Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. TNF/LT and Lung Cancer
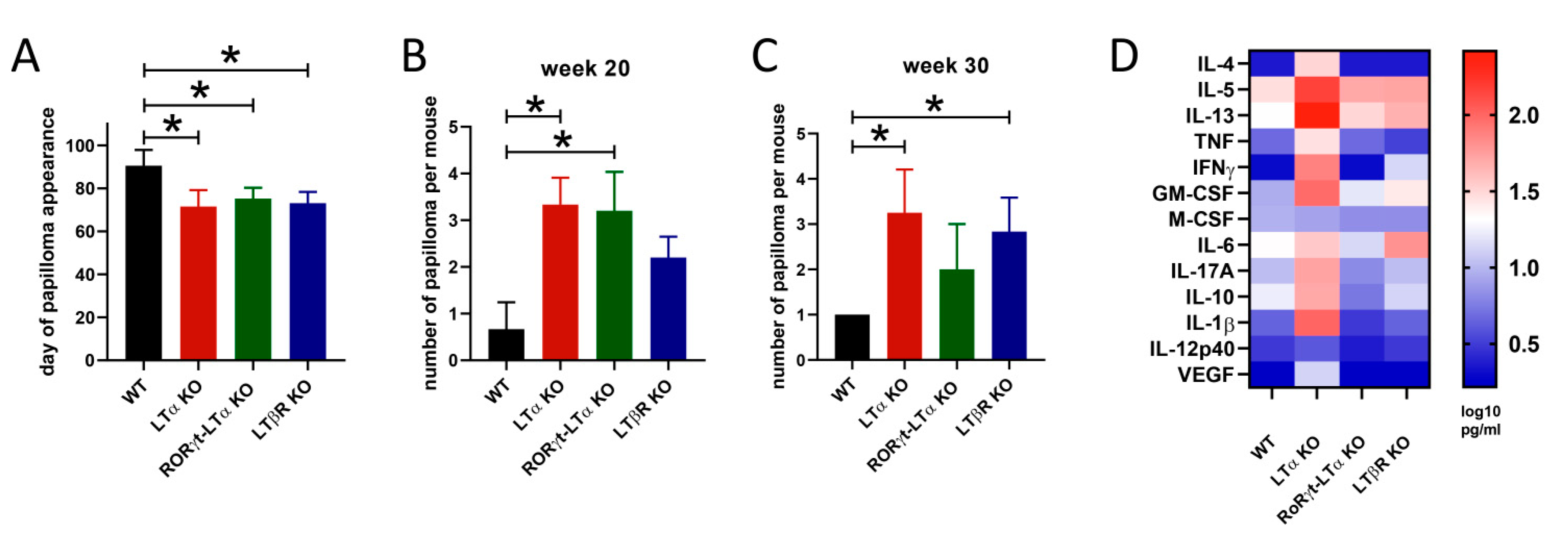
3. TNF/LT and Skin Cancer
4. TNF/LT and Liver Cancer
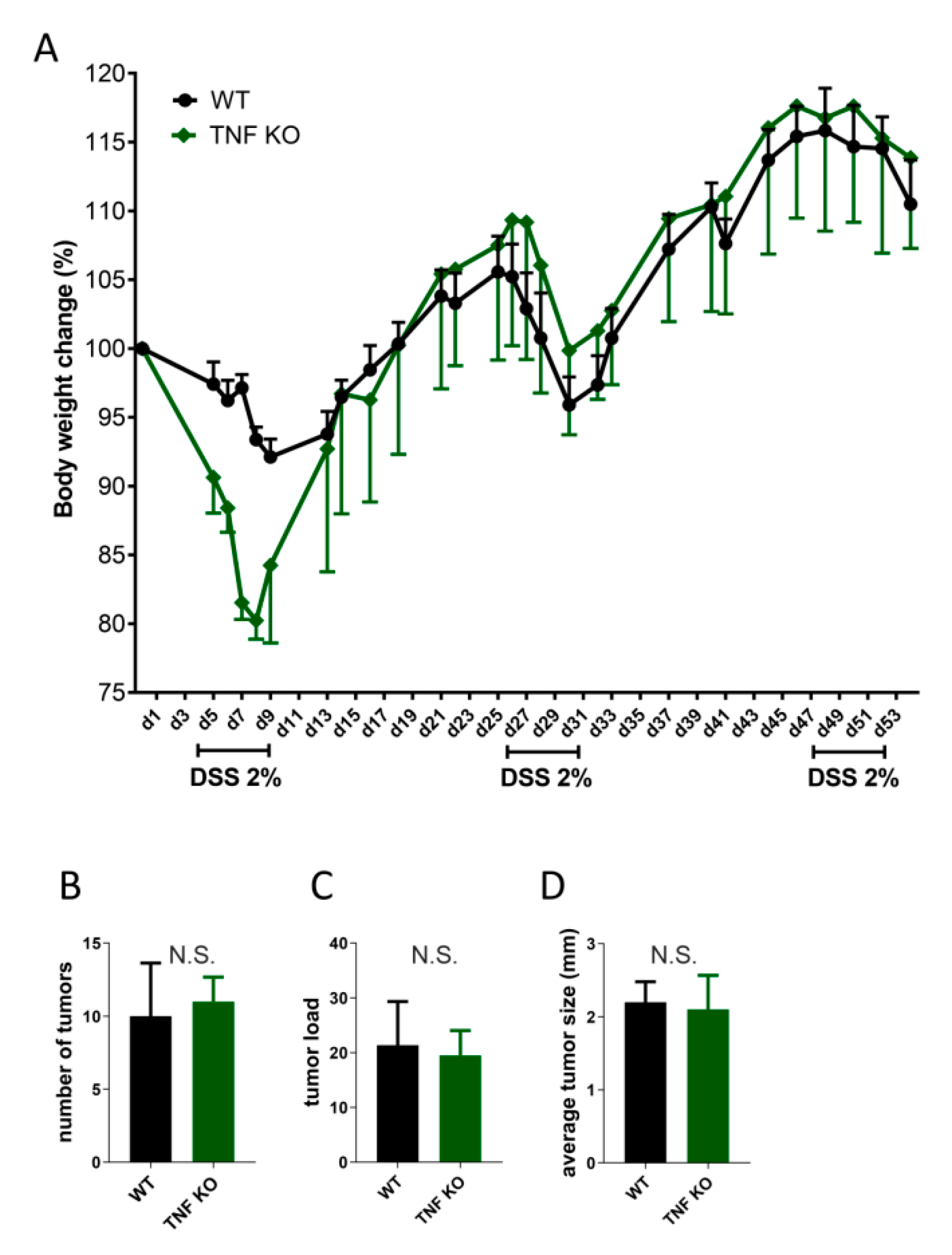
5. TNF/LT and Colorectal Cancer
6. TNF/LT and Hematological Malignancies
7. TNFR2 in Cancer Progression
8. Peculiarities of the LT System and Cancer
9. Immune System–Microbiota Interactions in Cancer Progression—A Clue to Resolving Earlier Controversies?
10. TNF and LT as Prognostic Markers and Their Polymorphisms
11. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Browning, J.L.; Dougas, I.; Ngam-ek, A.; Bourdon, P.R.; Ehrenfels, B.N.; Miatkowski, K.; Zafari, M.; Yampaglia, A.M.; Lawton, P.; Meier, W.; et al. Characterization of surface lymphotoxin forms. Use of specific monoclonal antibodies and soluble receptors. J. Immunol. 1995, 154, 33–46. [Google Scholar]
- Browning, J.L.; Ngam-ek, A.; Lawton, P.; DeMarinis, J.; Tizard, R.; Chow, E.P.; Hession, C.; O’Brine-Greco, B.; Foley, S.F.; Ware, C.F. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 1993, 72, 847–856. [Google Scholar] [CrossRef]
- Kuprash, D.V.; Tumanov, A.V.; Liepinsh, D.J.; Koroleva, E.P.; Drutskaya, M.S.; Kruglov, A.A.; Shakhov, A.N.; Southon, E.; Murphy, W.J.; Tessarollo, L.; et al. Novel tumor necrosis factor-knockout mice that lack Peyer’s patches. Eur. J. Immunol. 2005, 35, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Alexopoulou, L.; Episkopou, V.; Kollias, G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996, 184, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Korner, H.; Cook, M.; Riminton, D.S.; Lemckert, F.A.; Hoek, R.M.; Ledermann, B.; Kontgen, F.; Fazekas de St Groth, B.; Sedgwick, J.D. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 1997, 27, 2600–2609. [Google Scholar] [CrossRef]
- De Togni, P.; Goellner, J.; Ruddle, N.H.; Streeter, P.R.; Fick, A.; Mariathasan, S.; Smith, S.C.; Carlson, R.; Shornick, L.P.; Strauss-Schoenberger, J.; et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 1994, 264, 703–707. [Google Scholar] [CrossRef]
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc. R Soc. Med. 1910, 3, 1–48. [Google Scholar] [CrossRef]
- Ruddle, N.H.; Waksman, B.H. Cytotoxic effect of lymphocyte-antigen interaction in delayed hypersensitivity. Science 1967, 157, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Granger, G.A.; Williams, T.W. Lymphocyte cytotoxicity in vitro: Activation and release of a cytotoxic factor. Nature 1968, 218, 1253–1254. [Google Scholar] [CrossRef]
- Williams, T.W.; Granger, G.A. Lymphocyte in vitro cytotoxicity: Lymphotoxins of several mammalian species. Nature 1968, 219, 1076–1077. [Google Scholar] [CrossRef][Green Version]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Gray, P.W.; Aggarwal, B.B.; Benton, C.V.; Bringman, T.S.; Henzel, W.J.; Jarrett, J.A.; Leung, D.W.; Moffat, B.; Ng, P.; Svedersky, L.P.; et al. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature 1984, 312, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Li, C.B.; Gray, P.W.; Lin, P.F.; McGrath, K.M.; Ruddle, F.H.; Ruddle, N.H. Cloning and expression of murine lymphotoxin cDNA. J. Immunol. 1987, 138, 4496–4501. [Google Scholar]
- Pennica, D.; Hayflick, J.S.; Bringman, T.S.; Palladino, M.A.; Goeddel, D.V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc. Natl. Acad. Sci. USA 1985, 82, 6060–6064. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Nedwin, G.E.; Hayflick, J.S.; Seeburg, P.H.; Derynck, R.; Palladino, M.A.; Kohr, W.J.; Aggarwal, B.B.; Goeddel, D.V. Human tumour necrosis factor: Precursor structure, expression and homology to lymphotoxin. Nature 1984, 312, 724–729. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhou, S.; Diaz, L.A., Jr.; Holdhoff, M. Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget 2011, 2, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, N.; Diao, Z.; Chen, Y.; Zhang, Y. Therapeutic potential of TNFalpha inhibitors in chronic inflammatory disorders: Past and future. Genes Dis. 2021, 8, 38–47. [Google Scholar] [CrossRef]
- Wang, J.L.; Yin, W.J.; Zhou, L.Y.; Zhou, G.; Liu, K.; Hu, C.; Zuo, X.C.; Wang, Y.F. Risk of non-melanoma skin cancer for rheumatoid arthritis patients receiving TNF antagonist: A systematic review and meta-analysis. Clin. Rheumatol. 2020, 39, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Orosz, P.; Echtenacher, B.; Falk, W.; Ruschoff, J.; Weber, D.; Mannel, D.N. Enhancement of experimental metastasis by tumor necrosis factor. J. Exp. Med. 1993, 177, 1391–1398. [Google Scholar] [CrossRef]
- Im, S.Y.; Ko, H.M.; Kim, J.W.; Lee, H.K.; Ha, T.Y.; Lee, H.B.; Oh, S.J.; Bai, S.; Chung, K.C.; Lee, Y.B.; et al. Augmentation of tumor metastasis by platelet-activating factor. Cancer Res. 1996, 56, 2662–2665. [Google Scholar]
- Tomita, Y.; Yang, X.; Ishida, Y.; Nemoto-Sasaki, Y.; Kondo, T.; Oda, M.; Watanabe, G.; Chaldakov, G.N.; Fujii, C.; Mukaida, N. Spontaneous regression of lung metastasis in the absence of tumor necrosis factor receptor p55. Int. J. Cancer 2004, 112, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Sasi, S.P.; Bae, S.; Song, J.; Perepletchikov, A.; Schneider, D.; Carrozza, J.; Yan, X.; Kishore, R.; Enderling, H.; Goukassian, D.A. Therapeutic non-toxic doses of TNF induce significant regression in TNFR2-p75 knockdown Lewis lung carcinoma tumor implants. PLoS ONE 2014, 9, e92373. [Google Scholar] [CrossRef] [PubMed]
- Schrama, D.; thor Straten, P.; Fischer, W.H.; McLellan, A.D.; Brocker, E.B.; Reisfeld, R.A.; Becker, J.C. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001, 14, 111–121. [Google Scholar] [CrossRef]
- Brouckaert, P.G.; Leroux-Roels, G.G.; Guisez, Y.; Tavernier, J.; Fiers, W. In vivo anti-tumour activity of recombinant human and murine TNF, alone and in combination with murine IFN-gamma, on a syngeneic murine melanoma. Int. J. Cancer 1986, 38, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vincent, A.; Cates, J.; Brantley-Sieders, D.M.; Polk, D.B.; Young, P.P. Low levels of tumor necrosis factor alpha increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009, 69, 338–348. [Google Scholar] [CrossRef]
- Ito, D.; Back, T.C.; Shakhov, A.N.; Wiltrout, R.H.; Nedospasov, S.A. Mice with a targeted mutation in lymphotoxin-alpha exhibit enhanced tumor growth and metastasis: Impaired NK cell development and recruitment. J. Immunol. 1999, 163, 2809–2815. [Google Scholar]
- Teng, M.N.; Park, B.H.; Koeppen, H.K.; Tracey, K.J.; Fendly, B.M.; Schreiber, H. Long-term inhibition of tumor growth by tumor necrosis factor in the absence of cachexia or T-cell immunity. Proc. Natl. Acad. Sci. USA 1991, 88, 3535–3539. [Google Scholar] [CrossRef]
- Hehlgans, T.; Stoelcker, B.; Stopfer, P.; Muller, P.; Cernaianu, G.; Guba, M.; Steinbauer, M.; Nedospasov, S.A.; Pfeffer, K.; Mannel, D.N. Lymphotoxin-beta receptor immune interaction promotes tumor growth by inducing angiogenesis. Cancer Res. 2002, 62, 4034–4040. [Google Scholar] [PubMed]
- Kitakata, H.; Nemoto-Sasaki, Y.; Takahashi, Y.; Kondo, T.; Mai, M.; Mukaida, N. Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res. 2002, 62, 6682–6687. [Google Scholar]
- Germanova, D.; Keirsse, J.; Kohler, A.; Hastir, J.F.; Demetter, P.; Delbauve, S.; Elkrim, Y.; Verset, L.; Larbanoix, L.; Preyat, N.; et al. Myeloid tumor necrosis factor and heme oxygenase-1 regulate the progression of colorectal liver metastases during hepatic ischemia-reperfusion. Int. J. Cancer 2021, 148, 1276–1288. [Google Scholar] [CrossRef]
- Kempski, J.; Giannou, A.D.; Riecken, K.; Zhao, L.; Steglich, B.; Lucke, J.; Garcia-Perez, L.; Karstens, K.F.; Wostemeier, A.; Nawrocki, M.; et al. IL22BP Mediates the Antitumor Effects of Lymphotoxin Against Colorectal Tumors in Mice and Humans. Gastroenterology 2020, 159, 1417–1430.e13. [Google Scholar] [CrossRef]
- Jiao, S.F.; Sun, K.; Chen, X.J.; Zhao, X.; Cai, N.; Liu, Y.J.; Xu, L.M.; Kong, X.M.; Wei, L.X. Inhibition of tumor necrosis factor alpha reduces the outgrowth of hepatic micrometastasis of colorectal tumors in a mouse model of liver ischemia-reperfusion injury. J. Biomed. Sci. 2014, 21, 1. [Google Scholar] [CrossRef]
- Lukashev, M.; LePage, D.; Wilson, C.; Bailly, V.; Garber, E.; Lukashin, A.; Ngam-ek, A.; Zeng, W.; Allaire, N.; Perrin, S.; et al. Targeting the lymphotoxin-beta receptor with agonist antibodies as a potential cancer therapy. Cancer Res. 2006, 66, 9617–9624. [Google Scholar] [CrossRef]
- Verma, D.; Zanetti, C.; Godavarthy, P.S.; Kumar, R.; Minciacchi, V.R.; Pfeiffer, J.; Metzler, M.; Lefort, S.; Maguer-Satta, V.; Nicolini, F.E.; et al. Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia 2020, 34, 1540–1552. [Google Scholar] [CrossRef]
- Markey, K.A.; Burman, A.C.; Banovic, T.; Kuns, R.D.; Raffelt, N.C.; Rowe, V.; Olver, S.D.; Don, A.L.; Morris, E.S.; Pettit, A.R.; et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood 2010, 115, 122–132. [Google Scholar] [CrossRef]
- Hopner, S.S.; Raykova, A.; Radpour, R.; Amrein, M.A.; Koller, D.; Baerlocher, G.M.; Riether, C.; Ochsenbein, A.F. LIGHT/LTbetaR signaling regulates self-renewal and differentiation of hematopoietic and leukemia stem cells. Nat. Commun. 2021, 12, 1065. [Google Scholar] [CrossRef] [PubMed]
- Gloger, M.; Menzel, L.; Grau, M.; Vion, A.C.; Anagnostopoulos, I.; Zapukhlyak, M.; Gerlach, K.; Kammertons, T.; Hehlgans, T.; Zschummel, M.; et al. Lymphoma Angiogenesis Is Orchestrated by Noncanonical Signaling Pathways. Cancer Res. 2020, 80, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Chen, Q.Q.; Cao, J.; Xu, L.Q.; Tang, X.; Wang, J.; Zhang, J.; Dong, L.X. Expression of tumor necrosis factor receptor 2 in human non-small cell lung cancer and its role as a potential prognostic biomarker. Thorac. Cancer 2019, 10, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Keatings, V.M.; Collins, P.D.; Scott, D.M.; Barnes, P.J. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 1996, 153, 530–534. [Google Scholar] [CrossRef]
- Gessner, C.; Scheibe, R.; Wotzel, M.; Hammerschmidt, S.; Kuhn, H.; Engelmann, L.; Hoheisel, G.; Gillissen, A.; Sack, U.; Wirtz, H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 1229–1240. [Google Scholar] [CrossRef]
- Gong, L.; da Silva Caetano, M.; Cumpian, A.M.; Daliri, S.; Garza Flores, A.; Chang, S.H.; Ochoa, C.E.; Evans, C.M.; Yu, Z.; Moghaddam, S.J. Tumor necrosis factor links chronic obstructive pulmonary disease and K-ras mutant lung cancer through induction of an immunosuppressive pro-tumor microenvironment. Oncoimmunology 2016, 5, e1229724. [Google Scholar] [CrossRef]
- Gong, K.; Guo, G.; Gerber, D.E.; Gao, B.; Peyton, M.; Huang, C.; Minna, J.D.; Hatanpaa, K.J.; Kernstine, K.; Cai, L.; et al. TNF-driven adaptive response mediates resistance to EGFR inhibition in lung cancer. J. Clin. Investig. 2018, 128, 2500–2518. [Google Scholar] [CrossRef] [PubMed]
- Conlon, T.M.; John-Schuster, G.; Heide, D.; Pfister, D.; Lehmann, M.; Hu, Y.; Ertuz, Z.; Lopez, M.A.; Ansari, M.; Strunz, M.; et al. Inhibition of LTbetaR signalling activates WNT-induced regeneration in lung. Nature 2020, 588, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Dobrzanski, M.J.; Reome, J.B.; Hollenbaugh, J.A.; Hylind, J.C.; Dutton, R.W. Effector cell-derived lymphotoxin alpha and Fas ligand, but not perforin, promote Tc1 and Tc2 effector cell-mediated tumor therapy in established pulmonary metastases. Cancer Res. 2004, 64, 406–414. [Google Scholar] [CrossRef]
- Yang, D.; Ud Din, N.; Browning, D.D.; Abrams, S.I.; Liu, K. Targeting lymphotoxin beta receptor with tumor-specific T lymphocytes for tumor regression. Clin. Cancer Res. 2007, 13, 5202–5210. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulos, F.D.; Kottorou, A.E.; Antonacopoulou, A.G.; Panagopoulos, N.; Scopa, C.; Kalofonou, M.; Dougenis, D.; Koutras, A.; Makatsoris, T.; Tzelepi, V.; et al. Expression of Immune System-Related Membrane Receptors CD40, RANK, BAFFR and LTbetaR is Associated with Clinical Outcome of Operated Non-Small-Cell Lung Cancer Patients. J. Clin. Med. 2019, 8, 741. [Google Scholar] [CrossRef]
- Das, R.; Coupar, J.; Clavijo, P.E.; Saleh, A.; Cheng, T.F.; Yang, X.; Chen, J.; Van Waes, C.; Chen, Z. Lymphotoxin-beta receptor-NIK signaling induces alternative RELB/NF-kappaB2 activation to promote metastatic gene expression and cell migration in head and neck cancer. Mol. Carcinog. 2019, 58, 411–425. [Google Scholar] [CrossRef]
- Kratz, A.; Campos-Neto, A.; Hanson, M.S.; Ruddle, N.H. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 1996, 183, 1461–1472. [Google Scholar] [CrossRef]
- van Lumig, P.P.; Menting, S.P.; van den Reek, J.M.; Spuls, P.I.; van Riel, P.L.; van de Kerkhof, P.C.; Fransen, J.; Kievit, W.; de Jong, E.M. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 752–760. [Google Scholar] [CrossRef]
- McKenna, M.R.; Stobaugh, D.J.; Deepak, P. Melanoma and non-melanoma skin cancer in inflammatory bowel disease patients following tumor necrosis factor-alpha inhibitor monotherapy and in combination with thiopurines: Analysis of the Food and Drug Administration Adverse Event Reporting System. J. Gastrointestin. Liver Dis. 2014, 23, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Okabe, S.; Marino, M.W.; Sakai, A.; Sueoka, E.; Fujiki, H. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999, 59, 4516–4518. [Google Scholar] [PubMed]
- Moore, R.J.; Owens, D.M.; Stamp, G.; Arnott, C.; Burke, F.; East, N.; Holdsworth, H.; Turner, L.; Rollins, B.; Pasparakis, M.; et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 1999, 5, 828–831. [Google Scholar] [CrossRef]
- Arnott, C.H.; Scott, K.A.; Moore, R.J.; Hewer, A.; Phillips, D.H.; Parker, P.; Balkwill, F.R.; Owens, D.M. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene 2002, 21, 4728–4738. [Google Scholar] [CrossRef]
- Scott, K.A.; Moore, R.J.; Arnott, C.H.; East, N.; Thompson, R.G.; Scallon, B.J.; Shealy, D.J.; Balkwill, F.R. An antitumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol. Cancer Ther. 2003, 2, 445–451. [Google Scholar] [PubMed]
- Johansen, C.; Vestergaard, C.; Kragballe, K.; Kollias, G.; Gaestel, M.; Iversen, L. MK2 regulates the early stages of skin tumor promotion. Carcinogenesis 2009, 30, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667. [Google Scholar] [CrossRef] [PubMed]
- Arnott, C.H.; Scott, K.A.; Moore, R.J.; Robinson, S.C.; Thompson, R.G.; Balkwill, F.R. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene 2004, 23, 1902–1910. [Google Scholar] [CrossRef]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar] [CrossRef]
- Onizawa, M.; Nagaishi, T.; Kanai, T.; Nagano, K.; Oshima, S.; Nemoto, Y.; Yoshioka, A.; Totsuka, T.; Okamoto, R.; Nakamura, T.; et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G850–G859. [Google Scholar] [CrossRef]
- Yang, Y.; Gharaibeh, R.Z.; Newsome, R.C.; Jobin, C. Amending microbiota by targeting intestinal inflammation with TNF blockade attenuates development of colorectal cancer. Nat. Cancer 2020, 1, 723–734. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Alderton, G.K. Inflammation: The gut takes a toll on liver cancer. Nat. Rev. Cancer 2012, 12, 379. [Google Scholar] [CrossRef]
- Ubeda, C.; Lipuma, L.; Gobourne, A.; Viale, A.; Leiner, I.; Equinda, M.; Khanin, R.; Pamer, E.G. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med. 2012, 209, 1445–1456. [Google Scholar] [CrossRef]
- Briesemeister, D.; Friese, C.; Isern, C.C.; Dietz, E.; Blankenstein, T.; Thoene-Reineke, C.; Kammertoens, T. Differences in serum cytokine levels between wild type mice and mice with a targeted mutation suggests necessity of using control littermates. Cytokine 2012, 60, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Quarcoo, D.; Chashchina, A.A.; Bozrova, S.V.; Qin, Z.; Nedospasov, S.A.; Blankenstein, T.; Kammertoens, T.; Drutskaya, M.S. IL-13 but not IL-4 signaling via IL-4Ralpha protects mice from papilloma formation during DMBA/TPA two-step skin carcinogenesis. Cancer Med. 2013, 2, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, J.; Yang, T.; Li, Y.; Jiang, W.; Guan, Z.; Wang, Z.; Tan, J.; Wu, J.; Li, G.; et al. Platinums sensitize human epithelial tumor cells to lymphotoxin alpha by inhibiting NFkappaB-dependent transcription. Cancer Biol. Ther. 2008, 7, 1407–1414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, F.H.; Wang, Y.; Sun, G.P.; Chen, J.H.; Lin, Y.C.; Liu, W.; Zheng, R.S.; Chen, J.; Zhang, H.L.; Lan, H.T.; et al. Efficacy and safety of recombinant human lymphotoxin-alpha derivative with cisplatin and fluorouracil in patients with metastatic esophageal squamous cell carcinoma: A randomized, multicenter, open-label, controlled, phase 2b trial. Cancer 2017, 123, 3986–3994. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Wang, W.M.; Xia, H.F.; Yu, Z.L.; Li, H.M.; Ren, J.G.; Chen, G.; Wang, B.K.; Jia, J.; Zhang, W.; et al. Lymphotoxin-alpha promotes tumor angiogenesis in HNSCC by modulating glycolysis in a PFKFB3-dependent manner. Int. J. Cancer 2019, 145, 1358–1370. [Google Scholar] [CrossRef]
- Mariathasan, S.; Matsumoto, M.; Baranyay, F.; Nahm, M.H.; Kanagawa, O.; Chaplin, D.D. Absence of lymph nodes in lymphotoxin-alpha(LT alpha)-deficient mice is due to abnormal organ development, not defective lymphocyte migration. J. Inflamm. 1995, 45, 72–78. [Google Scholar]
- Futterer, A.; Mink, K.; Luz, A.; Kosco-Vilbois, M.H.; Pfeffer, K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 1998, 9, 59–70. [Google Scholar] [CrossRef]
- Liepinsh, D.J.; Grivennikov, S.I.; Klarmann, K.D.; Lagarkova, M.A.; Drutskaya, M.S.; Lockett, S.J.; Tessarollo, L.; McAuliffe, M.; Keller, J.R.; Kuprash, D.V.; et al. Novel lymphotoxin alpha (LTalpha) knockout mice with unperturbed tumor necrosis factor expression: Reassessing LTalpha biological functions. Mol. Cell Biol. 2006, 26, 4214–4225. [Google Scholar] [CrossRef][Green Version]
- Kruglov, A.A.; Grivennikov, S.I.; Kuprash, D.V.; Winsauer, C.; Prepens, S.; Seleznik, G.M.; Eberl, G.; Littman, D.R.; Heikenwalder, M.; Tumanov, A.V.; et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science 2013, 342, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Tumanov, A.V.; Koroleva, E.P.; Guo, X.; Wang, Y.; Kruglov, A.; Nedospasov, S.; Fu, Y.X. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 2011, 10, 44–53. [Google Scholar] [CrossRef]
- Grist, J.J.; Marro, B.S.; Skinner, D.D.; Syage, A.R.; Worne, C.; Doty, D.J.; Fujinami, R.S.; Lane, T.E. Induced CNS expression of CXCL1 augments neurologic disease in a murine model of multiple sclerosis via enhanced neutrophil recruitment. Eur. J. Immunol. 2018, 48, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Zindl, C.L.; Lai, J.F.; Lee, Y.K.; Maynard, C.L.; Harbour, S.N.; Ouyang, W.; Chaplin, D.D.; Weaver, C.T. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 12768–12773. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016, 64, S84–S101. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Marino, M.W.; Dunn, A.; Grail, D.; Inglese, M.; Noguchi, Y.; Richards, E.; Jungbluth, A.; Wada, H.; Moore, M.; Williamson, B.; et al. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Tarrats, N.; Moles, A.; Morales, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C.; Mari, M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology 2011, 54, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wheeler, M.D.; Kono, H.; Bradford, B.U.; Gallucci, R.M.; Luster, M.I.; Thurman, R.G. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 1999, 117, 942–952. [Google Scholar] [CrossRef]
- Mauad, T.H.; van Nieuwkerk, C.M.; Dingemans, K.P.; Smit, J.J.; Schinkel, A.H.; Notenboom, R.G.; van den Bergh Weerman, M.A.; Verkruisen, R.P.; Groen, A.K.; Oude Elferink, R.P.; et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am. J. Pathol. 1994, 145, 1237–1245. [Google Scholar] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Haybaeck, J.; Zeller, N.; Wolf, M.J.; Weber, A.; Wagner, U.; Kurrer, M.O.; Bremer, J.; Iezzi, G.; Graf, R.; Clavien, P.A.; et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009, 16, 295–308. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef]
- Teoh, N.; Leclercq, I.; Pena, A.D.; Farrell, G. Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: Implications for preconditioning. Hepatology 2003, 37, 118–128. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar] [CrossRef]
- Trinchieri, G. TNF-shaped microbiota promotes cancer. Nat. Cancer 2020, 1, 667–669. [Google Scholar] [CrossRef]
- Kuprash, D.V.; Qin, Z.; Ito, D.; Grivennikov, S.I.; Abe, K.; Drutskaya, L.N.; Blankenstein, T.; Nedospasov, S.A. Ablation of TNF or lymphotoxin signaling and the frequency of spontaneous tumors in p53-deficient mice. Cancer Lett. 2008, 268, 70–75. [Google Scholar] [CrossRef]
- Takeda, K.; Iwamoto, S.; Sugimoto, H.; Takuma, T.; Kawatani, N.; Noda, M.; Masaki, A.; Morise, H.; Arimura, H.; Konno, K. Identity of differentiation inducing factor and tumour necrosis factor. Nature 1986, 323, 338–340. [Google Scholar] [CrossRef]
- Jacobsen, F.W.; Rothe, M.; Rusten, L.; Goeddel, D.V.; Smeland, E.B.; Veiby, O.P.; Slordal, L.; Jacobsen, S.E. Role of the 75-kDa tumor necrosis factor receptor: Inhibition of early hematopoiesis. Proc. Natl. Acad. Sci. USA 1994, 91, 10695–10699. [Google Scholar] [CrossRef]
- Mizrahi, K.; Stein, J.; Yaniv, I.; Kaplan, O.; Askenasy, N. TNF-alpha has tropic rather than apoptotic activity in human hematopoietic progenitors: Involvement of TNF receptor-1 and caspase-8. Stem Cells 2013, 31, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Rebel, V.I.; Hartnett, S.; Hill, G.R.; Lazo-Kallanian, S.B.; Ferrara, J.L.; Sieff, C.A. Essential role for the p55 tumor necrosis factor receptor in regulating hematopoiesis at a stem cell level. J. Exp. Med. 1999, 190, 1493–1504. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Wang, J.; Lin, X.; Wang, Y.; Pegg, L.E.; Futterer, A.; Pfeffer, K.; Fu, Y.X. Signal via lymphotoxin-beta R on bone marrow stromal cells is required for an early checkpoint of NK cell development. J. Immunol. 2001, 166, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.J.; Baldwin, A.S. Deletion of the NF-kappaB subunit p65/RelA in the hematopoietic compartment leads to defects in hematopoietic stem cell function. Blood 2013, 121, 5015–5024. [Google Scholar] [CrossRef] [PubMed]
- Cachaco, A.S.; Carvalho, T.; Santos, A.C.; Igreja, C.; Fragoso, R.; Osorio, C.; Ferreira, M.; Serpa, J.; Correia, S.; Pinto-do, O.P.; et al. TNF-alpha regulates the effects of irradiation in the mouse bone marrow microenvironment. PLoS ONE 2010, 5, e8980. [Google Scholar] [CrossRef]
- Fernandes, M.T.; Ghezzo, M.N.; Silveira, A.B.; Kalathur, R.K.; Povoa, V.; Ribeiro, A.R.; Brandalise, S.R.; Dejardin, E.; Alves, N.L.; Ghysdael, J.; et al. Lymphotoxin-beta receptor in microenvironmental cells promotes the development of T-cell acute lymphoblastic leukaemia with cortical/mature immunophenotype. Br. J. Haematol. 2015, 171, 736–751. [Google Scholar] [CrossRef]
- Johrer, K.; Janke, K.; Krugmann, J.; Fiegl, M.; Greil, R. Transendothelial migration of myeloma cells is increased by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and autocrine up-regulation of MCP-1. Clin. Cancer Res. 2004, 10, 1901–1910. [Google Scholar] [CrossRef]
- Bharti, A.C.; Shishodia, S.; Reuben, J.M.; Weber, D.; Alexanian, R.; Raj-Vadhan, S.; Estrov, Z.; Talpaz, M.; Aggarwal, B.B. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 2004, 103, 3175–3184. [Google Scholar] [CrossRef]
- Roy, P.; Mukherjee, T.; Chatterjee, B.; Vijayaragavan, B.; Banoth, B.; Basak, S. Non-canonical NFkappaB mutations reinforce pro-survival TNF response in multiple myeloma through an autoregulatory RelB:p50 NFkappaB pathway. Oncogene 2017, 36, 1417–1429. [Google Scholar] [CrossRef]
- Kumar, S.; Witzig, T.E.; Timm, M.; Haug, J.; Wellik, L.; Kimlinger, T.K.; Greipp, P.R.; Rajkumar, S.V. Bone marrow angiogenic ability and expression of angiogenic cytokines in myeloma: Evidence favoring loss of marrow angiogenesis inhibitory activity with disease progression. Blood 2004, 104, 1159–1165. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Via, M.C.; Cicco, S.; Leone, P.; Di Lernia, G.; Giannico, D.; Desantis, V.; Frassanito, M.A.; Morizio, A.; Delgado Tascon, J.; et al. High-Risk Multiple Myeloma: Integrated Clinical and Omics Approach Dissects the Neoplastic Clone and the Tumor Microenvironment. J. Clin. Med. 2019, 8, 997. [Google Scholar] [CrossRef]
- Leone, P.; Solimando, A.G.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune Cells in the Myeloma Niche. Front. Oncol. 2020, 10, 599098. [Google Scholar] [CrossRef]
- Morris, E.V.; Suchacki, K.J.; Hocking, J.; Cartwright, R.; Sowman, A.; Gamez, B.; Lea, R.; Drake, M.T.; Cawthorn, W.P.; Edwards, C.M. Myeloma Cells Down-Regulate Adiponectin in Bone Marrow Adipocytes Via TNF-Alpha. J. Bone Miner. Res. 2020, 35, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Leone, P.; Frassanito, M.A.; Brunetti, C.; Perosa, F.; Ferrone, S.; Dammacco, F. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood 2010, 115, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Fhu, C.W.; Graham, A.M.; Yap, C.T.; Al-Salam, S.; Castella, A.; Chong, S.M.; Lim, Y.C. Reed-Sternberg cell-derived lymphotoxin-alpha activates endothelial cells to enhance T-cell recruitment in classical Hodgkin lymphoma. Blood 2014, 124, 2973–2982. [Google Scholar] [CrossRef]
- von Hoff, L.; Kargel, E.; Franke, V.; McShane, E.; Schulz-Beiss, K.W.; Patone, G.; Schleussner, N.; Kolesnichenko, M.; Hubner, N.; Daumke, O.; et al. Autocrine LTA signaling drives NF-kappaB and JAK-STAT activity and myeloid gene expression in Hodgkin lymphoma. Blood 2019, 133, 1489–1494. [Google Scholar] [CrossRef]
- Abegunde, S.O.; Buckstein, R.; Wells, R.A.; Rauh, M.J. An inflammatory environment containing TNFalpha favors Tet2-mutant clonal hematopoiesis. Exp. Hematol. 2018, 59, 60–65. [Google Scholar] [CrossRef]
- Mead, A.J.; Neo, W.H.; Barkas, N.; Matsuoka, S.; Giustacchini, A.; Facchini, R.; Thongjuea, S.; Jamieson, L.; Booth, C.A.G.; Fordham, N.; et al. Niche-mediated depletion of the normal hematopoietic stem cell reservoir by Flt3-ITD-induced myeloproliferation. J. Exp. Med. 2017, 214, 2005–2021. [Google Scholar] [CrossRef] [PubMed]
- Atretkhany, K.N.; Mufazalov, I.A.; Dunst, J.; Kuchmiy, A.; Gogoleva, V.S.; Andruszewski, D.; Drutskaya, M.S.; Faustman, D.L.; Schwabenland, M.; Prinz, M.; et al. Intrinsic TNFR2 signaling in T regulatory cells provides protection in CNS autoimmunity. Proc. Natl. Acad. Sci. USA 2018, 115, 13051–13056. [Google Scholar] [CrossRef] [PubMed]
- Polz, J.; Remke, A.; Weber, S.; Schmidt, D.; Weber-Steffens, D.; Pietryga-Krieger, A.; Muller, N.; Ritter, U.; Mostbock, S.; Mannel, D.N. Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immun. Inflamm. Dis. 2014, 2, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Vanamee, E.S.; Faustman, D.L. TNFR2: A Novel Target for Cancer Immunotherapy. Trends Mol. Med. 2017, 23, 1037–1046. [Google Scholar] [CrossRef]
- Santoiemma, P.P.; Powell, D.J., Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol. Ther. 2015, 16, 807–820. [Google Scholar] [CrossRef]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Du, R.; Wei, F.; Zhao, H.; Yu, J.; Wang, C.; Zhan, Z.; Ding, T.; Ren, X.; Chen, X.; et al. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol. Immunother. 2015, 64, 1475–1485. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, C.; Ma, G.; Sun, X.; Liu, J.; Li, S.; Liu, K.; Wang, J.; Yang, D. High expression of tumor necrosis factor receptor 2 in tissue is associated with progression and prognosis of esophageal squamous cell carcinoma. Hum. Pathol. 2018, 80, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.E.; Simmons, J.G.; Ding, S.; Van Landeghem, L.; Lund, P.K. Cytokine induction of tumor necrosis factor receptor 2 is mediated by STAT3 in colon cancer cells. Mol. Cancer Res. 2011, 9, 1718–1731. [Google Scholar] [CrossRef]
- Mizoguchi, E.; Mizoguchi, A.; Takedatsu, H.; Cario, E.; de Jong, Y.P.; Ooi, C.J.; Xavier, R.J.; Terhorst, C.; Podolsky, D.K.; Bhan, A.K. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology 2002, 122, 134–144. [Google Scholar] [CrossRef]
- Govindaraj, C.; Scalzo-Inguanti, K.; Madondo, M.; Hallo, J.; Flanagan, K.; Quinn, M.; Plebanski, M. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin. Immunol. 2013, 149, 97–110. [Google Scholar] [CrossRef]
- Nakayama, S.; Yokote, T.; Tsuji, M.; Akioka, T.; Miyoshi, T.; Hirata, Y.; Hiraoka, N.; Iwaki, K.; Takayama, A.; Nishiwaki, U.; et al. Expression of tumour necrosis factor-alpha and its receptors in Hodgkin lymphoma. Br. J. Haematol. 2014, 167, 574–577. [Google Scholar] [CrossRef]
- Heemann, C.; Kreuz, M.; Stoller, I.; Schoof, N.; von Bonin, F.; Ziepert, M.; Loffler, M.; Jung, W.; Pfreundschuh, M.; Trumper, L.; et al. Circulating levels of TNF receptor II are prognostic for patients with peripheral T-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2012, 18, 3637–3647. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Wang, J.; Yang, J.; Burrows, N.; Maxwell, P.H.; Eisen, T.; Warren, A.Y.; Vanharanta, S.; Pacey, S.; Vandenabeele, P.; et al. Tumor necrosis factor receptor 2-signaling in CD133-expressing cells in renal clear cell carcinoma. Oncotarget 2016, 7, 24111–24124. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Sadler, T.J.; Wang, J.; Reid, M.J.; Warren, A.Y.; Movassagh, M.; Lu, W.; Mills, I.G.; Neal, D.E.; Burge, J.; et al. Tumor necrosis factor receptor expression and signaling in renal cell carcinoma. Am. J. Pathol. 2010, 177, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; Ahmad, S.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. A Perspective Review on the Role of Nanomedicine in the Modulation of TNF-TNFR2 Axis in Breast Cancer Immunotherapy. J. Oncol. 2019, 2019, 6313242. [Google Scholar] [CrossRef] [PubMed]
- Torrey, H.; Khodadoust, M.; Tran, L.; Baum, D.; Defusco, A.; Kim, Y.H.; Faustman, D.L. Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sezary syndrome. Leukemia 2019, 33, 1206–1218. [Google Scholar] [CrossRef]
- Torrey, H.; Butterworth, J.; Mera, T.; Okubo, Y.; Wang, L.; Baum, D.; Defusco, A.; Plager, S.; Warden, S.; Huang, D.; et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Case, K.; Tran, L.; Yang, M.; Zheng, H.; Kuhtreiber, W.M.; Faustman, D.L. TNFR2 blockade alone or in combination with PD-1 blockade shows therapeutic efficacy in murine cancer models. J. Leukoc. Biol. 2020, 107, 981–991. [Google Scholar] [CrossRef]
- Klocke, K.; Sakaguchi, S.; Holmdahl, R.; Wing, K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. USA 2016, 113, E2383–E2392. [Google Scholar] [CrossRef]
- Yang, M.; Tran, L.; Torrey, H.; Song, Y.; Perkins, H.; Case, K.; Zheng, H.; Takahashi, H.; Kuhtreiber, W.M.; Faustman, D.L. Optimizing TNFR2 antagonism for immunotherapy with tumor microenvironment specificity. J. Leukoc. Biol. 2020, 107, 971–980. [Google Scholar] [CrossRef]
- He, J.; Li, R.; Chen, Y.; Hu, Y.; Chen, X. TNFR2-expressing CD4(+)Foxp3(+) regulatory T cells in cancer immunology and immunotherapy. Prog. Mol. Biol. Transl. Sci. 2019, 164, 101–117. [Google Scholar] [CrossRef]
- Banks, T.A.; Rouse, B.T.; Kerley, M.K.; Blair, P.J.; Godfrey, V.L.; Kuklin, N.A.; Bouley, D.M.; Thomas, J.; Kanangat, S.; Mucenski, M.L. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 1995, 155, 1685–1693. [Google Scholar]
- Koni, P.A.; Sacca, R.; Lawton, P.; Browning, J.L.; Ruddle, N.H.; Flavell, R.A. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity 1997, 6, 491–500. [Google Scholar] [CrossRef]
- Alimzhanov, M.B.; Kuprash, D.V.; Kosco-Vilbois, M.H.; Luz, A.; Turetskaya, R.L.; Tarakhovsky, A.; Rajewsky, K.; Nedospasov, S.A.; Pfeffer, K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 9302–9307. [Google Scholar] [CrossRef]
- Fernandes, M.T.; Dejardin, E.; dos Santos, N.R. Context-dependent roles for lymphotoxin-beta receptor signaling in cancer development. Biochim. Biophys. Acta 2016, 1865, 204–219. [Google Scholar] [CrossRef]
- Zhou, P.; Fang, X.; McNally, B.A.; Yu, P.; Zhu, M.; Fu, Y.X.; Wang, L.; Liu, Y.; Zheng, P. Targeting lymphotoxin-mediated negative selection to prevent prostate cancer in mice with genetic predisposition. Proc. Natl. Acad. Sci. USA 2009, 106, 17134–17139. [Google Scholar] [CrossRef]
- Rothe, M.; Wong, S.C.; Henzel, W.J.; Goeddel, D.V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 1994, 78, 681–692. [Google Scholar] [CrossRef]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; Van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Oshima, H.; Ishikawa, T.; Yoshida, G.J.; Naoi, K.; Maeda, Y.; Naka, K.; Ju, X.; Yamada, Y.; Minamoto, T.; Mukaida, N.; et al. TNF-alpha/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 2014, 33, 3820–3829. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Franco, A.T.; Israel, D.A.; Washington, M.K.; Krishna, U.; Fox, J.G.; Rogers, A.B.; Neish, A.S.; Collier-Hyams, L.; Perez-Perez, G.I.; Hatakeyama, M.; et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 2005, 102, 10646–10651. [Google Scholar] [CrossRef]
- Salcedo, R.; Worschech, A.; Cardone, M.; Jones, Y.; Gyulai, Z.; Dai, R.M.; Wang, E.; Ma, W.; Haines, D.; O’HUigin, C.; et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: Role of interleukin 18. J. Exp. Med. 2010, 207, 1625–1636. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Medzhitov, R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 2007, 317, 124–127. [Google Scholar] [CrossRef]
- Fukata, M.; Chen, A.; Vamadevan, A.S.; Cohen, J.; Breglio, K.; Krishnareddy, S.; Hsu, D.; Xu, R.; Harpaz, N.; Dannenberg, A.J.; et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 2007, 133, 1869–1881. [Google Scholar] [CrossRef]
- Lowe, E.L.; Crother, T.R.; Rabizadeh, S.; Hu, B.; Wang, H.; Chen, S.; Shimada, K.; Wong, M.H.; Michelsen, K.S.; Arditi, M. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS ONE 2010, 5, e13027. [Google Scholar] [CrossRef]
- van Heel, D.A.; Udalova, I.A.; De Silva, A.P.; McGovern, D.P.; Kinouchi, Y.; Hull, J.; Lench, N.J.; Cardon, L.R.; Carey, A.H.; Jewell, D.P.; et al. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(-kappa)B transcription factors. Hum. Mol. Genet. 2002, 11, 1281–1289. [Google Scholar] [CrossRef]
- Breese, E.J.; Michie, C.A.; Nicholls, S.W.; Murch, S.H.; Williams, C.B.; Domizio, P.; Walker-Smith, J.A.; MacDonald, T.T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 1994, 106, 1455–1466. [Google Scholar] [CrossRef]
- Murch, S.H.; Lamkin, V.A.; Savage, M.O.; Walker-Smith, J.A.; MacDonald, T.T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut 1991, 32, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.; Galvao, I.; Vieira, A.T. Mechanisms by Which the Gut Microbiota Influences Cytokine Production and Modulates Host Inflammatory Responses. J. Interferon Cytokine Res. 2019, 39, 393–409. [Google Scholar] [CrossRef]
- Jones-Hall, Y.L.; Kozik, A.; Nakatsu, C. Ablation of tumor necrosis factor is associated with decreased inflammation and alterations of the microbiota in a mouse model of inflammatory bowel disease. PLoS ONE 2015, 10, e0119441. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; Paulino, D.S.; Brambilla, S.R.; Camargo, J.A.; Persinoti, G.F.; Carvalheira, J.B.C. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 2018, 24, 1995–2008. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-alpha and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Wang, Y.; Koroleva, E.P.; Kruglov, A.A.; Kuprash, D.V.; Nedospasov, S.A.; Fu, Y.X.; Tumanov, A.V. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 2010, 32, 403–413. [Google Scholar] [CrossRef]
- Upadhyay, V.; Poroyko, V.; Kim, T.J.; Devkota, S.; Fu, S.; Liu, D.; Tumanov, A.V.; Koroleva, E.P.; Deng, L.; Nagler, C.; et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat. Immunol. 2012, 13, 947–953. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar] [CrossRef]
- Dalaveris, E.; Kerenidi, T.; Katsabeki-Katsafli, A.; Kiropoulos, T.; Tanou, K.; Gourgoulianis, K.I.; Kostikas, K. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer 2009, 64, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O.; Oztopuz, O.; Ozkan, O.F. Determination of IL-6, TNF-alpha and VEGF levels in the serums of patients with colorectal cancer. Cell Mol. Biol. 2017, 63, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.D.; Zheng, Y.Q.; Wang, L.P.; Zhao, H.T.; Yang, S. Long noncoding RNA UCA1 promotes cell proliferation, migration and invasion of human leukemia cells via sponging miR-126. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2233–2245. [Google Scholar] [CrossRef]
- Aguayo, A.; Kantarjian, H.; Manshouri, T.; Gidel, C.; Estey, E.; Thomas, D.; Koller, C.; Estrov, Z.; O’Brien, S.; Keating, M.; et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 2000, 96, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-alpha as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zhao, M.; Liu, B.; Han, X.; Li, Y.; Wang, W.; Zhang, Q.; Lv, P.; Xing, L.; Shen, H.; et al. TNFalphamediated upregulation of SOD2 contributes to cell proliferation and cisplatin resistance in esophageal squamous cell carcinoma. Oncol. Rep. 2019, 42, 1497–1506. [Google Scholar] [CrossRef]
- Tan, W.; Luo, X.; Li, W.; Zhong, J.; Cao, J.; Zhu, S.; Chen, X.; Zhou, R.; Shang, C.; Chen, Y. TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine 2019, 40, 446–456. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.; Guo, Y.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. PTTG1 is involved in TNF-alpha-related hepatocellular carcinoma via the induction of c-myc. Cancer Med. 2019, 8, 5702–5715. [Google Scholar] [CrossRef]
- Zhang, G.P.; Yue, X.; Li, S.Q. Cathepsin C Interacts with TNF-alpha/p38 MAPK Signaling Pathway to Promote Proliferation and Metastasis in Hepatocellular Carcinoma. Cancer Res. Treat. 2020, 52, 10–23. [Google Scholar] [CrossRef]
- Moller, T.; James, J.P.; Holmstrom, K.; Sorensen, F.B.; Lindebjerg, J.; Nielsen, B.S. Co-Detection of miR-21 and TNF-alpha mRNA in Budding Cancer Cells in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 1907. [Google Scholar] [CrossRef]
- Sanchez, N.C.; Medrano-Jimenez, E.; Aguilar-Leon, D.; Perez-Martinez, L.; Pedraza-Alva, G. Tumor Necrosis Factor-Induced miR-146a Upregulation Promotes Human Lung Adenocarcinoma Metastasis by Targeting Merlin. DNA Cell Biol. 2020, 39, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Lazariotou, M.; Kircher, S.; Hofelmayr, A.; Germer, C.T.; von Rahden, B.H.; Waaga-Gasser, A.M.; Gasser, M. Tumor necrosis factor-alpha is associated with positive lymph node status in patients with recurrence of colorectal cancer-indications for anti-TNF-alpha agents in cancer treatment. Cell Oncol. 2011, 34, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Ferrer, A.; Larramendy, M.L.; Galimberti, S.; Aalto, Y.; Casas, S.; Vilpo, J.; Ruutu, T.; Vettenranta, K.; Franssila, K.; et al. Lymphotoxin beta expression is high in chronic lymphocytic leukemia but low in small lymphocytic lymphoma: A quantitative real-time reverse transcriptase polymerase chain reaction analysis. Haematologica 2003, 88, 654–658. [Google Scholar]
- Cornett, W.R.; McCall, L.M.; Petersen, R.P.; Ross, M.I.; Briele, H.A.; Noyes, R.D.; Sussman, J.J.; Kraybill, W.G.; Kane, J.M., 3rd; Alexander, H.R.; et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J. Clin. Oncol. 2006, 24, 4196–4201. [Google Scholar] [CrossRef]
- Fraker, D.L.; Alexander, H.R.; Ross, M.; Bartlett, D.L.; Tyler, D.; Libutti, L.S.; Boddie, A.; Briele, H.; Karakousis, G. A phase III trial of isolated limb perfusion for extremity melanoma comparing melphalan alone versus melphalan plus tumor necrosis factor (TNF-α) plus interferon-gamma (IFN). Ann. Surg. Oncol. 2002, 9, S8. [Google Scholar] [CrossRef]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA 2017, 318, 1679–1686. [Google Scholar] [CrossRef]
- Chupin, A.; Perduca, V.; Meyer, A.; Bellanger, C.; Carbonnel, F.; Dong, C. Systematic review with meta-analysis: Comparative risk of lymphoma with anti-tumour necrosis factor agents and/or thiopurines in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Herrinton, L.J.; Liu, L.; Weng, X.; Lewis, J.D.; Hutfless, S.; Allison, J.E. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am. J. Gastroenterol. 2011, 106, 2146–2153. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, X.; Wang, Q.; Zhao, F. The association of TNF-308 (G/A) gene polymorphisms and hepatocellular carcinoma risk: A meta-analysis. Chin. J. Cancer Res. 2016, 28, 536–542. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Yu, M.; Li, Z. Tumor Necrosis Factor-alpha T-857C (rs1799724) Polymorphism and Risk of Cancers: A Meta-Analysis. Dis. Markers 2016, 2016, 4580323. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.F.; Chen, S.C.; Lin, Z.Y.; Dai, C.Y.; Huang, J.F.; Yu, M.L.; Chuang, W.L. Independent and additive interaction between polymorphisms of tumor necrosis factor alpha-308 and lymphotoxin alpha+252 on risk of hepatocellular carcinoma related to hepatitis B. Kaohsiung J. Med. Sci. 2017, 33, 453–457. [Google Scholar] [CrossRef]
- Xie, H.; Yao, H.; Huo, Y.; Li, N.; Cheng, Y. Association between TNF-alpha gene 308G>A polymorphism and lung cancer risk: A meta-analysis. Tumour. Biol. 2014, 35, 9693–9699. [Google Scholar] [CrossRef]
- Kaabachi, S.; Kaabachi, W.; Rafrafi, A.; Belkis, H.; Hamzaoui, K.; Sassi, F.H. Tumor necrosis factor gene polymorphisms in Tunisian patients with non-small cell lung cancer. Clin. Lab. 2013, 59, 1389–1395. [Google Scholar] [CrossRef]
- Takei, K.; Ikeda, S.; Arai, T.; Tanaka, N.; Muramatsu, M.; Sawabe, M. Lymphotoxin-alpha polymorphisms and presence of cancer in 1536 consecutive autopsy cases. BMC Cancer 2008, 8, 235. [Google Scholar] [CrossRef]
- Sobjanek, M.; Zablotna, M.; Michajlowski, I.; Nedoszytko, B.; Lesiak, A.; Nowicki, R. -308 G/A TNF-alpha gene polymorphism influences the course of basal cell carcinoma in a Polish population. Arch. Med. Sci. 2015, 11, 599–604. [Google Scholar] [CrossRef]
- Rizzato, C.; Canzian, F.; Rudnai, P.; Gurzau, E.; Stein, A.; Koppova, K.; Hemminki, K.; Kumar, R.; Campa, D. Interaction between functional polymorphic variants in cytokine genes, established risk factors and susceptibility to basal cell carcinoma of skin. Carcinogenesis 2011, 32, 1849–1854. [Google Scholar] [CrossRef]
- Huang, X.; Qin, S.; Liu, Y.; Tao, L.; Jiang, H. Associations of tumor necrosis factor-alpha polymorphisms with the risk of colorectal cancer: A meta-analysis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, J.; Wang, G.; Qiao, W. Association between tumor necrosis factor alpha gene polymorphisms and multiple myeloma risk: An updated meta-analysis. Hematology 2019, 24, 216–224. [Google Scholar] [CrossRef]
- Zhai, K.; Ding, J.; Zhou, Y. Different role of tumor necrosis factor-alpha polymorphism in non-Hodgkin lymphomas among Caucasian and Asian populations: A meta-analysis. Int. J. Mol. Sci. 2014, 15, 7684–7698. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhu, J.H.; Huang, S.Y.; Cui, Z.; He, J.; Jia, W.H. The association between the polymorphisms of TNF-alpha and non-Hodgkin lymphoma: A meta-analysis. Tumour. Biol. 2014, 35, 12509–12517. [Google Scholar] [CrossRef]
- Gong, L.L.; Han, F.F.; Lv, Y.L.; Liu, H.; Wan, Z.R.; Zhang, W.; Shi, M.B.; Pei, L.X.; Liu, L.H. TNF-alpha and LT-alpha polymorphisms and the risk of leukemia: A meta-analysis. Tumori J. 2017, 103, 53–59. [Google Scholar] [CrossRef]



| Transplantable Tumor Cell Type and Injection Site | Genetic Background of Recipient Mice | Additional Experimental Procedures | Reported Phenotype | Ref. | |
|---|---|---|---|---|---|
| Meth A sarcoma | s.c. | (BALB/c x C57BL/6) F1 hybrid | Single administration of TNF-positive serum (i.v.) | Hemorrhagic tumor necrosis | [11] |
| CFS1-fibrosarcoma | i.v. | C3H/He, DBA/2 | Single injection with rhTNF or mTNF (i.p.) 5 h before or 1 h after tumor cell inoculation | Enhanced lung metastasis, dose- and time-dependent effect | [19] |
| Renca RCC | i.v. | TNFR1 knockout in BALB/c | None | Regression of lung metastasis | [21] |
| B16F10 melanoma | i.v. | C57BL/6 | Single injection with rmTNF (i.v.) 1 h before tumor cell inoculation | Enhanced lung metastasis | [20] |
| TNFR2–/–LLC | s.c. | No effect | [22] | ||
| Low-dose injections with rmTNF (i.t.) for 6 days | Tumor regression | ||||
| LLC | s.c. | Low-dose injections with rmTNF (i.t.) for 6 days | Increased tumor growth | ||
| GD2-expressing B16 melanoma | i.v. | Daily injections with αGD2–LTα fusion protein (i.p.) for 5 consecutive days | Reduced growth and number of lung metastasis foci | [23] | |
| s.c. | Daily injections with αGD2–LTα fusion protein (i.v.) for 7 consecutive days | Tumor flattening and necrosis | |||
| B16BL6 melanoma | s.c. | Daily injections (i.p. or p.l.) with mTNF or rhTNF | Reduced tumor growth | [24] | |
| Low TNF-expressing B16F10 melanoma or LLC | s.c | None | Enhanced tumor growth, reduced necrosis | [25] | |
| High TNF-expressing B16F10 melanoma or LLC | s.c. | None | No effect | ||
| TNF-expressing B16F10 melanoma or LLC cells | s.c. | TNFR1/TNFR2 double knockout in C57BL/6 | None | No effect in the case of B16F10 and even reduced tumor growth in the case of LLC (compared with control cells) | |
| B16F10 melanoma | s.c. | LTα knockout in C57BL/6 | Enhanced tumor growth | [26] * | |
| B16F10 melanoma or LLC | i.v. | None | Increased incidence of metastasis | ||
| hTNF-expressing murine 1591-RE cells | s.c. | athymic NCR nude mice | None | Reduced tumor growth | [27] |
| BFS-1 fibrosarcoma | i.d. | LTα/LTβ double knockout in C57BL/6 | None | Reduced tumor growth in both cases | [28] |
| sLTβR-Fc fusion protein-expressing BFS-1 fibrosarcoma | I.d. | C57BL/6 | None | ||
| CT26 colorectal carcinoma | i.s. | TNFR1 knockout in BALB/c | None | Reduced incidence of liver metastasis | [29] |
| MC-38 colorectal carcinoma | i.s. | TNFflox/flox LysMcre/wt in C57BL/6 | Hepatic ischemia-reperfusion injury | Increased liver metastasis | [30] |
| i.c. | C57BL/6 | Multiple injections with neutralizing LTβR-Fc fusion protein (i.p.) | Increased tumor number and load | [31] | |
| CT26 colorectal carcinoma | i.s. | BALB/c | Single injection with etanercept or TNF (i.p.) followed by hepatic ischemia-reperfusion injury | Reduced liver metastasis | [32] |
| CT26 colorectal carcinoma | s.c. | Single injection with α-mLTβR agonistic Ab (i.p.) | Tumor necrosis | [33] | |
| Tnf shRNA-expressing B-ALL | i.v. | C57BL/6 | None | Increased survival | [34] |
| BCR/ABL myeloma | i.v. | C57BL/6 as donors, B6C3F1 as recipients | Transfer of BM from TNF, LTα or TNF/LTα double knockout mice into lethally irradiated mice | Increased survival, especially in the case of TNF/LTα double knockout mice | [35] |
| LTβR knockout BCR/ABL myeloma | i.v. | C57BL/6 | None | Increased survival | [36] |
| Eμ-myc B-cell lymphoma | i.v. | C57BL/6 | Two injections of α-mLTβR neutralizing Ab (i.p.) | Decreased tumor growth | [37] |
| LtβRflox/flox Cdh5cre/ERT2 in C57BL/6 | None | ||||
| Chemically Induced Cancer Mouse Model | Genetic Background | Additional Experimental Procedures | Resulting Phenotype | Ref. |
|---|---|---|---|---|
| DMBA/TPA-induced skin carcinogenesis | TNF knockout in 129/Svj (CD-1 mice as controls) | None | Reduced tumor number | [52] * |
| TNF knockout in mixed 129Sv × C57BL/6 background or BALB/c | None | [53] | ||
| TNF knockout in BALB/c | None | [54] | ||
| TNF knockout in C57BL/6, 129/SvEv, BALB/c | None | [55] | ||
| C57BL/6 | Injections of α-TNF (i.p.) 1 day prior to DMBA treatment and once a week during TPA promotion | |||
| TNF knockout in C57BL/6 | None | [56] | ||
| TNF knockout in C57BL/6 | None | [57] | ||
| Tissue-specific B-cell TNF knockout in C57BL/6 | None | Reduced tumor number, less pronounced effect | ||
| C57BL/6 | Adoptive transfer of splenic B-cells from DMBA/TPA-treated WT mice into DMBA/TPA-treated TNF knockout mice | Increased tumor number compared with TNF knockout mice | ||
| TNFR1 or TNFR2 knockout in C57BL/6 | None | Reduced tumor number, especially in TNFR1 knockout mice | [58] | |
| DMBA/okadaic-acid-induced skin carcinogenesis | TNF knockout in 129/Svj (CD-1 mice as controls) | None | Reduced tumor number | [52] * |
| AOM/DSS-induced colorectal cancer | TNFR1 knockout in BALB/c | Daily injections of Etanercept (i.p.) from day 56 to day 60 | Reduced tumor number and growth | [59] |
| C57BL/6 | Weekly injections of α-TNF (i.p.) following the first DSS cycle | [60] | ||
| C57BL/6 | Multiple injections of neutralizing LTβR-Fc fusion protein (i.p.) | Increased tumor number and load | [31] | |
| Colibactin/DSS-induced colorectal cancer | APCmin/-in 129/SvE | Injections of α-TNF (i.p.) every other day for up to 6 times immediately after DSS | Reduced tumor number, no effect when co-housed with control mice | [61] * |
| Cecal microbiota transplantation from colibactin/DSS-exposed mice treated with α-TNF to germ-free mice followed by DSS exposure | Reduced tumor number as compared to germ-free mice transplanted with cecal microbiota from colibactin/DSS-exposed mice treated with PBS | |||
| Colibactin-induced colorectal cancer | APCmin/-IL-10 knockout in 129/SvE | Twice-weekly injections of α-TNF (i.p.) twice a week starting at 8 weeks after Escherichia coli gavage and until the endpoint | Reduced tumor number, no effect when co-housed with control mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubernatorova, E.O.; Polinova, A.I.; Petropavlovskiy, M.M.; Namakanova, O.A.; Medvedovskaya, A.D.; Zvartsev, R.V.; Telegin, G.B.; Drutskaya, M.S.; Nedospasov, S.A. Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice. Cancers 2021, 13, 1775. https://doi.org/10.3390/cancers13081775
Gubernatorova EO, Polinova AI, Petropavlovskiy MM, Namakanova OA, Medvedovskaya AD, Zvartsev RV, Telegin GB, Drutskaya MS, Nedospasov SA. Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice. Cancers. 2021; 13(8):1775. https://doi.org/10.3390/cancers13081775
Chicago/Turabian StyleGubernatorova, Ekaterina O., Almina I. Polinova, Mikhail M. Petropavlovskiy, Olga A. Namakanova, Alexandra D. Medvedovskaya, Ruslan V. Zvartsev, Georgij B. Telegin, Marina S. Drutskaya, and Sergei A. Nedospasov. 2021. "Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice" Cancers 13, no. 8: 1775. https://doi.org/10.3390/cancers13081775
APA StyleGubernatorova, E. O., Polinova, A. I., Petropavlovskiy, M. M., Namakanova, O. A., Medvedovskaya, A. D., Zvartsev, R. V., Telegin, G. B., Drutskaya, M. S., & Nedospasov, S. A. (2021). Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice. Cancers, 13(8), 1775. https://doi.org/10.3390/cancers13081775





