; Zhu, C.; Ding, Z.; Huang, X.; et al. Chaperonin containing TCP1, subunit 8 (CCT8) is upregulated in hepatocellular carcinoma and promotes HCC proliferation. APMIS 2014, 122, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, H.; Shao, Y.; Sun, Y.; Zhang, L.; Li, H.; Zhang, X.; Yang, X.; Yu, W.; Fu, J. Chaperonin-containing T-complex protein 1 subunit 8 promotes cell migration and invasion in human esophageal squamous cell carcinoma by regulating α-actin and β-tubulin expression. Int. J. Oncol. 2018, 52, 2021–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; He, X.; Huang, Q.; Liu, X.; Sun, G.; Guo, J.; Yuan, D.; Yang, L.; Ban, N.; Fan, S.; et al. Overexpression of CCT8 and its significance for tumor cell proliferation, migration and invasion in glioma. Pathol. Res. Pract. 2015, 211, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, R.; Nemec, K.N.; Iketani, A.; Flores, O.; Showalter, A.; Khaled, A.S.; Vishnubhotla, P.; Sprung, R.W.; Kaittanis, C.; Perez, J.M.; et al. Chaperonin Containing TCP-1 Protein Level in Breast Cancer Cells Predicts Therapeutic Application of a Cytotoxic Peptide. Clin. Cancer Res. 2016, 22, 4366–4379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Khaled, A.S.; Bassiouni, R.; Flores, O.; Nierenberg, D.; Bhatti, H.; Vishnubhotla, P.; Perez, J.M.; Santra, S.; Khaled, A.R. Targeting chaperonin containing TCP1 (CCT) as a molecular therapeutic for small cell lung cancer. Oncotarget 2017, 8, 110273–110288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-F.; Tsai, W.-P.; Liu, H.-G.; Liang, P.-H. Intracellular β-Tubulin/Chaperonin Containing TCP1-β Complex Serves as a Novel Chemotherapeutic Target against Drug-Resistant Tumors. Cancer Res. 2009, 69, 6879–6888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y.; Wei, Y.; Wu, J.; Zhang, P.; Shen, S.; Saiyin, H.; Wumaier, R.; Yang, X.; Wang, C.; et al. Molecular chaperone CCT3 supports proper mitotic progression and cell proliferation in hepatocellular carcinoma cells. Cancer Lett. 2016, 372, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Ho, J.Y.; Lee, H.W.; Baik, M.W.; Kim, O.; Choi, Y.J.; Hur, S.Y. Inhibition of Phosphatidylinositol 3-kinase (PI3K) Signaling Synergistically Potentiates Antitumor Efficacy of Paclitaxel and Overcomes Paclitaxel-Mediated Resistance in Cervical Cancer. Int. J. Mol. Sci. 2019, 20, 3383. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.A.; El-Sokkary, G.H.; Saber, S.H. Low doses of Paclitaxel repress breast cancer invasion through DJ-1/KLF17 signalling pathway. Clin. Exp. Pharmacol. Physiol. 2018, 45, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Torres, A.; Fernández-Retana, J.; Peralta-Zaragoza, O.; Jacobo-Herrera, N.; de Leon, D.C.; Cerna-Cortés, J.F.; Lopez-Camarillo, C.; Pérez-Plasencia, C. A microRNA expression signature for clinical response in locally advanced cervical cancer. Gynecol. Oncol. 2016, 142, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Mendaza, S.; Fernández-Irigoyen, J.; Santamaría, E.; Zudaire, T.; Guarch, R.; Guerrero-Setas, D.; Vidal, A.; Santos-Salas, J.; Matias-Guiu, X.; Ausín, K.; et al. Absence of Nuclear p16 Is a Diagnostic and Independent Prognostic Biomarker in Squamous Cell Carcinoma of the Cervix. Int. J. Mol. Sci. 2020, 21, 2125. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Guo, C.; Zhao, H.; Pan, Z.; Zhu, F.; Zhang, L.; Wang, Q. TIPE3 differentially modulates proliferation and migration of human non-small-cell lung cancer cells via distinct subcellular location. BMC Cancer 2018, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cuello-Carrión, F.D.; Shortrede, J.E.; Alvarez-Olmedo, D.; Cayado-Gutiérrez, N.; Castro, G.N.; Zoppino, F.C.M.; Guerrero, M.; Martinis, E.; Wuilloud, R.; Gomez, N.N.; et al. HER2 and β-catenin protein location: Importance in the prognosis of breast cancer patients and their correlation when breast cancer cells suffer stressful situations. Clin. Exp. Metastasis 2015, 32, 151–168. [Google Scholar] [CrossRef]
- López-Knowles, E.; Zardawi, S.J.; McNeil, C.M.; Millar, E.K.; Crea, P.; Musgrove, E.A.; Sutherland, R.L.; O’Toole, S.A. Cytoplasmic Localization of β-Catenin is a Marker of Poor Outcome in Breast Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2010, 19, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omatsu, M.; Kunimura, T.; Mikogami, T.; Shiokawa, A.; Masunaga, A.; Nagai, T.; Kitami, A.; Suzuki, T.; Kadokura, M. Cyclin-dependent kinase inhibitors, p16 and p27, demonstrate different expression patterns in thymoma and thymic carcinoma. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 678–684. [Google Scholar] [CrossRef]
- Duncan, T.J.; Al-Attar, A.; Rolland, P.; Harper, S.; Spendlove, I.; Durrant, L.G. Cytoplasmic p27 Expression is an Independent Prognostic Factor in Ovarian Cancer. Int. J. Gynecol. Pathol. 2010, 29, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, A.; Schlomm, T.; Huland, H.; Köllermann, J.; Simon, P.; Mirlacher, M.; Salomon, G.; Chun, F.H.; Steuber, T.; Simon, R.; et al. Distinct Subcellular Expression Patterns of Neutral Endopeptidase (CD10) in Prostate Cancer Predict Diverging Clinical Courses in Surgically Treated Patients. Clin. Cancer Res. 2008, 14, 7838–7842. [Google Scholar] [CrossRef] [Green Version]
- Martín-Sánchez, E.; Mendaza, S.; Ulazia-Garmendia, A.; Monreal-Santesteban, I.; Blanco-Luquin, I.; Córdoba, A.; Vicente-García, F.; Pérez-Janices, N.; Escors, D.; Megías, D.; et al. CHL1 hypermethylation as a potential biomarker of poor prognosis in breast cancer. Oncotarget 2017, 8, 15789–15801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M.J. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The Paragon Algorithm, a Next Generation Search Engine That Uses Sequence Temperature Values and Feature Probabilities to Identify Peptides from Tandem Mass Spectra. Mol. Cell. Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Shilov, I.V.; Seymour, S.L. Nonlinear Fitting Method for Determining Local False Discovery Rates from Decoy Database Searches. J. Proteome Res. 2008, 7, 3661–3667. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- A Vizcaíno, J.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; A Dianes, J.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]
- da Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Thomas, P. PANTHER Pathway: An Ontology-Based Pathway Database Coupled with Data Analysis Tools. Methods Mol. Biol. 2009, 563, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Martín-Sánchez, E.; Odqvist, L.; Rodríguez-Pinilla, S.M.; Sánchez-Beato, M.; Roncador, G.; Domínguez-González, B.; Blanco-Aparicio, C.; Collazo, A.M.G.; Cantalapiedra, E.G.; Fernández, J.P.; et al. PIM Kinases as Potential Therapeutic Targets in a Subset of Peripheral T Cell Lymphoma Cases. PLoS ONE 2014, 9, e112148. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, X.; Languino, L.R.; Altieri, D.C. Evaluation of Drug Combination Effect Using a Bliss Independence Dose–Response Surface Model. Stat. Biopharm. Res. 2018, 10, 112–122. [Google Scholar] [CrossRef]
- Demidenko, E.; Miller, T.W. Statistical determination of synergy based on Bliss definition of drugs independence. PLoS ONE 2019, 14, e0224137. [Google Scholar] [CrossRef] [PubMed]




| Cervical Tissue | miR-877-3p Expression | ||
|---|---|---|---|
| ≤Median | >Median | NA | |
| BL | 18 (78%) | 2 (9%) | 3 (13%) |
| HSIL | 20 (48%) | 18 (43%) | 4 (9%) |
| SCCC | 14 (27%) | 32 (62%) | 6 (11%) |
| Cervical Tissue | Nuclear ZNF177 | Cytoplasmic ZNF177 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | NA | 0 | 1 | 2 | 3 | NA | |
| BL | 7 (30%) | 13 (57%) | 1 (4%) | 2 (9%) | 0 (0%) | 19 (83%) | 4 (17%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HSIL | 19 (45%) | 12 (29%) | 2 (5%) | 0 (0%) | 9 (21%) | 10 (24%) | 20 (48%) | 3 (7%) | 0 (0%) | 9 (21%) |
| SCCC | 34 (65%) | 8 (15%) | 0 (0%) | 0 (0%) | 10 (19%) | 24 (46%) | 15 (29%) | 3 (6%) | 0 (0%) | 10 (19%) |
| Effect | Drug | C-33A | SiHa | HeLa | |||
|---|---|---|---|---|---|---|---|
| NC | anti-miR-877-3p | NC | anti-miR-877-3p | NC | anti-miR-877-3p | ||
| Cell viability | DMSO | 0.00 | 0.18 | 0.00 | 0.24 | 0.00 | 0.17 |
| Paclitaxel | 0.67 | 0.67 | 0.16 | 0.40 | 0.14 | 0.36 | |
| PEcombination | 0.73 | 0.37 | 0.29 | ||||
| Cell migration | DMSO | 0.00 | 0.54 | 0.00 | 0.77 | 0.00 | 0.23 |
| Paclitaxel | 0.92 | 1.52 | 0.26 | 1.10 | −0.17 | 0.97 | |
| PEcombination | 0.96 | 0.83 | 0.09 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendaza, S.; Fernández-Irigoyen, J.; Santamaría, E.; Arozarena, I.; Guerrero-Setas, D.; Zudaire, T.; Guarch, R.; Vidal, A.; Salas, J.-S.; Matias-Guiu, X.; et al. Understanding the Molecular Mechanism of miR-877-3p Could Provide Potential Biomarkers and Therapeutic Targets in Squamous Cell Carcinoma of the Cervix. Cancers 2021, 13, 1739. https://doi.org/10.3390/cancers13071739
Mendaza S, Fernández-Irigoyen J, Santamaría E, Arozarena I, Guerrero-Setas D, Zudaire T, Guarch R, Vidal A, Salas J-S, Matias-Guiu X, et al. Understanding the Molecular Mechanism of miR-877-3p Could Provide Potential Biomarkers and Therapeutic Targets in Squamous Cell Carcinoma of the Cervix. Cancers. 2021; 13(7):1739. https://doi.org/10.3390/cancers13071739
Chicago/Turabian StyleMendaza, Saioa, Joaquín Fernández-Irigoyen, Enrique Santamaría, Imanol Arozarena, David Guerrero-Setas, Tamara Zudaire, Rosa Guarch, August Vidal, José-Santos Salas, Xavier Matias-Guiu, and et al. 2021. "Understanding the Molecular Mechanism of miR-877-3p Could Provide Potential Biomarkers and Therapeutic Targets in Squamous Cell Carcinoma of the Cervix" Cancers 13, no. 7: 1739. https://doi.org/10.3390/cancers13071739
APA StyleMendaza, S., Fernández-Irigoyen, J., Santamaría, E., Arozarena, I., Guerrero-Setas, D., Zudaire, T., Guarch, R., Vidal, A., Salas, J.-S., Matias-Guiu, X., Ausín, K., Gil, C., Hernández-Alcoceba, R., & Martín-Sánchez, E. (2021). Understanding the Molecular Mechanism of miR-877-3p Could Provide Potential Biomarkers and Therapeutic Targets in Squamous Cell Carcinoma of the Cervix. Cancers, 13(7), 1739. https://doi.org/10.3390/cancers13071739








