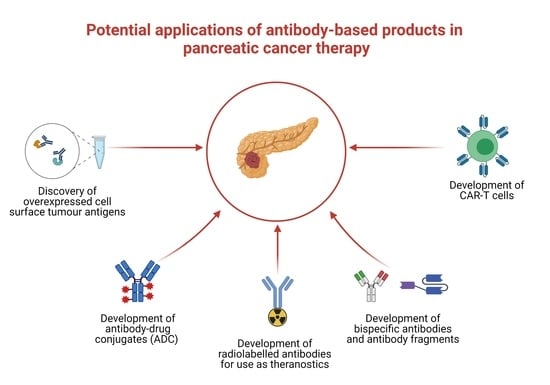
Therapeutic Application of Monoclonal Antibodies in Pancreatic Cancer: Advances, Challenges and Future Opportunities
Abstract
:Simple Summary
Abstract
1. Introduction
2. Therapeutic Antibodies Approved in Cancer
3. Preclinical Studies
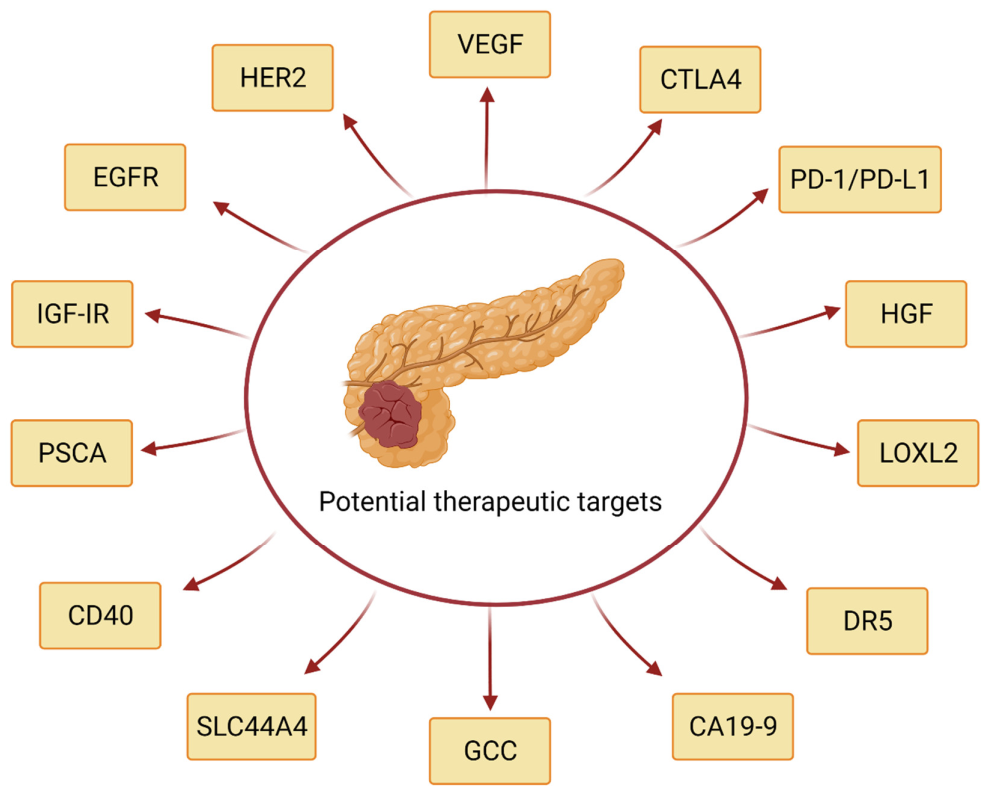
4. Clinical Trials Evaluating the Diagnostic and Therapeutic Potential of Monoclonal Antibodies in Pancreatic Cancer
4.1. Clinical Trials with Antibodies Targeting Insulin-Like Growth Factor Receptor (IGF-IR)
4.2. Clinical Trials with Anti-Epidermal Growth Factor Receptor (EGFR) Antibodies
4.3. Clinical Trials with the Anti-Human Epidermal Growth Factor Receptor 2 (HER2) Antibody Trastuzumab
4.4. Clinical Trials with the Anti-Vascular Endothelial Growth Factor (VEGF) Antibody Bevacizumab
4.5. Clinical Trials with Anti-Cytotoxic Lymphocyte-Associated Antigen-4 (CTLA-4)
4.6. Clinical Trials with Anti-Programmed Cell Death-1 (PD-1) Receptor and Anti-PD-L1 Ligand Antibodies
4.7. Clinical Trial with Anti-Hepatocyte Growth Factor (HGF) Antibody Ficlatuzumab
4.8. Clinical Trial with Anti-Lysyl Oxidase-Like 2 (LOXL2) Antibody Simtuzumab
4.9. Clinical Trials with Anti-Death Receptor 5 (DR5) Antibodies
4.10. Clinical Trials with Anti-CA19-9 Antigen (CA19-9) Antibodies
4.11. Clinical Trial with Anti-Guanylyl Cyclase C (GCC) Antibody
4.12. Clinical Trial with Anti-SLC44A4 Antibody ASG-5ME
4.13. Clinical Trial with Anti-CD40 Antibody Selicrelumab
4.14. Clinical Trial with Anti-Prostate Stem Cell Antigen (PSCA) Antibody AGS-1C4D4
5. Challenges and Future Opportunities with Antibody Therapeutics in Pancreatic Cancer
6. Summary and Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [Green Version]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Modjtahedi, H.; Ali, S.; Essapen, S. Therapeutic application of monoclonal antibodies in cancer: Advances and challenges. Br. Med. Bull. 2012, 104, 41–59. [Google Scholar] [CrossRef] [Green Version]
- Pillay, V.; Gan, H.K.; Scott, A.M. Antibodies in oncology. N. Biotechnol. 2011, 28, 518–529. [Google Scholar] [CrossRef]
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012, 12, 14. [Google Scholar]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Huang, S.; van Duijnhoven, S.M.J.; Sijts, A.; van Elsas, A. Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. J. Cancer. Res. Clin. Oncol. 2020, 146, 3111–3122. [Google Scholar] [CrossRef]
- Buss, N.A.P.S.; Henderson, S.J.; McFarlane, M.; Shenton, J.M.; de Haan, L. Monoclonal antibody therapeutics: History and future. Curr. Opin. Pharmacol. 2012, 12, 615–622. [Google Scholar] [CrossRef]
- Modjtahedi, H. Monoclonal Antibodies as Therapeutic Agents: Advances and Challenges. Iran. J. Immunol. 2005, 2, 3–20. [Google Scholar]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2021. mAbs 2021, 13, 1860476. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Bauer, C.A.; Öhlund, D.; Lauth, M.; Buchholz, M.; Michl., P.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut 2019, 68, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Raffenne, J.; Couvelard, A.; Poté, N. Tumor Heterogeneity in Pancreatic Adenocarcinoma. Pathobiology 2018, 85, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Crown, J. Companion biomarkers: Paving the pathway to personalized treatment for cancer. Clin. Chem. 2013, 59, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Ressler, D.; Snyder, G. The current and future state of companion diagnostics. Pharmgenom. Pers. Med. 2015, 8, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Arias-Pinilla, G.A.; Dalgleish, A.G.; Mudan, S.; Bagwan, I.; Walker, A.J.; Modjtahedi, H. Development and application of two novel monoclonal antibodies against overexpressed CD26 and integrin α3 in human pancreatic cancer. Sci. Rep. 2020, 10, 53. [Google Scholar] [CrossRef]
- Aithal, A.; Orzechowski, C.; Junker, W.M.; Kshirsagar, P.; Shah, A.; Gautam, S.K.; Varshney, G.C.; Batra, S.K.; Jain, M. (Eds.) Targeting MUC4 in pancreatic cancer using non-shed cell surface bound antigenic epitopes. Abstracts of Papers Submitted to the Joint 50th Anniversary Meeting of the American Pancreatic Association and Japan Pancreas Society. Pancreas 2019, 48, 1401–1402. [Google Scholar] [CrossRef]
- Bose, M.; Mukherjee, P. A novel antibody blocks anti-apoptotic activity of MUC1 in pancreatic cancer cell lines. Cancer Res. 2019, 79 (Suppl. S13), 2052. [Google Scholar] [CrossRef]
- Nishii, Y.; Yamaguchi, M.; Kimura, Y.; Hasegawa, T.; Aburatani, H.; Uchida, H.; Hirata, K.; Sakuma, Y. A newly developed anti-Mucin 13 monoclonal antibody targets pancreatic ductal adenocarcinoma cells. Int. J. Oncol. 2015, 46, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Aghevlian, S.; Cai, Z.; Hedley, D.; Winnik, M.A.; Reilly, R.M. Radioimmunotherapy of PANC-1 human pancreatic cancer xenografts in NOD/SCID or NRG mice with Panitumumab labeled with Auger electron emitting, (111)In or β-particle emitting, (177)Lu. EJNMMI Radiopharm. Chem. 2020, 5, 22. [Google Scholar] [CrossRef]
- Nishimura, T.; Mitsunaga, M.; Sawada, R.; Saruta, M.; Kobayashi, H.; Matsumoto, N.; Kanke, T.; Yanai, H.; Nakamura, K. Photoimmunotherapy targeting biliary-pancreatic cancer with humanized anti-TROP2 antibody. Cancer Med. 2019, 8, 7781–7792. [Google Scholar] [CrossRef] [Green Version]
- Aung, W.; Tsuji, A.B.; Sudo, H.; Sugyo, A.; Ukai, Y.; Kouda, K.; Kurosawa, Y.; Furukawa, T.; Saga, T. Radioimmunotherapy of pancreatic cancer xenografts in nude mice using 90Y-labeled anti-α6β4 integrin antibody. Oncotarget 2016, 7, 38835–38844. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.; Karve, A.; Matiash, K.; Stone, T.; Desai, P.; Bogdanov, V. Preclinical in vivo characterization of a first-in-class, fully humanized antibody targeting alternatively spliced tissue factor. Res. Pract. Thromb. Haemost. 2020, 4, 1–44. [Google Scholar]
- Tsumura, R.; Anzai, T.; Manabe, S.; Takashima, H.; Koga, Y.; Yasunaga, M.; Matsumura, Y. Antitumor effect of humanized anti-tissue factor antibody-drug conjugate in a model of peritoneal disseminated pancreatic cancer. Oncol. Rep. 2021, 45, 329–336. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ehlerding, E.B.; Rosenkrans, Z.T.; Jiang, D.; Sun, T.; Aluicio-Sarduy, E.; Engle, J.W.; Ni, D.; Cai, W. 86/90Y-Labeled Monoclonal Antibody Targeting Tissue Factor for Pancreatic Cancer Theranostics. Mol. Pharm. 2020, 17, 1697–1705. [Google Scholar] [CrossRef]
- Aung, W.; Tsuji, A.B.; Sugyo, A.; Takashima, H.; Yasunaga, M.; Matsumura, Y.; Higashi, T. Near-infrared photoimmunotherapy of pancreatic cancer using an indocyanine green-labeled anti-tissue factor antibody. World J. Gastroenterol. 2018, 24, 5491–5504. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Ohishi, T.; Kawada, M.; Kato, Y. A cancer-specific anti-podocalyxin monoclonal antibody (60-mG2a-f) exerts antitumor effects in mouse xenograft models of pancreatic carcinoma. Biochem. Biophys. Rep. 2020, 24, 100826. [Google Scholar] [CrossRef]
- Kato, Y.; Ohishi, T.; Sano, M.; Asano, T.; Sayama, Y.; Takei, J.; Kawada, M.; Kaneko, M.K. H(2)Mab-19 Anti-Human Epidermal Growth Factor Receptor 2 Monoclonal Antibody Therapy Exerts Antitumor Activity in Pancreatic Cancer Xenograft Models. Monoclon. Antib. Immunodiagn. Immunother. 2020, 39, 61–65. [Google Scholar] [CrossRef]
- Nishigaki, T.; Takahashi, T.; Serada, S.; Fujimoto, M.; Ohkawara, T.; Hara, H.; Sugase, T.; Otsuru, T.; Saito, Y.; Tsujiiet, S.; et al. Anti-glypican-1 antibody-drug conjugate is a potential therapy against pancreatic cancer. Br. J. Cancer 2020, 122, 1333–1341. [Google Scholar] [CrossRef]
- Dimastromatteo, J.; Poisonnier, A.; Perez, S.; Coussens, L.; Kelly, K. Therapeutic targeting of cell surface plectin induces anti-cancer immune response and pancreatic cancer regression. Cancer Res. 2019, 79 (Suppl. S13), 1558. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Koide, A.; Bolen, J.; Miller, G.; Koide, S. First in class immunotherapy targeting Galectin-9 promotes T-cell activation and anti-tumor response against pancreatic cancer and other solid tumors. Cancer Res. 2019, 79 (Suppl. S13), 1551. [Google Scholar] [CrossRef]
- Yao, H.-P.; Feng, L.; Suthe, S.R.; Chen, L.-H.; Weng, T.-H.; Hu, C.-Y.; Jun, E.S.; Wu, Z.G.; Wang, W.L.; Kim, S.C.; et al. Therapeutic efficacy, pharmacokinetic profiles, and toxicological activities of humanized antibody-drug conjugate Zt/g4-MMAE targeting RON receptor tyrosine kinase for cancer therapy. J. Immunother. Cancer 2019, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Basile, A.; De Marco, M.; Festa, M.; Falco, A.; Iorio, V.; Guerriero, L.; Eletto, D.; Rea, D.; Arra, C.; Lamolinara, A.; et al. Development of an anti-BAG3 humanized antibody for treatment of pancreatic cancer. Mol. Oncol. 2019, 13, 1388–1399. [Google Scholar] [CrossRef] [Green Version]
- Türeci, Ӧ.; Mitnacht-Kraus, R.; Wöll, S.; Yamada, T.; Sahin, U. Characterization of zolbetuximab in pancreatic cancer models. Oncoimmunology 2018, 8, e1523096. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, T.; Kamachi, H.; Fujii, Y.; Matsuzawa, F.; Einama, T.; Kawamata, F.; Kobayashi, N.; Hatanaka, Y.; Taketomi, A. The anti-mesothelin monoclonal antibody amatuximab enhances the anti-tumor effect of gemcitabine against mesothelin-high expressing pancreatic cancer cells in a peritoneal metastasis mouse model. Oncotarget 2018, 9, 33844–33852. [Google Scholar] [CrossRef] [Green Version]
- Babic, I.; Nomura, N.; Glassy, E.; Nurmemmedov, E.; Yenugonda, V.; Glassy, M.; Kesari, S. Abstract 3828: Pritumumab mAb binds cell surface expressed vimentin on pancreatic cancer cells and inhibits tumor growth. Cancer Res. 2018, 78 (Suppl. S13), 3828. [Google Scholar] [CrossRef]
- Weygant, N.; Qu, D.; May, R.; Chandrakesan, P.; Ge, Y.; Ryan, C.D.; An, G.; Schlosser, M.J.; Bannerman-Menson, E.; Houchen, C.W. Systemic delivery of CBT-15G DCLK1-targeted monoclonal antibody dramatically decreases tumorigenesis in a xenograft model of pancreatic cancer. Cancer Res. (Chic. Ill.) 2016, 76 (Suppl. S14), 577. [Google Scholar] [CrossRef]
- Moek, K.L.; Giesen, D.; Kok, I.C.; de Groot, D.J.A.; Jalving, M.; Fehrmann, R.S.N.; Lub-de Hooge, M.N.; Brouwers, A.H.; de Vries, E.G. Theranostics Using Antibodies and Antibody-Related Therapeutics. J. Nucl. Med. 2017, 58, 83s–90s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, C.G.; Hernandez, R.; Eddine, S.B.; Cai, W. Molecular Imaging of Pancreatic Cancer with Antibodies. Mol. Pharm. 2016, 13, 8–24. [Google Scholar] [CrossRef]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.R.; Kurosawa, Y.; Saga, T. Evaluation of 89Zr-Labeled Human Anti-CD147 Monoclonal Antibody as a Positron Emission Tomography Probe in a Mouse Model of Pancreatic Cancer. PLoS ONE 2013, 8, e61230. [Google Scholar] [CrossRef] [Green Version]
- Poty, S.; Mandleywala, K.; O’Neill, E.; Knight, J.C.; Cornelissen, B.; Lewis, J.S. 89Zr-PET imaging of DNA double-strand breaks for the early monitoring of response following α- and β-particle radioimmunotherapy in a mouse model of pancreatic ductal adenocarcinoma. Theranostics 2020, 10, 5802–5814. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Park, J.A.; Kwon, H.J.; Lee, Y. Targeting TM4SF5 with anti-TM4SF5 monoclonal antibody suppresses the growth and motility of human pancreatic cancer cells. Oncol. Lett. 2020, 19, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.-W.; Pan, H.-C.; Hsu, Y.-H.; Chang, K.-C.; Wu, L.-W.; Chen, W.-Y.; Chang, M.S. IL-20 antagonist suppresses PD-L1 expression and prolongs survival in pancreatic cancer models. Nat. Commun. 2020, 11, 4611. [Google Scholar] [CrossRef] [PubMed]
- Dréau, D.; Moore, L.J.; Wu, M.; Roy, L.D.; Dillion, L.; Porter, T.; Puri, R.; Momin, N.; Wittrup, K.D.; Mukherjee, P. Combining the Specific Anti-MUC1 Antibody TAB004 and Lip-MSA-IL-2 Limits Pancreatic Cancer Progression in Immune Competent Murine Models of Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Smeets, E.; Dorst, D.; van Lith, S.; Freimoser-Grundschober, A.; Klein, C.; Trajkovic-Arsic, M.; Gotthardt, M.; Siveke, J.; Aarntzen, E.H. A dual-labeled anti-FAP antibody for imaging and targeted photodynamic therapy of cancer associated fibroblasts in a pancreatic cancer mouse model. Nuklearmedizin 2019, 58, 93. [Google Scholar]
- Li, Z.; Wang, M.; Yao, X.; Luo, W.; Qu, Y.; Yu, D.; Li, X.; Fang, J.; Huang, C. Development of a Novel EGFR-Targeting Antibody-Drug Conjugate for Pancreatic Cancer Therapy. Target. Oncol. 2019, 14, 93–105. [Google Scholar] [CrossRef]
- Sugyo, A.; Tsuji, A.; Sudo, H.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Saga, T.; Higashi, T. Efficacy Evaluation of Combination Treatment Using Gemcitabine and Radioimmunotherapy with 90Y-Labeled Fully Human Anti-CD147 Monoclonal Antibody 059-053 in a BxPC-3 Xenograft Mouse Model of Refractory Pancreatic Cancer. Int. J. Mol. Sci. 2018, 19, 2979. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Maharjan, S.; Kim, D.; Kim, J.N.; Park, B.K.; Koh, H.; Moon, K.; Lee, Y.; Kwon, H.J. A Novel Monoclonal Antibody Targets Mucin1 and Attenuates Growth in Pancreatic Cancer Model. Int. J. Mol. Sci. 2018, 19, 2004. [Google Scholar] [CrossRef] [Green Version]
- Ogier, C.; Colombo, P.-E.; Bousquet, C.; Canterel-Thouennon, L.; Sicard, P.; Garambois, V.; Thomas, G.; Gaborit, N.; Jarlier, M.; Pirot, N.; et al. Targeting the NRG1/HER3 pathway in tumor cells and cancer-associated fibroblasts with an anti-neuregulin 1 antibody inhibits tumor growth in pre-clinical models of pancreatic cancer. Cancer Lett. 2018, 432, 227–236. [Google Scholar] [CrossRef]
- Mattie, M.; Raitano, A.; Morrison, K.; Morrison, K.; An, Z.; Capo, L.; Verlinsky, A.; Leavitt, M.; Ou, J.; Nadell, R.; et al. The Discovery and Preclinical Development of ASG-5ME, an Antibody-Drug Conjugate Targeting SLC44A4-Positive Epithelial Tumors Including Pancreatic and Prostate Cancer. Mol. Cancer Ther. 2016, 15, 2679–2687. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, T.; Deng, D.; Bover, L.; Wang, H.; Logsdon, C.D.; Ramachandran, V. New Blocking Antibodies against Novel AGR2-C4.4A Pathway Reduce Growth and Metastasis of Pancreatic Tumors and Increase Survival in Mice. Mol. Cancer Ther. 2015, 14, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Okada, M.; Koizumi, M.; Satoh, H.; Kurosawa, G.; Kurosawa, Y.; Saga, T. Evaluation of Efficacy of Radioimmunotherapy with 90Y-Labeled Fully Human Anti-Transferrin Receptor Monoclonal Antibody in Pancreatic Cancer Mouse Models. PLoS ONE 2015, 10, e0123761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaei, F.; Walsh, C.; Smith, K.; Menendez, C.; Lopez, P.; Norton, J.; Iglesias, J.; Hidalgo, M.; Reyes, C.; Chu, P. The LGR5 monoclonal antibody BNC101 has anti-tumor and anti-cancer stem cell activity in pancreatic cancer. Cancer Res. 2015, 75 (Suppl. S15). [Google Scholar] [CrossRef]
- Tung, K.H.; Lin, C.W.; Kuo, C.C.; Li, L.T.; Kuo, Y.H.; Wu, H.C. CHC promotes tumor growth and angiogenesis through regulation of HIF-1α and VEGF signaling. Cancer Lett. 2013, 331, 58–67. [Google Scholar] [CrossRef]
- Lwin, T.; Hollandsworth, H.M.; Bouvet, M.; Amirfakhri, S.; Filemoni, F.; Hoffman, R.M.; Singer, B.; Bouvet, M. Fluorescent anti-carcinoembryonic antigen-related cell adhesion molecule (CEACAM) detects pancreatic cancer at sub-millimeter resolution in mouse models. Society of Surgical Oncology SSO 2020 - International Conference on Surgical Cancer Care. Ann. Surg. Oncol. 2020, 27, S163. [Google Scholar] [CrossRef]
- Arias-Pinilla, G.A.; Dalgleish, A.G.; Mudan, S.; Bagwan, I.; Walker, A.J.; Modjtahedi, H. Development of novel monoclonal antibodies against CD109 overexpressed in human pancreatic cancer. Oncotarget 2018, 9, 19994–20007. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, R.; England, C.G.; Yang, Y.; Valdovinos, H.F.; Liu, B.; Wong, H.C.; Barnhart, T.E.; Cai, W. ImmunoPET imaging of tissue factor expression in pancreatic cancer with 89Zr-Df-ALT-836. J. Control. Release 2017, 264, 160–168. [Google Scholar] [CrossRef]
- Simpson, A.; Petnga, W.; Macaulay, V.M.; Weyer-Czernilofsky, U.; Bogenrieder, T. Insulin-Like Growth Factor (IGF) Pathway Targeting in Cancer: Role of the IGF Axis and Opportunities for Future Combination Studies. Target. Oncol. 2017, 12, 571–597. [Google Scholar] [CrossRef] [Green Version]
- Subramani, R.; Lopez-Valdez, R.; Arumugam, A.; Nandy, S.; Boopalan, T.; Lakshmanaswamy, R. Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PLoS ONE 2014, 9, e97016. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, N.; Seddon, A.M.; Dalgleish, A.; Mackintosh, D.; Modjtahedi, H. Expression pattern and targeting of HER family members and IGF-IR in pancreatic cancer. Front. Biosci. 2012, 17, 2698–2724. [Google Scholar] [CrossRef] [Green Version]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef]
- Qu, X.; Wu, Z.; Dong, W.; Zhang, T.; Wang, L.; Pang, Z.; Ma, W.; Du, J. Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy. Oncotarget 2017, 8, 29501–29518. [Google Scholar] [CrossRef] [Green Version]
- Kindler, H.L.; Richards, D.A.; Garbo, L.E.; Garon, E.B.; Stephenson, J.J.; Rocha-Lima, C.M.; Safran, H.; Chan, D.; Kocs, D.M.; Galimi, F.; et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann. Oncol. 2012, 23, 2834–2842. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Azevedo, S.; Okusaka, T.; Van Laethem, J.L.; Lipton, L.R.; Riess, H.; Szczylik, C.; Moore, M.J.; Peeters, M.; Bodoky, G.; et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: The GAMMA trial. Ann. Oncol. 2015, 26, 921–927. [Google Scholar] [CrossRef] [PubMed]
- McKian, K.P.; Haluska, P. Cixutumumab. Expert Opin. Investig. Drugs 2009, 18, 1025–1033. [Google Scholar] [CrossRef]
- Philip, P.A.; Goldman, B.; Ramanathan, R.K.; Lenz, H.J.; Lowy, A.M.; Whitehead, R.P.; Wakatsuki, T.; Iqbal, S.; Gaur, R.; Benedetti, J.K.; et al. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor–1 signaling in metastatic pancreatic cancer: Phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer 2014, 120, 2980–2985. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, R.; Varadhachary, G.R.; Bhosale, P.R.; Wang, X.; Fogelman, D.R.; Shroff, R.T.; Overman, M.J.; Wolff, R.A.; Javle, M. Randomized, phase I/II study of gemcitabine plus IGF-1R antagonist (MK-0646) versus gemcitabine plus erlotinib with and without MK-0646 for advanced pancreatic adenocarcinoma. J. Hematol. Oncol. 2018, 11, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; George, B.; Campbell, M.R.; Verma, N.; Paul, A.M.; Melo-Alvim, C.; Ribeiro, L.; Pillai, M.R.; da Costa, L.M.; Moasser, M.M. HER family in cancer progression: From discovery to 2020 and beyond. Adv. Cancer. Res. 2020, 147, 109–152. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, J.M.; Butterfield, L.H.; Tarhini, A.A.; Zarour, H.; Kalinski, P.; Ferrone, S. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 2012, 62, 309–335. [Google Scholar] [CrossRef]
- Fensterer, H.; Schade-Brittinger, C.; Müller, H.H.; Tebbe, S.; Fass, J.; Lindig, U.; Settmacher, U.; Schmidt, W.E.; Märten, A.; Ebert, M.P.; et al. Multicenter phase II trial to investigate safety and efficacy of gemcitabine combined with cetuximab as adjuvant therapy in pancreatic cancer (ATIP). Ann. Oncol. 2013, 24, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Powell, M.; Catalano, P.; Berlin, J.; Liles, D.; Chapman, A.; Mitchell, E.; Benson, A.B. Randomized Phase II Trial of Irinotecan/Docetaxel or Irinotecan/Docetaxel Plus Cetuximab for Metastatic Pancreatic Cancer: An Eastern Cooperative Oncology Group Study. Am. J. Clin. Oncol. 2016, 39, 340. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.A.; Benedetti, J.; Corless, C.L.; Wong, R.; O’Reilly, E.M.; Flynn, P.J.; Rowland, K.M.; Atkins, J.N.; Mirtsching, B.C.; Rivkin, S.E.; et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J. Clin. Oncol. 2010, 28, 3605–3610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, C.-J.; Huang, M.-T.; Wu, C.-H.; Wang, C.-K.; Tai, C.-J.; Chang, C.-C.; Hsieh, C.-I.; Chang, Y.-J.; Wu, C.-J.; Kuo, L.-J.; et al. Combination of Two Targeted Medications (Bevacizumab Plus Cetuximab) Improve the Therapeutic Response of Pancreatic Carcinoma. Medicine (Baltim.) 2016, 95, e3259. [Google Scholar] [CrossRef]
- Forster, T.; Huettner, F.J.; Springfeld, C.; Loehr, M.; Kalkum, E.; Hackbusch, M.; Hackert, T.; Diener, M.K.; Probst, P. Cetuximab in Pancreatic Cancer Therapy: A Systematic Review and Meta-Analysis. Oncology 2020, 98, 53–60. [Google Scholar] [CrossRef]
- Adler, M.J.; Dimitrov, D.S. Therapeutic antibodies against cancer. Hematol. Oncol. Clin. North. Am. 2012, 26, 447–481. [Google Scholar] [CrossRef] [Green Version]
- van Zweeden, A.A.; van der Vliet, H.J.; Wilmink, J.W.; Meijerink, M.R.; Meijer, O.W.M.; Bruynzeel, A.M.E.; van Tienhoven, G.; Giovannetti, E.; Kazemier, G.; Jacobs, M.A.; et al. Phase I Clinical Trial to Determine the Feasibility and Maximum Tolerated Dose of Panitumumab to Standard Gemcitabine-Based Chemoradiation in Locally Advanced Pancreatic. Cancer Clin. Cancer Res. 2015, 21, 4569–4575. [Google Scholar] [CrossRef] [Green Version]
- Halfdanarson, T.R.; Foster, N.R.; Kim, G.P.; Meyers, J.P.; Smyrk, T.C.; McCullough, A.E.; Ames, M.M.; Jaffe, J.P.; Alberts, S.R. A Phase II Randomized Trial of Panitumumab, Erlotinib, and Gemcitabine Versus Erlotinib and Gemcitabine in Patients with Untreated, Metastatic Pancreatic Adenocarcinoma: North Central Cancer Treatment Group Trial N064B (Alliance). Oncologist 2019, 24, 589–e160. [Google Scholar] [CrossRef] [Green Version]
- van Keulen, S.; van den Berg, N.S.; Nishio, N.; Birkeland, A.; Zhou, Q.; Lu, G.; Wang, H.W.; Middendorf, L.; Forouzanfar, T.; Martin, B.A.; et al. Rapid, non-invasive fluorescence margin assessment: Optical specimen mapping in oral squamous cell carcinoma. Oral Oncol. 2019, 88, 58–65. [Google Scholar] [CrossRef]
- Lu, G.; van den Berg, N.S.; Martin, B.A.; Nishio, N.; Hart, Z.P.; van Keulen, S.; Fakurnejad, S.; Chirita, S.U.; Raymundo, R.C.; Yi, G.; et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: A phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol. Hepatol. 2020, 5, 753–764. [Google Scholar] [CrossRef]
- Mazorra, Z.; Chao, L.; Lavastida, A.; Sanchez, B.; Ramos, M.; Iznaga, N.; Crombet, T. Nimotuzumab: Beyond the EGFR signaling cascade inhibition. Semin. Oncol. 2018, 45, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, B.; Reuter, D.; Ebert, M.P.; Siveke, J.; Kerkhoff, A.; Berdel, W.E.; Hofheinz, R.; Behringer, D.M.; Schmidt, W.E.; Goker, E.; et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: A multicenter, randomized phase IIb study. Ann. Oncol. 2017, 28, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Strumberg, D.; Schultheis, B.; Scheulen, M.E.; Hilger, R.A.; Krauss, J.; Marschner, N.; Lordick, F.; Bach, F.; Reuter, D.; Edler, L.; et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest. New Drugs 2012, 30, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Graeven, U.; Kremer, B.; Südhoff, T.; Killing, B.; Rojo, F.; Weber, D.; Tillner, J.; Ünal, C.; Schmiegel, W. Phase I study of the humanised anti-EGFR monoclonal antibody matuzumab (EMD 72000) combined with gemcitabine in advanced pancreatic cancer. Br. J. Cancer 2006, 94, 1293–1299. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Gu, J.; Zheng, L. Prognostic role of HER2 amplification based on fluorescence in situ hybridization (FISH) in pancreatic ductal adenocarcinoma (PDAC): A meta-analysis. World J. Surg. Oncol. 2016, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.; Claret, F.X. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef] [Green Version]
- Assenat, E.; Azria, D.; Mollevi, C.; Guimbaud, R.; Tubiana-Mathieu, N.; Smith, D.; Delord, J.P.; Samalin, E.; Portales, F.; Larbouret, C.; et al. Dual targeting of HER1/EGFR and HER2 with cetuximab and trastuzumab in patients with metastatic pancreatic cancer after gemcitabine failure: Results of the “THERAPY”phase 1-2 trial. Oncotarget 2015, 6, 12796–12808. [Google Scholar] [CrossRef] [Green Version]
- Harder, J.; Ihorst, G.; Heinemann, V.; Hofheinz, R.; Moehler, M.; Buechler, P.; Kloeppel, G.; Röcken, C.; Bitzer, M.; Boeck, S.; et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br. J. Cancer 2012, 106, 1033–1038. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug. Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Vervenne, W.L.; Bennouna, J.; Humblet, Y.; Gill, S.; Van Laethem, J.L.; Verslype, C.; Scheithauer, W.; Shang, A.; Cosaert, J.; et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2009, 27, 2231–2237. [Google Scholar] [CrossRef]
- Kindler, H.L.; Niedzwiecki, D.; Hollis, D.; Sutherland, S.; Schrag, D.; Hurwitz, H.; Innocenti, F.; Mulcahy, M.F.; O’Reilly, E.; Wozniak, T.F.; et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 2010, 28, 3617–3622. [Google Scholar] [CrossRef] [Green Version]
- Johansson, H.; Andersson, R.; Bauden, M.; Hammes, S.; Holdenrieder, S.; Ansari, D. Immune checkpoint therapy for pancreatic cancer. World J. Gastroenterol. 2016, 22, 9457–9476. [Google Scholar] [CrossRef]
- Bengsch, F.; Knoblock, D.M.; Liu, A.; McAllister, F.; Beatty, G.L. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol. Immunother. 2017, 66, 1609–1617. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz Jr, L.A.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of Ipilimumab in Combination with Allogeneic Pancreatic Tumor Cells Transfected With a GM-CSF Gene in Previously Treated Pancreatic Cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Aglietta, M.; Barone, C.; Sawyer, M.B.; Moore, M.J.; Miller, W.H.; Bagalà, C.; Colombi, F.; Cagnazzo, C.; Gioeni, L.; Wang, E.; et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann. Oncol. 2014, 25, 1750–1755. [Google Scholar] [CrossRef]
- Sharma, P.; Dirix, L.; De Vos, F.Y.F.L.; Allison, J.P.; Decoster, L.; Zaucha, R.; Park, J.O.; Vanderwalde, A.M.; Kataria, R.S.; Ferro, S.; et al. Efficacy and tolerability of tremelimumab in patients with metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2018, 36, 470. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8–18. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: A Phase 1b study. Clin. Cancer Res. 2020, 26, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schütz, E.; Khemka, V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest. New Drugs 2017, 36, 96–102. [Google Scholar] [CrossRef]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Hochster, H.S.; Kim, E.J.; George, B.; Kaylan, A.; Chiorean, E.G.; Waterhouse, D.M.; Guiterrez, M.; Parikh, A.; Jain, R.; et al. Open-label, Phase I Study of Nivolumab Combined with nab-Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4814–4822. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Unceta, N.; Burgueño, I.; Jiménez, E.; Paz-Ares, L. Durvalumab in NSCLC: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2018, 10, 175883591880415–1758835918804151. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.M.; Oh, D.-Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.C.; Vlahovic, G.; et al. Durvalumab With or Without Tremelimumab for Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef]
- Jakubowski, C.; Thompson, E.D.; Wang, H.; Walker, R.; Jaffee, E.M.; Azad, N.S. A phase II trial of PD-1 inhibition with INCMGA00012 in patients with previously treated unresectable or metastatic adenosquamous pancreatic cancer. J. Clin. Oncol. 2020, 38, TPS4662. [Google Scholar] [CrossRef]
- Srinivasa, P.P.; Zhihong, X.; David, G.; Romano, C.P.; Jeremy, S.W.; Minoti, V.A. Targeting HGF/c-MET Axis in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 9170. [Google Scholar] [CrossRef]
- Xu, Z.; Pang, T.C.Y.; Liu, A.C.; Pothula, S.P.; Mekapogu, A.R.; Perera, C.J.; Murakami, T.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; et al. Targeting the HGF/c-MET pathway in advanced pancreatic cancer: A key element of treatment that limits primary tumour growth and eliminates metastasis. Br. J. Cancer 2020, 122, 1486–1495. [Google Scholar] [CrossRef]
- Rizwani, W.; Allen, A.E.; Trevino, J.G. Hepatocyte growth factor from a clinical perspective: A pancreatic cancer challenge. Cancers 2015, 7, 1785–1805. [Google Scholar] [CrossRef]
- Patnaik, A.; Weiss, G.J.; Papadopoulos, K.P.; Hofmeister, C.C.; Tibes, R.; Tolcher, A.; Isaacs, R.; Jac, J.; Han, M.; Payumo, F.C.; et al. Phase I ficlatuzumab monotherapy or with erlotinib for refractory advanced solid tumours and multiple myeloma. Br. J. Cancer 2014, 111, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Yamada, S.; Sonohara, F.; Suenaga, M.; Hayashi, M.; Takami, H.; Niwa, Y.; Hattori, N.; Iwata, N.; Kanda, M.; et al. Clinical Implications of Lysyl Oxidase-Like Protein 2 Expression in Pancreatic Cancer. Sci. Rep. 2018, 8, 9846–9849. [Google Scholar] [CrossRef]
- Benson, A.B.; Wainberg, Z.A.; Hecht, J.R.; Vyushkov, D.; Dong, H.; Bendell, J.; Kudrik, F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. Oncologist 2017, 22, 241. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Lee, J.-H.; Lee, Y.S.; Kim, J.K.; Dong, S.M.; Yoon, D.S. Emerging role of LOXL2 in the promotion of pancreas cancer metastasis. Oncotarget 2016, 7, 42539–42552. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]
- Kaplan-Lefko, P.J.; Graves, J.D.; Zoog, S.J.; Pan, Y.; Wall, J.; Branstetter, D.G.; Moriguchi, J.; Coxon, A.; Huard, J.N.; Xu, R.; et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol. Ther. 2010, 9, 618–631. [Google Scholar] [CrossRef] [Green Version]
- Forero-Torres, A.; Shah, J.; Wood, T.; Posey, J.; Carlisle, R.; Copigneaux, C.; Luo, F.; Wojtowicz-Praga, S.; Percent, I.; Saleh, M. Phase I Trial of Weekly Tigatuzumab, an Agonistic Humanized Monoclonal Antibody Targeting Death Receptor 5 (DR5). Cancer Biother. Radiopharm. 2010, 25, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Forero-Torres, A.; Infante, J.R.; Waterhouse, D.; Wong, L.; Vickers, S.; Arrowsmith, E.; He, A.R.; Hart, L.; Trent, D.; Wade, J.; et al. Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013, 2, 925–932. [Google Scholar] [CrossRef]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta Rev. Cancer 2020, 188409. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [CrossRef]
- Lohrmann, C.; O’Reilly, E.M.; O’Donoghue, J.A.; Pandit-Taskar, N.; Carrasquillo, J.A.; Lyashchenko, S.K.; Ruan, S.; Teng, R.; Scholz, W.; Maffuid, P.W.; et al. Retooling a Blood-Based Biomarker: Phase I Assessment of the High-Affinity CA19-9 Antibody HuMab-5B1 for Immuno-PET Imaging of Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 7014–7023. [Google Scholar] [CrossRef] [Green Version]
- Almhanna, K.; Kalebic, T.; Cruz, C.; Faris, J.E.; Ryan, D.P.; Jung, J.; Wyant, T.; Fasanmade, A.A.; Messersmith, W.; Rodon, J. Phase I study of the investigational anti-guanylyl cyclase antibody-drug conjugate TAK-264 (MLN0264) in adult patients with advanced gastrointestinal malignancies. Clin. Cancer Res. 2016, 22, 5049–5057. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, A.R.; Nguyen, A.; Bagby, S.M.; Arcaroli, J.J.; Yacob, B.W.; Quackenbush, K.; Guy, J.L.; Crowell, T.; Stringer, B.; Danaee, H.; et al. Evaluation of TAK-264, an Antibody-Drug Conjugate in Pancreatic Cancer Cell Lines and Patient-Derived Xenograft Models. Clin. Cancer Drugs 2018, 5, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Almhanna, K.; Wright, D.; Mercade, T.M.; Van Laethem, J.-L.; Gracian, A.C.; Guillen-Ponce, C.; Faris, J.; Lopez, C.M.; Hubner, R.A.; Bendell, J.; et al. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Invest. New Drugs 2017, 35, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Coveler, A.L.; Ko, A.H.; Catenacci, D.V.T.; Von Hoff, D.; Becerra, C.; Whiting, N.C.; Yang, J.; Wolpin, B. A phase 1 clinical trial of ASG-5ME, a novel drug-antibody conjugate targeting SLC44A4, in patients with advanced pancreatic and gastric cancers. Investig. New Drugs 2016, 34, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Piechutta, M.; Berghoff, A.S. New emerging targets in cancer immunotherapy: The role of Cluster of Differentiation 40 (CD40/TNFR5). ESMO Open 2019, 4, e000510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, G.L.; Torigian, D.A.; Chiorean, E.G.; Saboury, B.; Brothers, A.; Alavi, A.; Troxel, A.B.; Sun, W.; Teitelbaum, U.R.; Vonderheide, R.H.; et al. A Phase I Study of an Agonist CD40 Monoclonal Antibody (CP-870,893) in Combination with Gemcitabine in Patients with Advanced Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2013, 19, 6286–6295. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Guo, Z.; Liu, Y.; Si, T.; Yu, H.; Li, B.; Tian, W. Prostate stem cell antigen and cancer risk, mechanisms and therapeutic implications. Expert Rev. Anticancer Ther. 2014, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Saeki, N.; Gu, J.; Yoshida, T.; Wu, X. Prostate stem cell antigen: A Jekyll and Hyde molecule? Clin. Cancer Res. 2010, 16, 3533–3538. [Google Scholar] [CrossRef] [Green Version]
- Wolpin, B.M.; O’Reilly, E.M.; Ko, Y.J.; Blaszkowsky, L.S.; Rarick, M.; Rocha-Lima, C.M.; Ritch, P.; Chan, E.; Spratlin, J.; Macarulla, T.; et al. Global, multicenter, randomized, phase II trial of gemcitabine and gemcitabine plus AGS-1C4D4 in patients with previously untreated, metastatic pancreatic cancer. Ann. Oncol. 2013, 24, 1792–1801. [Google Scholar] [CrossRef]
- Pelzer, U.; Bendell, J.C.; Womack, M.S.; Bahary, N.; Macarulla, T.; Borazanci, E.H.; Levy, D.E.; Mo, G.; Ramage, S.C.; Garrido-Laguna, I. A phase Ib study evaluating olaratumab in combination with nab-paclitaxel and gemcitabine in first-line treatment of metastatic pancreatic cancer. J. Clin. Oncol. 2019, 37, 330. [Google Scholar] [CrossRef]
- Reilly, E.M.; Wang, J.S.-Z.; Yu, K.H.; Lowery, M.A.; Varghese, A.M.; Bendell, J.C.; Borazanci, E.H.; Estrella, H.; Fowler, K.; Hoskins, M.; et al. Abstract LB-B25: Preliminary phase I data comparing HuMab-5B1 (MVT-5873), a monoclonal antibody targeting sLea, as a single agent and in combination with first line nab-paclitaxel and gemcitabine in patients with CA19-9 positive pancreatic cancer. Mol. Cancer Ther. 2018, 17 (Suppl. S1). [Google Scholar] [CrossRef]
- Berlin, J.D.; Feng, Y.; Catalano, P.; Abbruzzese, J.L.; Philip, P.A.; McWilliams, R.R.; Lowy, A.M.; Benson III, A.B.; Blackstock, A.W. An Intergroup Randomized Phase II Study of Bevacizumab or Cetuximab in Combination with Gemcitabine and in Combination with Chemoradiation in Patients with Resected Pancreatic Carcinoma: A Trial of the ECOG-ACRIN Cancer Research Group (E2204). Oncology 2018, 94, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.S.; Azad, N.S.; Patel, S.P.; Torrealba, J.; Mavroukakis, S.; Beatson, M.A.; Wang, X.P.; Arlen, P.M.; Morse, M.A. A phase 1 dose-escalation study of NEO-102 in patients with refractory colon and pancreatic cancer. Cancer Chemother. Pharmacol. 2016, 78, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadha, A.S.; Skinner, H.D.; Gunther, J.R.; Munsell, M.F.; Das, P.; Minsky, B.D.; Delclos, M.E.; Chatterjee, D.; Wang, H.; Clemons, M.; et al. Phase i trial of consolidative radiotherapy with concurrent bevacizumab, erlotinib and capecitabine for unresectable pancreatic cancer. PLoS ONE 2016, 11, e0156910. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Cunningham, D.; Peckitt, C.; Barton, S.; Tait, D.; Hawkins, M.; Watkins, D.; Starling, N.; Rao, S.; Begum, R.; et al. miR-21 expression and clinical outcome in locally advanced pancreatic cancer: Exploratory analysis of the pancreatic cancer Erbitux, radiotherapy and UFT (PERU) trial. Oncotarget 2016, 7, 12672–12681. [Google Scholar] [CrossRef] [Green Version]
- Fiore, M.; Trodella, L.; Valeri, S.; Borzomati, D.; Floreno, B.; Ippolito, E.; Trecca, P.; Trodella, L.E.; D’Angelillo, R.M.; Ramella, S.; et al. Prospective study of cetuximab and gemcitabine in combination with radiation therapy: Feasibility and efficacy in locally advanced pancreatic head cancer. Radiat. Oncol. 2015, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Picozzi, V.J.; Ramanathan, R.K.; Lowery, M.A.; Ocean, A.J.; Mitchel, E.P.; O’Neil, B.H.; Guarino, M.J.; Conkling, P.R.; Cohen, S.J.; Bahary, N.; et al. (90)Y-clivatuzumab tetraxetan with or without low-dose gemcitabine: A phase Ib study in patients with metastatic pancreatic cancer after two or more prior therapies. Eur. J. Cancer 2015, 51, 1857. [Google Scholar] [CrossRef] [Green Version]
- Okusaka, T.; Ikeda, M.; Fukutomi, A.; Kobayashi, Y.; Shibayama, K.; Takubo, T.; Gansert, J. Safety, Tolerability, Pharmacokinetics and Antitumor Activity of Ganitumab, an Investigational Fully Human Monoclonal Antibody to Insulin-like Growth Factor Type 1 Receptor, Combined with Gemcitabine as First-line Therapy in Patients with Metastatic Pancreatic Cancer: A Phase 1b Study. Jpn. J. Clin. Oncol. 2014, 44, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Sahora, K.; Schindl, M.; Kuehrer, I.; Eisenhut, A.; Werba, G.; Brostjan, C.; Telek, B.; Ba’ssalamah, A.; Stift, J.; Schoppmann, S.F.; et al. A phase II trial of two durations of Bevacizumab added to neoadjuvant gemcitabine for borderline and locally advanced pancreatic cancer. Anticancer Res. 2014, 34, 2377–2384. [Google Scholar]
- Watkins, D.J.; Starling, N.; Cunningham, D.; Thomas, J.; Webb, J.; Brown, G.; Barbachano, Y.; Oates, J.; Chau, I. The combination of a chemotherapy doublet (gemcitabine and capecitabine) with a biological doublet (bevacizumab and erlotinib) in patients with advanced pancreatic adenocarcinoma. The results of a phase I/II study. Eur. J. Cancer 2014, 50, 1422–1429. [Google Scholar] [CrossRef]
- Su, D.; Jiao, S.-C.; Wang, L.-J.; Shi, W.-W.; Long, Y.-Y.; Li, J.; Bai, L. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour Biol. 2014, 35, 2313–2318. [Google Scholar] [CrossRef]
- Van Buren Ii, G.; Ramanathan, R.K.; Krasinskas, A.M.; Smith, R.P.; Abood, G.J.; Bahary, N.; Lembersky, B.C.; Shuai, Y.; Potter, D.M.; Bartlett, D.L.; et al. Phase II Study of Induction Fixed-Dose Rate Gemcitabine and Bevacizumab Followed by 30 Gy Radiotherapy as Preoperative Treatment for Potentially Resectable Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2013, 20, 3787–3793. [Google Scholar] [CrossRef]
- Kordes, S.; Richel, D.J.; Klümpen, H.-J.; Weterman, M.J.; Stevens, A.J.W.M.; Wilmink, J.W. A phase I/II, non-randomized, feasibility/safety and efficacy study of the combination of everolimus, cetuximab and capecitabine in patients with advanced pancreatic cancer. Invest. New Drugs 2013, 31, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.K.; Li, X.; Kleiber, B.; Ellison, E.C.; Bloomston, M.; Zalupski, M.; Bekaii-Saab, T.S. VEGF remains an interesting target in advanced pancreas cancer (APCA): Results of a multi-institutional phase II study of bevacizumab, gemcitabine, and infusional 5-fluorouracil in patients with APCA. Ann. Oncol. 2012, 23, 2812–2820. [Google Scholar] [CrossRef]
- Pipas, J.M.; Zaki, B.I.; McGowan, M.M.; Tsapakos, M.J.; Ripple, G.H.; Suriawinata, A.A.; Tsongalis, G.J.; Colacchio, T.A.; Gordon, S.R.; Sutton, J.E.; et al. Neoadjuvant cetuximab, twice-weekly gemcitabine, and intensity-modulated radiotherapy (IMRT) in patients with pancreatic adenocarcinoma. Ann. Oncol. 2012, 23, 2820–2827. [Google Scholar] [CrossRef]
- Ocean, A.J.; Pennington, K.L.; Guarino, M.J.; Sheikh, A.; Bekaii-Saab, T.; Serafini, A.N. Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer 2012, 118, 5497. [Google Scholar] [CrossRef] [Green Version]
- Ko, A.H.; Youssoufian, H.; Gurtler, J.; Dicke, K.; Kayaleh, O.; Lenz, H.-J.; Keaton, M.; Katz, T.; Ballal, S.; Rowinsky, E.K. A phase II randomized study of cetuximab and bevacizumab alone or in combination with gemcitabine as first-line therapy for metastatic pancreatic adenocarcinoma. Invest. New Drugs 2012, 30, 1597–1606. [Google Scholar] [CrossRef]
- Merchan, J.R.; Ferrell, A.; Macintyre, J.; Ciombor, K.K.; Levi, J.; Ribeiro, A.; Sleeman, D.; Flores, A.; Lopes, G.; Rocha-Lima, C.M. Phase II study of gemcitabine, oxaliplatin, and cetuximab in advanced pancreatic cancer. Am. J. Clin. Oncol. 2012, 35, 446–450. [Google Scholar] [CrossRef]
- Kullmann, F.; Hartmann, A.; Stöhr, R.; Messmann, H.; Dollinger, M.M.; Trojan, J.; Fuchs, M.; Hollerbach, S.; Harder, J.; Troppmann, M.; et al. KRAS mutation in metastatic pancreatic ductal adenocarcinoma: Results of a multicenter phase II study evaluating efficacy of cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line therapy. Oncology 2011, 81, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Crane, C.H.; Varadhachary, G.R.; Yordy, J.S.; Staerkel, G.A.; Javle, M.M.; Safran, H.; Haque, W.; Hobbs, B.D.; Krishnan, S.; Fleming, J.B.; et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: Correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J. Clin. Oncol. 2011, 29, 3037–3043. [Google Scholar] [CrossRef] [Green Version]
- Gulec, S.A.; Cohen, S.J.; Pennington, K.L.; Zuckier, L.S.; Hauke, R.J.; Horne, H.; Wegener, W.A.; Teoh, N.; Gold, D.V.; Sharkey, R.M.; et al. Treatment of advanced pancreatic carcinoma with 90Y-clivatuzumab tetraxetan: A phase I single-dose escalation trial. Clin. Cancer Res. 2011, 17, 4091–4100. [Google Scholar] [CrossRef] [Green Version]
- Fogelman, D.; Jafari, M.; Varadhachary, G.R.; Xiong, H.; Bullock, S.; Ozer, H.; Lin, E.; Morris, J.; Cunningham, P.; Bennett, B.; et al. Bevacizumab plus gemcitabine and oxaliplatin as first-line therapy for metastatic or locally advanced pancreatic cancer: A phase II trial. Cancer Chemother. Pharmacol. 2011, 68, 1431–1438. [Google Scholar] [CrossRef]
- Small, W.; Mulcahy, M.F.; Rademaker, A.; Bentrem, D.J.; Benson, A.B.; Weitner, B.B.; Talamonti, M.S. Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 476–482. [Google Scholar] [CrossRef]
- Arnoletti, J.P.; Frolov, A.; Eloubeidi, M.; Keene, K.; Posey, J.; Wood, T.; Greeno, E.; Jhala, N.; Varadarajulu, S.; Russo, S.; et al. A phase I study evaluating the role of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 67, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Astsaturov, I.; Meropol, N.; Alpaugh, R.; Burtness, B.; Cheng, J.; McLaughlin, S.; Rogatko, A.; Xu, Z.; Watson, J.C.; Weiner, L.M.; et al. Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. Am. J. Clin. Oncol. 2011, 34, 70. [Google Scholar] [CrossRef] [Green Version]
- Ko, A.H.; Venook, A.P.; Bergsland, E.K.; Kelley, R.K.; Korn, W.M.; Dito, E.; Schillinger, B.; Scott, J.; Hwang, J.; Tempero, M.A. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2010, 66, 1051–1057. [Google Scholar] [CrossRef]
- Starling, N.; Watkins, D.; Cunningham, D.; Thomas, J.; Webb, J.; Brown, G.; Thomas, K.; Oates, J.; Chau, I. Dose finding and early efficacy study of gemcitabine plus capecitabine in combination with bevacizumab plus erlotinib in advanced pancreatic cancer. J. Clin. Oncol. 2009, 27, 5499–5505. [Google Scholar] [CrossRef]
- Crane, C.H.; Winter, K.; Regine, W.F.; Safran, H.; Rich, T.A.; Curran, W.; Wolff, R.A.; Willett, C.G. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J. Clin. Oncol. 2009, 27, 4096–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javle, M.; Yu, J.; Garrett, C.; Pande, A.; Kuvshinoff, B.; Litwin, A.; Phelan, J.; Gibbs, J.; Iyer, R. Bevacizumab combined with gemcitabine and capecitabine for advanced pancreatic cancer: A phase II study. Br. J. Cancer 2009, 100, 1842–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullmann, F.; Hollerbach, S.; Dollinger, M.M.; Harder, J.; Fuchs, M.; Messmann, H.; Trojan, J.; Gäbele, E.; Hinke, A.; Hollerbach, C.; et al. Cetuximab plus gemcitabine oxaliplatin (GEMOXCET) in first-line metastatic pancreatic cancer: A multicentre phase II study. Br. J. Cancer 2009, 100, 1032–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, A.; Shore, S.; Raraty, M.G.; Vinjamuri, S.; Evans, J.E.; Smith, C.T.; Lane, S.; Chauhan, S.; Bosonnet, L.; Garvey, C.; et al. Randomised Phase I/II trial assessing the safety and efficacy of radiolabelled anti-carcinoembryonic antigen I(131) KAb201 antibodies given intra-arterially or intravenously in patients with unresectable pancreatic adenocarcinoma. BMC Cancer 2009, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, A.H.; Dito, E.; Schillinger, B.; Venook, A.P.; Xu, Z.; Bergsland, E.K.; Wong, D.; Scott, J.; Hwang, J.; Tempero, M.A. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: Is an anti-VEGF strategy still applicable? Invest. New Drugs 2008, 26, 463–471. [Google Scholar] [CrossRef]
- Cascinu, S.; Berardi, R.; Labianca, R.; Siena, S.; Falcone, A.; Aitini, E.; Barni, S.; Di Costanzo, F.; Dapretto, E.; Tonini, G.; et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: A randomised, multicentre, phase II trial. Lancet Oncol. 2008, 9, 39–44. [Google Scholar] [CrossRef]
- Crane, C.H.; Ellis, L.M.; Abbruzzese, J.L.; Amos, C.; Xiong, H.Q.; Ho, L.; Evans, D.B.; Tamm, E.P.; Ng, C.; Pisters, P.W.; et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J. Clin. Oncol. 2006, 24, 1145–1151. [Google Scholar] [CrossRef]
- Kindler, H.L.; Friberg, G.; Singh, D.A.; Locker, G.; Nattam, S.; Kozloff, M.; Taber, D.A.; Karrison, T.; Dachman, A.; Stadler, W.M.; et al. Phase II Trial of Bevacizumab Plus Gemcitabine in Patients with Advanced Pancreatic Cancer. J. Clin. Oncol. 2005, 23, 8033–8040. [Google Scholar] [CrossRef]
- Xiong, H.Q.; Rosenberg, A.; LoBuglio, A.; Schmidt, W.; Wolff, R.A.; Deutsch, J.; Needle, M.; Abbruzzese, J.L. Cetuximab, a Monoclonal Antibody Targeting the Epidermal Growth Factor Receptor, in Combination With Gemcitabine for Advanced Pancreatic Cancer: A Multicenter Phase II Trial. J. Clin. Oncol. 2004, 22, 2610–2616. [Google Scholar] [CrossRef]
- Weiner, L.M.; Harvey, E.; Padavic-Shaller, K.; Willson, J.K.V.; Walsh, C.; LaCreta, F.; Khazaeli, M.B.; Kirkwood, J.M.; Haller, D.G. Phase II Multicenter Evaluation of Prolonged Murine Monoclonal Antibody 17-1A Therapy in Pancreatic Carcinoma. J. Immunother. 1993, 13, 110–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Cheng, H.; Fan, Z.; Huang, Q.; Lu, Y.; Fan, K.; Luo, G.; Jin, K.; Wang, Z.; et al. Novel agents for pancreatic ductal adenocarcinoma: Emerging therapeutics and future directions. J. Hematol. Oncol. 2018, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Roth, M.T.; Cardin, D.B.; Berlin, J.D. Recent advances in the treatment of pancreatic cancer. F1000Research 2020, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.; Seddon, A.M.; Dalgleish, A.G.; Khelwatty, S.; Ioannou, N.; Mudan, S.; Modjtahedi, H. Synergistic activity of agents targeting growth factor receptors, CDKs and downstream signaling molecules in a panel of pancreatic cancer cell lines and the identification of antagonistic combinations: Implications for future clinical trials in pancreatic cancer. Oncol. Rep. 2020, 44, 2581–2594. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Akce, M.; Zaidi, M.Y.; Waller, E.K.; El-Rayes, B.F.; Lesinski, G.B. The Potential of CAR T Cell Therapy in Pancreatic Cancer. Front. Immunol. 2018, 9, 2166. [Google Scholar] [CrossRef]
- Neesse, A.; Algül, H.; Tuveson, A.D.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Zhou, L.; Lu, J.; Wang, Y.; Liu, C.; You, L.; Guo, J. Stroma-Targeting Therapy in Pancreatic Cancer: One Coin with Two Sides? Front. Oncol. 2020, 10, 576399. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Wang, W.-Q.; Yu, X.-J.; Liu, L. Killing the “BAD”: Challenges for immunotherapy in pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer. 2020, 1874, 188384. [Google Scholar] [CrossRef] [PubMed]
- Sleightholm, R.L.; Neilsen, B.K.; Li, J.; Steele, M.M.; Singh, R.K.; Hollingsworth, M.A.; Oupicky, D. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol. Ther. 2017, 179, 158–170. [Google Scholar] [CrossRef]
- Leonardi, A.J.; Kotlyar, D.S.; Proenca, R.B. CAR T cell therapy. Cancers. (In progress).
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.A.; Casulo, C.; Munshi, P.N.; Maloney, D.G.; De Vos, S.; et al. Interim analysis of ZUMA-5: A phase II study of axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed/refractory indolent non-Hodgkin lymphoma (R/R iNHL). J. Clin. Oncol. 2020, 38, 8008. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef]
- Li, N.; Li, D.; Ren, H.; Torres, M.; Ho, M. Chimeric antigen receptor T-cell therapy targeting glypican-1 in pancreatic cancer. Cancer Res. 2019, 2309. [Google Scholar]
- Ma, H.S.; Poudel, B.; Torres, E.R.; Sidhom, J.-W.; Robinson, T.M.; Christmas, B.; Scott, B.; Cruz, K.; Woolman, S.; Wall, V.Z.; et al. A CD40 Agonist and PD-1 Antagonist Antibody Reprogram the Microenvironment of Nonimmunogenic Tumors to Allow T-cell–Mediated Anticancer Activity. Cancer Immunol. Res. 2019, 7, 428–442. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Go, V.L.W.; Hu, S. Membrane proteomic analysis of pancreatic cancer cells. J. Biomed. Sci. 2010, 17, 74. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.I.; Bendell, J.C.; Bullock, A.; LoConte, N.K.; Hatoum, H.; Ritch, P.; Hool, H.; Leach, J.W.; Sanchez, J.; Sohal, D.P.S.; et al. A randomized phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019, 8, 5148–5157. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Moore, K.N.; Birrer, M.J.; Berlin, S.; Matulonis, U.A.; Infante, J.R.; Wolpin, B.; Poon, K.A.; Firestein, R.; Xu, J.; et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody–drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann. Oncol. 2016, 27, 2124–2130. [Google Scholar] [CrossRef]
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Bsc, B.G.S.M.; Mieog, J.S.D.; Swijnenburg, R.J.; Van De Velde, C.J.H.; Sarasqueta, A.F.; Bonsing, B.A.; Framery, B.; Pèlegrin, A.; et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018, 25, 3350–3357. [Google Scholar] [CrossRef]
- King, J.; Bouvet, M.; Singh, G.; Williams, J. Improving theranostics in pancreatic cancer. J. Surg. Oncol. 2017, 116, 104–113. [Google Scholar] [CrossRef]
- Dammes, N.; Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 2020, 10, 938–955. [Google Scholar] [CrossRef]
- Knutson, S.; Raja, E.; Bomgarden, R.; Nlend, M.; Chen, A.; Kalyanasundaram, R.; Desai, S. Development and Evaluation of a Fluorescent Antibody-Drug Conjugate for Molecular Imaging and Targeted Therapy of Pancreatic Cancer. PLoS ONE 2016, 11, e0157762. [Google Scholar] [CrossRef] [Green Version]
- Moroz, A.; Wang, Y.-H.; Sharib, J.M.; Wei, J.; Zhao, N.; Huang, Y.; Chen, Z.; Martinko, A.J.; Zhuo, J.; Lim, S.A.; et al. Theranostic Targeting of CUB Domain Containing Protein 1 (CDCP1) in Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 3608–3615. [Google Scholar] [CrossRef]
- Escorcia, F.E.; Houghton, J.L.; Abdel-Atti, D.; Pereira, P.R.; Cho, A.; Gutsche, N.T.; Baidoo, K.E.; Lewis, J.S. ImmunoPET Predicts Response to Met-targeted Radioligand Therapy in Models of Pancreatic Cancer Resistant to Met Kinase Inhibitors. Theranostics 2020, 10, 151–165. [Google Scholar] [CrossRef]
- Sutcliffe, J.L. Abstract IA-13: Molecularly targeted imaging and treatment via the integrin αvβ6. Cancer Res. 2020, 80 (Suppl. S22). [Google Scholar] [CrossRef]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64Cu- and 225Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2019, 61, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Altmann, A.; Kraemer, S.; Kleist, C.; Loktev, A.; Kratochwil, C.; Giesel, F.; Mier, W.; Marme, F.; Debus, J.; et al. Design and Development of 99mTc-Labeled FAPI Tracers for SPECT Imaging and 188Re Therapy. J. Nucl. Med. 2020, 61, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhang, H.; Chen, X.; Song, L.; Cui, W.; Ren, S.; Wang, Y.; Guo, K.; Li, D.; Chen, R.; et al. A GPC1-targeted and gemcitabine-loaded biocompatible nanoplatform for pancreatic cancer multimodal imaging and therapy. Nanomedicine 2019, 14, 2339–2353. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, W.; Uckun, F.M.; Wang, L.; Wang, Y.A.; Chen, H.; Kooby, D.; Yu, Q.; Lipowska, M.; Staley, C.A.; et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano 2015, 9, 7976–7991. [Google Scholar] [CrossRef] [Green Version]
- Cruz, E.; Kayser, V. Monoclonal antibody therapy of solid tumors: Clinical limitations and novel strategies to enhance treatment efficacy. Biologics 2019, 13, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted Nanobody-Based Molecular Tracers for Nuclear Imaging and Image-Guided Surgery. Antibodies 2019, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ning, Q.; Mo, Z.; Tang, S. A promising cancer diagnosis and treatment strategy: Targeted cancer therapy and imaging based on antibody fragment. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3621–3630. [Google Scholar] [CrossRef] [Green Version]

| MAb Generic Name (Trade Name) | Target Antigen/Isotype | Cancer Type Indication | Date of Approval |
|---|---|---|---|
| Rituximab (Rituxan®) | CD20/Chimeric IgG1 | B-cell lymphoma, NHL | 1997 |
| Diffuse large B-cell, CD20+, NHL | 2006 | ||
| CLL | 2010 | ||
| Previously untreated follicular, CD20+, B-cell NHL | 2011 | ||
| Trastuzumab (Herceptin®) | HER-2/Humanised IgG1 | Metastatic breast cancer | 1998 |
| Early-stage breast cancer | 2006 | ||
| HER2 overexpressing metastatic gastric or GEJ adenoca | 2010 | ||
| Gentuzumab ozogamicin (Mylotarg®) | CD33/Humanised IgG4 | AML | 2000 * |
| Newly diagnosed, relapsed or refractory CD33+ AML | 2017 | ||
| Alemtuzumab (Campath®) | CD52/Humanised IgG1 | B-CLL | 2001 |
| Ibritumomab tiuxetan (Zevalin®) | CD20/Murine IgG1; conjugated to 90Y | NHL | 2002 |
| Tositumomab-I131 (Bexxar®) | CD20/Murine IgG2a; conjugated to 131I | NHL | 2003 * |
| Cetuximab (Erbitux®) | EGFR/Chimeric IgG1 | Metastatic CRC | 2004 |
| Locally or regionally advanced HNSCC or recurrent or metastatic HNSCC | 2006 | ||
| Recurrent locoregional disease and/or metastatic HNSCC (first-line) | 2011 | ||
| K-ras wild-type, EGFR-expressing metastatic CRC (first-line) | 2012 | ||
| Bevacizumab (Avastin®) | VEGF/Humanised IgG1 | Metastatic CRC | 2004 |
| Locally advanced, metastatic or recurrent NSCLC | 2006 | ||
| Metastatic HER-2 negative breast cancer | 2008 | ||
| Metastatic RCC | 2009 | ||
| GBM | 2009 | ||
| Metastatic CRC | 2013 | ||
| Persistent, recurrent or metastatic cervical cancer | 2014 | ||
| Platinum-resistant, recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer | 2014 | ||
| Stage III or IV epithelial ovarian, fallopian tube, or primary peritoneal cancer after initial surgical resection | 2018 | ||
| Panitumumab (Vectibix®) | EGFR/Human IgG2 | Metastatic CRC | 2006 |
| Ofatumumab (Arzerra®) | CD20/Human IgG1 | CLL | 2009 |
| CLL (previously untreated) | 2014 | ||
| Recurrent or progressive CLL | 2016 | ||
| Catumaxomab (Removab®) | EpCAM/ CD3/Bi-specific Rat IgG2b/mouse IgG2a | Malignant ascites (in Europe) | 2009 |
| Ipilimumab (Yervoy®) | CTLA-4/Human IgG1 | Unresectable or metastatic melanoma | 2011 |
| Cutaneous melanoma with pathological involvement of regional lymph nodes | 2015 | ||
| Intermediate or poor risk, previously untreated advanced RCC (in combination with nivolumab) | 2018 | ||
| MSI-H or dMMR metastatic CRC (in combination with nivolumab) | 2018 | ||
| Brentuximab vedotin (Adcentris®) | CD30/Chimeric IgG1; conjugated to monomethyl auristatin E | ALCL and HL | 2011 |
| cHL (as consolidation post-auto-HSCT) | 2015 | ||
| pcALCL or CD30-expressing MF | 2017 | ||
| Previously untreated stage III or IV cHL | 2018 | ||
| Previously untreated systemic ALCL or other CD30-expressing peripheral T-cell lymphomas | 2018 | ||
| Pertuzumab (Perjecta®) | HER-2/Humanised IgG1 | HER-2 positive metastatic breast cancer | 2012 |
| HER-2 positive, locally advanced, inflammatory, or early-stage breast cancer (in combination with trastuzumab as neoadjuvant therapy) | 2013 | ||
| HER-2 positive early breast cancer at high risk or recurrence | 2017 | ||
| Denosumab (Xgeva®) | RANKL/Human IgG2 | Unresectable giant cell tumour of bone | 2013 |
| Ado-trastuzumab emtansine (Kadcyla®) | HER-2/Humanised IgG1; conjugated to DM1 | HER-2 positive, metastatic breast cancer | 2013 |
| HER-2 positive breast cancer with residual invasive disease | 2019 | ||
| Obinutuzumab (Gazyva®) | CD20/Humanised IgG1 | CLL (previously untreated) | 2013 |
| FL | 2016 | ||
| Previously untreated stage II bulky, III or IV FL | 2017 | ||
| Ramucirumab (Cyramza®) | VEGFR-2/Recombinant IgG1 | Advanced or metastatic, gastric or GEJ adenocarcinoma | 2014 |
| Metastatic NSCLC | 2014 | ||
| Metastatic CRC | 2015 | ||
| HCC | 2019 | ||
| First-line treatment of metastatic NSCLC (in combination with erlotinib) | 2020 | ||
| Pembrolizumab (Keytruda®) | PD-1 receptor/Humanised IgG4 | Unresectable or metastatic melanoma and disease progression following ipilimumab | 2014 |
| Unresectable and metastatic melanoma (initial treatment) | 2015 | ||
| Metastatic NSCLC | 2016 | ||
| Recurrent or metastatic HNSCC | 2016 | ||
| Refractory cHL | 2017 | ||
| Previously untreated metastatic non-squamous NSCLC | 2017 | ||
| Locally advanced or metastatic urothelial carcinoma | 2017 | ||
| Unresectable or metastatic MSI-H or dMMR solid tumours | 2017 | ||
| Recurrent locally advanced or metastatic gastric or GEJ adenocarcinoma | 2017 | ||
| Recurrent or metastatic cervical cancer | 2018 | ||
| Refractory PMBCL | 2018 | ||
| First-line treatment metastatic non-squamous NSCLC | 2018 | ||
| First-line treatment metastatic squamous NSCLC | 2018 | ||
| HCC | 2018 | ||
| Recurrent locally advanced or metastatic Merkel cell carcinoma | 2018 | ||
| Melanoma with involvement of lymph nodes following complete resection | 2019 | ||
| First-line treatment stage III or metastatic NSCLC | 2019 | ||
| First-line treatment advanced RCC | 2019 | ||
| Metastatic or unresectable recurrent HNSCC | 2019 | ||
| Metastatic SCLC | 2019 | ||
| Advanced oesophageal squamous cell cancer | 2019 | ||
| Advanced endometrial carcinoma that is not MSI-H or dMMR | 2019 | ||
| BCG-unresponsive, high-risk, non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumours | 2020 | ||
| Unresectable or metastatic tumour mutational burden-high (TMB H) solid tumours | 2020 | ||
| Recurrent or metastatic CSCC not curable by surgery or radiation | 2020 | ||
| First-line treatment unresectable or metastatic MSI-H or dMMR CRC | 2020 | ||
| R/R cHL | 2020 | ||
| Locally recurrent unresectable or metastatic TNBC whose tumours express PD-L1 | 2020 | ||
| Blinatumomab (Blincyto®) | CD19/Bispecific CD19-directed CD3 T-cell engager | Philadelphia chromosome-negative R/R B-cell precursor ALL | 2014 |
| R/R B-cell precursor ALL | 2017 | ||
| B-cell precursor ALL in first or second complete remission with MRD >/= 0.1% | 2018 | ||
| Nivolumab (Opdivo®) | PD-1 receptor/Human IgG4 | Unresectable or metastatic melanoma | 2014 |
| BRAF V600 wild-type, unresectable or metastatic melanoma (in combination with ipilimumab) | 2015 | ||
| Metastatic NSCLC | 2015 | ||
| Advanced RCC | 2015 | ||
| cHL | 2016 | ||
| Recurrent or metastatic HNSCC | 2016 | ||
| Locally advanced or metastatic urothelial carcinoma | 2017 | ||
| MSI-H or dMMR metastatic CRC | 2017 | ||
| HCC | 2017 | ||
| Melanoma with involvement of lymph nodes or metastatic disease following complete resection | 2017 | ||
| Intermediate or poor risk, previously untreated advanced RCC (in combination with ipilimumab) | 2018 | ||
| Metastatic SCLC | 2018 | ||
| HCC (in combination with ipilimumab) | 2018 | ||
| First-line treatment metastatic NSCLC whose tumours express PD-L1 (in combination with ipilimumab) | 2020 | ||
| Unresectable advanced, recurrent or metastatic oesophageal squamous cell carcinoma (ESCC) | 2020 | ||
| Unresectable malignant pleural mesothelioma (first-line; in combination with ipilimumab) | 2020 | ||
| First-line treatment advanced renal cell carcinoma (in combination with cabozantinib) | 2021 | ||
| Dinutuximab (Unituxin®) | GD2/Chimeric IgG1 | High-risk neuroblastoma | 2015 |
| Daratumumab (Darzalex®) | CD38/Human IgG1 | MM | 2015 |
| Newly diagnosed MM ineligible for autologous SCT | 2019 | ||
| Newly diagnosed MM eligible for autologous SCT | 2019 | ||
| Necitumumab (Portrazza®) | EGFR/Human IgG1 | Metastatic squamous NSCLC (first-line) | 2015 |
| Elotuzumab (Empliciti®) | SLAMF7/Humanised IgG1 | MM | 2015 |
| Atezolizumab (Tecentriq®) | PD-L1/Humanised IgG1 | Locally advanced or metastatic urothelial carcinoma | 2016 |
| Metastatic NSCLC | 2016 | ||
| First-line treatment metastatic non-squamous NSCLC | 2018 | ||
| Unresectable locally-advanced or metastatic TNBC | 2019 | ||
| Extensive-stage SCLC | 2019 | ||
| Unresectable or metastatic HCC (in combination with bevacizumab) | 2020 | ||
| BRAF V600 mutation-positive unresectable or metastatic melanoma | 2020 | ||
| Olaratumab (Lartruvo®) | PDGFRα/Human IgG1 | Metastatic soft-tissue sarcoma | 2016 |
| Avelumab (Bavencio®) | PD-L1/Human IgG1 | Metastatic Merkel cell carcinoma | 2017 |
| Locally advanced or metastatic urothelial carcinoma | 2017 | ||
| Advanced RCC | 2019 | ||
| Maintenance treatment in locally advanced or metastatic urothelial carcinoma (UC) | 2020 | ||
| Durvalumab (Imfinzi®) | PD-L1/Human IgG1 | Locally advanced or metastatic urothelial carcinoma | 2017 |
| Unresectable stage III NSCLC | 2018 | ||
| Extensive-stage SCLC | 2020 | ||
| Rituximab+hyaluronidase human (Rituxan hycela®) | CD20/Chimeric IgG1 | Follicular lymphoma, DLBCL and CLL | 2017 |
| Inotuzumab ozogamicin (Besponsa®) | CD22/Humanised IgG4; conjugated with calicheamicin | R/R B-cell precursor ALL | 2017 |
| Mogamulizumab (Poteligeo®) | CCR4/Humanised IgG1 | R/R mycosis fungoides or Sezary syndrome | 2018 |
| Moxetumomab pasudotox-tdfk (Lumoxiti®) | CD-22/Immunotoxin; IgG1 fragment fused to Pseudomonas exotoxin PE38 | R/R hairy cell leukaemia | 2018 |
| Cemiplimab-rwlc (Libtayo®) | PD-1/Human IgG4 | Metastatic CSCC or locally advanced CSCC who are not candidates for curative surgery or curative radiation | 2018 |
| Locally advanced and metastatic basal cell carcinoma | 2021 | ||
| First-line treatment of advanced NSCLC whose tumors have high PD-L1 expression | 2021 | ||
| Trastuzumab + hyaluronidase oysk (Herceptin Hylecta®) | HER-2/Humanised IgG1 | HER-2 overexpressing breast cancer | 2019 |
| Polatuzumab vedotin-piiq (Polivy®) | CD79b-directed ADC; conjugated to MMAE | R/R DLBCL | 2019 |
| Enfortumab vedotin-ejfv (Padcev®) | Nectin-4-directed ADC; conjugated to MMAE | Locally advanced or metastatic urothelial cancer | 2019 |
| Fam-trastuzumab deruxtecan-nxki (Enhertu®) | HER-2 directed ADC; conjugated to topoisomerase inhibitor | Unresectable or metastatic HER2-positive breast cancer | 2019 |
| Locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma | 2021 | ||
| Isatuximab-irfc (Sarclisa®) | CD38 | MM | 2020 |
| Sacituzumab govitecan-hziy (Trodelvy®) | Trop-2 directed ADC; conjugated to SN-38 | Metastatic TNBC | 2020 |
| Daratumumab and hyaluronidase-fihj (Darzalex faspro®) | CD38/Human IgG1 | Newly diagnosed or R/R MM | 2020 |
| Tafasitamab-cxix (Monjuvi®) | CD19/Humanised Fc-modified cytolytic | R/R DLBCL | 2020 |
| Belantamab mafodotin-blmf (Blenrep®) | BCMA/ADC conjugated to microtubule inhibitor monomethyl auristatin F (MMAF) | R/R MM | 2020 |
| Naxitamab (Danyelza®) | GD2/Humanised IgG1 | R/R high-risk neuroblastoma in the bone or bone marrow (in combination with GM-CSF) | 2020 |
| Margetuximab-cmkb (Margenza®) | HER2/Fc engineered chimeric IgG1 | Metastatic HER2-positive breast cancer | 2020 |
| mAb Name | Target Antigen | Source/Immunogen | Finding | Ref. |
|---|---|---|---|---|
| Humanised anti-TF mAb conjugated with MMAE | TF | - | Significantly inhibited tumour growth in an orthotopic xenograft model, and extended survival in a murine peritoneal dissemination model. | [34] |
| h-RabMab1 | Alternatively spliced TF | - | Orthotopic pancreatic tumours in mice treated with h-RabMab1 were 60% smaller than tumours in control group. | [33] |
| Humanised anti-hTM4SF5 mAb | TM4SF5 | Established based on the mouse mAb mEC2-C obtained by immunisation with the cyclic peptide hTM4SF5EC2-C | Reduced cell viability and cell motility, and modulated the expression of EMT markers (vimentin and E-cadherin) in pancreatic cancer cells that endogenously express TM4SF5. | [52] |
| Panitumumab labelled with 111In or 177Lu - | EGFR | - | Radioimmunotherapy with panitumumab labelled with Auger electron-emitting 111In or β particle-emitting 177Lu inhibited the growth of subcutaneous PANC-1 human pancreatic cancer xenografts in mice. | [30] |
| PcMab-60 | PODXL | Soluble PODXL | Demonstrated antitumour activity in Mia PaCa-2 xenograft mouse models. | [37] |
| 7E | IL-20 | - | Prolongs survival and attenuates PD-L1 expression in a transgenic mouse and an orthotopic pancreatic cancer model. Combination of 7E and an anti-PD-1 mAb increases efficacy in inhibiting tumour growth in the orthotopic model. | [53] |
| H2Mab-19 | HER2 | Purified recombinant extracellular domain of HER2 | Reduces tumour development in MiaPaCa-2 xenograft model. | [38] |
| 90Y-DTPA-ALT836 | TF | - | Slow tumour growth compared to the control groups and had significantly smaller tumour volumes 1-day post-injection. | [35] |
| Anti-GPC-1 mAb conjugated with MMAF | Glypican-1 (GPC-1) | - | Induces significant tumour growth inhibition against GPC-1-positive pancreatic cell lines (BxPC-3 and T3M-4) and patient-derived tumour models. | [39] |
| KU44.22B | Integrin α3 | CFPAC-1 pancreatic cancer cells | Inhibits proliferation of Capan-2 pancreatic cancer cells and increases migration of CFPAC-1 and BxPC-3 cancer cells in vitro. | [26] |
| 6E8 and E9 | MUC4 | MUC4beta domain | Decreased proliferation and migration of MUC4 expressing pancreatic cancer cells. | [27] |
| TAB004 | MUC1 | Pancreatic tumour lysate isolated from an adenocarcinoma developed by a mouse that was transgenic for human MUC1 | TAB004 + Lip-MSA-IL-2 significantly improved survival and slowed tumour growth compared to controls in a human MUC1 transgenic mouse model of pancreatic cancer. | [54] |
| TROP2-IR700 | TROP2 | - | Photoimmunotherapy with the humanised anti-TROP2 mAb conjugated to the photosensitizer IR700 significantly inhibits tumour growth in pancreatic cancer and cholangiocarcinoma xenografts. | [31] |
| ZB131 | Cell surface plectin 1 | - | Decreases tumour volume 5-fold in xenografts, and induces complete tumour regression in subcutaneous syngeneic pancreatic cancer mouse models. | [40] |
| TAB004 | MUC1 | - | TAB004 treatment had minimal effect as single agent on most of pancreatic cancer cell lines except for Capan-2. However, when combined with gemcitabine, paclitaxel, or 5-FU, it significantly increased anti-tumour efficacy. | [28] |
| Anti-Gal-9 mAb | Galectin-9 | - | Induced significant reduction in tumour size in orthotopic pancreatic cancer mouse models. | [41] |
| 28H1 | FAP | - | The dual-labeled mAb (conjugated with either DTPA for imaging studies or with DTPA and the photosensitizer IRDye700DX for therapy studies) targets pancreatic tumours in mice with good signal-to-background ratios and favourable biodistribution, and it efficiently induces cell death. | [55] |
| RC68 | EGFR | - | The RC68-based antibody-drug conjugates induced death of EGFR-positive pancreatic cancer cell lines and inhibited the growth of BxPC-3 xenografts. | [56] |
| H-Zt/g4-MMAE | RON | - | Inhibited the growth of pancreatic cancer xenografts regardless of chemoresistance or metastatic status. | [42] |
| BAG3-H2L4 | BAG3 | BAG3- multiple antigenic peptides | Significantly inhibits the growth of Mia PaCa-2 pancreatic cancer cell xenografts | [43] |
| Zolbetuximab | CLDN18.2 | - | Slow tumour growth, improve survival and attenuate development of metastases in xenograft models. Gemcitabine enhanced zolbetuximab-induced ADCC. | [44] |
| 1849-ICG conjugate | TF | - | A single dose of 1849-ICG conjugate accompanied by NIR light exposure inhibited tumour growth in vivo without noticeable adverse effects. | [36] |
| 90Y-labeled 059-053 | CD147 | Human antibody library | Combined treatment using 90Y-labeled 059-053 with gemcitabine significantly suppressed tumour growth and prolonged survival with tolerable toxicity in a BxPC-3 xenograft mouse model of refractory pancreatic cancer. | [57] |
| Amatuximab | Mesothelin | - | Supressed the development of peritoneal metastases and exhibited synergistic killing in combination with gemcitabine in a peritoneal metastatic pancreatic cancer model. | [45] |
| Anti-hMUC1 antibody | MUC1 | rhMUC1-EC192 protein | Specifically targets MUC1-C pancreatic cancer cells in vitro and in vivo and suppresses the growth of tumours in Capan-2 xenogratfs mouse models. | [58] |
| Pritumumab | Vimentin | B lymphocytes isolated from a regional draining lymph node of a patient with cervical carcinoma | Inhibits subcutaneous pancreatic cancer xenografts models. The binding of the antibody to pancreatic cancer cells and tissue induces ADCC. | [46] |
| 7E3 | Neuregulin 1 (NRG1) | rhNRG1β1-EDC | Inhibits migration and growth of pancreatic cancer cells co-cultured with CAFs in orthotopic pancreatic tumour xenografts. | [59] |
| CBT-15G | Doublecortin-like kinase 1 (DCLK1) | KLH-linked peptides targeting the DCLK1 extracellular domain | Significantly inhibited SW1990 pancreatic cancer xenograft growth. | [47] |
| 90Y-ITGA6B4 | α6β4 | Human antibody library constructed using a phage-display system | A single dose of 90Y-ITGA6B4 inhibited tumour growth in mice bearing BxPC-3 human pancreatic cancer xenografts overexpressing α6β4. | [32] |
| ASG-5ME | SLC44A4 | B300.19 cells engineered to express SLC44A4 | The ADC induced potent antitumour activity in both cell line– and patient-derived xenograft models of pancreatic and prostate cancers. Combination studies of ASG5ME and nab-paclitaxel increased antitumour activity compared to single agents alone. | [60] |
| Novel mAbs against AGR2 and C4.4A | AGR2 and C4.4A | Unconjugated antigenic peptides | Reduced tumour growth and metastasis and led to regression of xenograft tumours in mice, resulting in increased survival. | [61] |
| 90Y-TSP-A01 | Transferrin receptor (TfR) | Human antibody libraries | Induced almost complete response in mice bearing Mia PaCa-2 tumours (high TfR expression), but it had limited efficacy on BxPC-3 tumours (low TfR expression). | [62] |
| BNC101 | LRG5 | - | BNC101 as single agent partially inhibited tumour growth in AsPC1 and PANC-1 pancreatic cancer models, while when used in combination with gemcitabine, it significantly inhibited tumour growth in both models. In contrast, BNC101 as monotherapy or in combination with chemotherapy had no antitumour activity in the LGR5 negative BxPC3 xenograft tumours. | [63] |
| TCC56 | MUC13 | PAN2 cells | The ADC can be efficiently internalised and induces cell death in TCC-PAN2 cells. | [29] |
| Pa65-2 | CHC | MIA PaCa-2 pancreatic cancer cells | Inhibited tumour growth and angiogenesis in NOD/SCID mice bearing MIA PaCa-2-derived pancreatic cancer xenografts. | [64] |
| mAb Name | Target Antigen | Source/Immunogen | Finding | Ref. |
|---|---|---|---|---|
| 89Zr-DFO-anti-γH2AX-TAT | CA19.9 | Fully human 5B1 mAb, generated from blood lymphocytes from a patient immunised with a sLea – KLH vaccine | PET imaging allows monitoring of tumour radiobiological response in a BxPC-3 pancreatic ductal adenocarcinoma subcutaneous xenograft mouse model. | [51] |
| 6G5j mAb conjugated to LICOR-IRDye800 | CEACAM | - | Selectively labels pancreatic cancer in nude mouse models. | [65] |
| KU44.22B | Integrin α3 | CFPAC-1 pancreatic cancer cells | Immunodetects integrin α3 by IHC in tumour cell pellets and pancreatic cancer tissue microarrays. | [26] |
| KU44.13A | CD26 | CFPAC-1 pancreatic cancer cells | Immunodetects CD26 by IHC in tumour cell pellets and pancreatic cancer tissue microarrays. | [26] |
| KU42.33C | CD109 | BxPC-3 pancreatic cancer cells | Immunodetects CD109 by Western blot and IHC in tumour cell pellets and pancreatic cancer tissue microarrays. | [66] |
| 89Zr-Df-ALT-836 | TF | - | Demonstrated high binding affinity and TF-specificity, and persistent accumulation in BxPC-3 xenografts in mice. | [67] |
| 89Zr-059-053 | CD147 | Human antibody phage-display library | 89Zr-059-053 highly accumulated in CD147-expressing tumours and clearly visualised subcutaneous and orthotopic xenografts. | [50] |
| Condition/Stage of Disease | Therapeutic Intervention | n | Ph | Outcomes | Ref. |
|---|---|---|---|---|---|
| Metastatic PC | Durvalumab + tremelimumab vs. durvalumab | 65 | II | ORR: 3.1 vs. 0% Not enrolled in part B because the threshold for efficacy was not met in part A | [114] |
| Metastatic PC | Olaratumab + nab-paclitaxel + gemcitabine | 10 | I | Well tolerated and manageable toxicity | [139] |
| Advanced PC | Gemcitabine + MK-0646 (arm A) vs. gemcitabine + MK-0646 + erlotinib (arm B) vs. gemcitabine + erlotinib (arm C) | 81 | I/II | PFS: 1.8 vs. 1.8 vs. 1.9 months OS: 10.4 vs. 7.1 vs. 5.7 months (p = 0.02) | [77] |
| CA19-9 positive PC | MVT-5873 + nab-paclitaxel + gemcitabine | 38 | I | Single agent MVT-5873 appears safe and tolerable at biologically active doses | [140] |
| Metastatic PC | Gemcitabine + nab-paclitaxel + pembrolizumab | 17 | Ib/II | mPFS: 9.1 months mOS: 15.0 months (in chemo-naive) | [110] |
| Resected PC | Cetuximab or bevacizumab + gemcitabine + chemoradiation | 127 | II | mOS: 17 months (both arms) DFS: 11 months | [141] |
| Locally advanced or metastatic PC | Gemcitabine + nimotuzumab vs. gemcitabine + placebo | 192 | IIb | mOS: 8.6 vs. 6.0 months (p = 0.03) mPFS: 5.1 vs. 3.6 months (p = 0.02) | [92] |
| Advanced or metastatic PC | TAK-264 (MLN0264) | 43 | II | ORR: 3% | [132] |
| Metastatic PC | Gemcitabine + simtuzumab (700 mg) vs. gemcitabine + simtuzumab (200 mg) vs. gemcitabine + placebo | 240 | II | mPFS: 3.7 (p = 0.73) vs. 3.5 (p = 0.61) vs. 3.7 months mOS: 7.6 (p = 0.28) vs. 5.9 (p = 0.69) vs. 5.7 months ORR: 13.9 (p = 0.16) vs. 14.5 (p = 0.20) vs. 23.5% | [121] |
| Refractory colon and PC | NEO-102 (ensituximab) | 19 (4 PC) | I | Safe and well tolerated | [142] |
| Unresectable PC | Bevacizumab + erlotinib + capecitabine + RT | 17 | I | Safe and well tolerated | [143] |
| PC not amenable to curative treatment | Bevacizumab + cetuximab + leucovorin + gemcitabine + cisplatin + fluorouracil vs. leucovorin + gemcitabine + cisplatin + fluorouracil | 59 | II | mOS: 13.2 vs. 6.8 months (p = 0.03) TTP: 10.7 vs. 3.1 months (p = 0.004) | [84] |
| Advanced pancreatic and gastric cancers | ASG-5ME | 50 | I | Well tolerated with limited evidence of antitumour activity | [133] |
| Locally advanced PC | Neoadjuvant gemcitabine plus capecitabine, followed by either: capecitabine or UFT + RT (A) or capecitabine or UFT + cetuximab + RT (B). | 17 | II | mOS: 15.8 vs. 22.0 months (p > 0.05) mPFS: 10.4 vs. 12.7 months (p > 0.05) | [144] |
| Metastatic PC | Irinotecan + docetaxel vs irinotecan + docetaxel + cetuximab | 87 | II | ORR: 4.5 vs. 7% mPFS: 3.9 vs. 4.5 monthsmOS: 6.5 vs. 5.3 months | [82] |
| Locally advanced PC | Cetuximab + gemcitabine + RT | 34 | II | mOS: 15.3 months | [145] |
| Metastatic PC | 90Y-clivatuzumab tetraxetan + gemcitabine vs. 90Y-clivatuzumab tetraxetan alone | 58 | Ib | mOS: 2.7 vs. 2.6 months (7.9 vs. 3.4 months in patients who received multiple cycles; p = 0.004) | [146] |
| Locally advanced PC | Panitumumab + gemcitabine-based CRT | 14 | I | Manageable toxicity mPFS: 8.9 months mOS: 12.3 months | [87] |
| Metastatic PC | Trastuzumab + cetuximab (after failure of first-line gemcitabine) | 49 | I/II | mPFS: 1.8 months mOS: 4.6 months | [97] |
| Metastatic PC | Gemcitabine + erlotinib + cixutumumab vs. gemcitabine + erlotinib | 116 | Ib/II | mPFS: 3.6 vs. 3.6 months (p = 0.97) mOS: 7.0 vs. 6.7 months (p = 0.64) | [76] |
| Metastatic PC | Tremelimumab + gemcitabine | 34 | I | Safe and acceptable tolerability profile | [106] |
| Metastatic PC | Ganitumab + gemcitabine | 6 | Ib | Tolerable and acceptable safety profile | [147] |
| Borderline and locally advanced PC | Neoadjuvant bevacizumab + gemcitabine | 30 | II | No survival benefit in patients undergoing resection | [148] |
| Locally advanced or metastatic PC | Gemcitabine + capecitabine + bevacizumab + erlotinib | 44 | II | ORR: 23% mPFS: 8.4 months mOS: 12.6 months | [149] |
| Unresectable locally advanced or metastatic PC | Gemcitabine + nimotuzumab | 18 | - | mOS: 9.3 months mPFS: 3.7 months | [150] |
| Unresectable or metastatic PC | Tigatuzumab (CS-1008) + gemcitabine | 62 | II | ORR: 13.1% mPFS: 3.9 months mOS: 8.2 months | [126] |
| Advanced PC | CP-870,893 + gemcitabine | 22 | I | Safe and well tolerated mPFS: 5.2 months mOS: 8.4 months ORR: 19% | [135] |
| Locally advanced or metastatic PC | Ipilimumab vs. ipilimumab + GVAX | 30 | Ib | mOS: 3.6 vs. 5.7 months (p = 0.07) | [105] |
| Potentially resectable PC | Gemcitabine + bevacizumab followed by RT + bevacizumab | 59 | II | mOS: 16.8 months (19.7 months after resection) mPFS: 6.6 months (12.9 months after resection) | [151] |
| R0 or R1-resected PC | Gemcitabine + cetuximab | 76 | II | DFS at 18 months: 27.1% mDFS: 10.0 months mOS: 22.4 months | [81] |
| Metastatic PC | Gemcitabine vs. gemcitabine + AGS-1C4D4 | 196 | II | 6-month SR: 44.4 vs. 60.9% (P = 0.03) mOS: 5.5 vs. 7.6 months (p = 0.12) mPFS: 3.2 vs. 3.8 months (p = 0.27) ORR: 13.1 vs. 21.6% | [138] |
| Advanced PC | Everolimus + cetuximab + capecitabine | 43 | I/II | ORR: 6.5% mOS: 5.0 months | [152] |
| Advanced PC | Bevacizumab + gemcitabine + 5-FU | 42 | II | PFS at 6 months: 49% mPFS: 5.9 months mOS: 7.4 months | [153] |
| Metastatic PC | Gemcitabine + ganitumab vs. gemcitabine + conatumumab vs. gemcitabine + placebo | 125 | II | 6-month SR: 57 vs. 59 vs. 50% mOS: 8.7 vs. 7.5 vs. 5.9 months mPFS: 5.1 vs.. 4.0 vs. 2.1 months ORR: 10 vs. 3 vs. 3% | [73] |
| Localized or locally advanced PC | Neoadjuvant cetuximab + gemcitabine + IMRT | 37 | II | mOS: 24.3 (resected patients) vs. 10 months (not resected) | [154] |
| Stage III or IV PC | 90Y-clivatuzumab tetraxetan + low-dose gemcitabine | 42 | - | The combination is feasible DCR: 58% mOS: 7.7 months | [155] |
| HER2 overexpressing metastatic PC | Trastuzumab + capecitabine | 17 | II | PFS after 12 weeks: 23.5% mOS: 6.9 months | [98] |
| Advanced PC | Bevacizumab + cetuximab + gemcitabine vs. bevacizumab + cetuximab | 61 | II | mPFS: 3.5 vs. 1.9 months mOS: 5.4 vs. 4.2 months | [156] |
| Locally advanced or metastatic PC | Gemcitabine + oxaliplatin + cetuximab | 41 | II | ORR: 24% mPFS: 6.9 months mOS: 11.3 months | [157] |
| Locally advanced or metastatic PC | Nimotuzumab | 56 | II | mPFS: 6.7 weeks PFS after 1 year: 10.3% mOS: 18.1 weeks | [93] |
| Metastatic PC | Cetuximab + gemcitabine + oxaliplatin | 64 | II | mOS: 263 vs.. 162 days (WT vs. KRAS mutation) mPFS: 104 vs. 118 days (WT vs. KRAS mutation) | [158] |
| Locally advanced PC | Cetuximab + gemcitabine + oxaliplatin followed by cetuximab + capecitabine + RT | 69 | II | mOS: 19.2 months 1-year OS: 66% | [159] |
| Advanced PC | 90Y-clivatuzumab tetraxetan | 21 | I | Well tolerated with manageable hematologic toxicity | [160] |
| Locally advanced or metastatic PC | Bevacizumab + gemcitabine + oxaliplatin | 55 | II | mPFS: 4.9 months mOS: 11.9 months ORR: 36% | [161] |
| Localised PC | Gemcitabine + bevacizumab + RT | 32 | II | mPFS: 9.9 months mOS: 11.8 months | [162] |
| Locally advanced PC | Gemcitabine + cetuximab + RT | 16 | I | Safe and well tolerated mOS: 10.5 months | [163] |
| Gemcitabine-refractory metastatic PC | Bevacizumab alone vs. bevacizumab + docetaxel | 32 | II | mPFS: 43 vs. 48 days mOS: 165 vs. 125 days The study was stopped due to futility. | [164] |
| Locally advanced or metastatic PC | Ipilimumab | 27 | II | No responders but one subject experienced a delayed response after initial progressive disease. | [104] |
| Gemcitabine-refractory metastatic PC | Bevacizumab + erlotinib | 36 | II | OS at 6 months: 22% | [165] |
| Locally advanced or metastatic PC | Gemcitabine + capecitabine + bevacizumab + erlotinib | 20 | I | mOS: 12.5 months mPFS: 9.0 months ORR: 50% | [166] |
| Locally advanced, unresectable PC | Bevacizumab + capecitabine + RT | 82 | II | mOS: 11.9 months 1-year survival: 47% mPFS: 8.6 months; RR: 26% | [167] |
| Metastatic or locally advanced unresectable PC | Bevacizumab + gemcitabine + capecitabine | 50 | II | ORR: 22% mPFS: 5.8 months mOS: 9.8 months | [168] |
| Metastatic PC | Cetuximab + gemcitabine + oxaliplatin | 64 | II | ORR: 33% mPFS: 3.9 months mOS: 7.1 months | [169] |
| Inoperable PC (head) | Radiolabelled anti-CEA I131 KAb201 mAb | 25 | I/II | ORR: 6% mOS: 5.2 months | [170] |
| Chemotherapy-naive metastatic PC | Gemcitabine + cisplatin + bevacizumab | 52 | II | mTTP: 6.6 months mOS: 8.2 months 1-year survival: 36% | [171] |
| Advanced PC | Cetuximab + gemcitabine + cisplatin vs. gemcitabine + cisplatin alone | 84 | II | ORR: 17.5 vs. 12.2% (p = 0.55) mPFS: 3.4 vs. 4.2 months (p = 0.85) mOS: 7.5 vs. 7.8 months (p = 0.74) | [172] |
| Untreated stage III or IV PC | Matuzumab + gemcitabine | 17 | I | Well tolerated mOS: 3.7 months | [94] |
| Locally Advanced PC | Bevacizumab + capecitabine-based chemoradiotherapy | 48 | I | Safe and well tolerated, required capecitabine dose reduction ORR: 20% mOS: 14.4 months | [173] |
| Advanced PC | Bevacizumab + gemcitabine | 52 | II | ORR: 21% mPFS: 5.4 months mOS: 8.8 months | [174] |
| Advanced PC | Cetuximab + gemcitabine | 41 | II | ORR: 12% mPFS: 3.8 months mOS: 7.1 months | [175] |
| Unresectable, measurable PC | Murine mAb 17-1A | 28 | II | Acceptable toxicity. Lack of efficacy of treatment | [176] |
| Condition/Stage of Disease | Therapeutic Intervention | n | Ph | Outcomes | Ref. |
|---|---|---|---|---|---|
| Metastatic PC (GAMMA trial) | Gemcitabine + placebo vs.. gemcitabine + ganitumab (12 mg/kg) vs. gemcitabine + ganitumab (20 mg/kg) | 800 | III | mOS: 7.2 vs. 7.0 (p = 0.49) vs. 7.1 months (p = 0.40) | [74] |
| Advanced PC (CALGB 80303 trial) | Gemcitabine + bevacizumab vs. gemcitabine + placebo | 602 | III | mOS: 5.8 vs. 5.9 months (p = 0.95) mPFS: 3.8 vs. 2.9 months (p = 0.07)ORR: 13 vs. 10% | [101] |
| Unresectable locally advanced or metastatic PC (SWOG S0205 trial) | Gemcitabine vs. gemcitabine + cetuximab | 745 | III | mOS: 5.9 vs. 6.3 months (p = 0.19) mPFS: 3.0 vs. 3.4 months (p < 0.18) mTTF: 1.8 vs. 2.3 months (p < 0.006) ORR: 14 vs. 12% (p = 0.59) | [83] |
| Metastatic PC | Gemcitabine + erlotinib + bevacizumab vs. gemcitabine + erlotinib + placebo | 607 | III | mOS: 7.1 vs. 6.0 months (p < 0.21) mPFS: 4.6 vs. 3.6 months (p < 0.0002) ORR: 13.5 vs. 8.6% (p < 0.06) | [100] |
| Trials Identifier | Therapeutic Intervention | n1 | Ph | Study Status (Completion Date) |
|---|---|---|---|---|
| Anti-PD-1/PD-L1 mAbs | ||||
| NCT02930902 | Pembrolizumab (anti-PD-1 mAb) + paricalcitol vs.. pembrolizumab + paricalcitol + chemotherapy | 10 | I | Active, not recruiting (12/2022) |
| NCT03723915 | Pembrolizumab (anti-PD-1 mAb) + pelareorep | 30 | II | Active, not recruiting (06/2021) |
| NCT04548752 | Olaparib + pembrolizumab (anti-PD-1 mAb) vs. olaparib | 88 | II | Not yet recruiting (03/2025) |
| NCT02907099 | BL-8040 + pembrolizumab (anti-PD-1 mAb) | 23 | II | Active, not recruiting (12/2022) |
| NCT03634332 | PEGPH20 + pembrolizumab (anti-PD-1 mAb) | 35 | II | Recruiting (01/2021) |
| NCT04477343 | SX-682 (dual-inhibitor CXCR1/CXCR2) + nivolumab (anti-PD-1 mAb) | 20 | I | Recruiting (10/2022) |
| NCT03970252 | Nivolumab (anti-PD-1 mAb) + mFOLFIRINOX | 36 | I, II | Recruiting (04/2022) |
| NCT04050085 | SD-101 (TLR9 agonist) + radiation therapy + nivolumab (anti-PD-1 mAb) | 6 | I | Recruiting (11/2021) |
| NCT03767582 | Nivolumab + CCR2/CCR5 dual antagonist vs. Nivolumab + GVAX + CCR2/CCR5 dual antagonist | 30 | I, II | Recruiting (03/2022) |
| NCT03214250 | APX005M (CD40 agonistic mAb) + nivolumab (anti-PD-1 mAb) + gemcitabine + nab-paclitaxel vs. APX005M + gemcitabine + nab-paclitaxel. | 129 | I, II | Active, not recruiting (09/2022) |
| NCT03373188 | Surgery vs. VX15/2503 + surgery vs. VX15/2503 + ipilimumab (anti-CTLA-4 mAb) + surgery vs. VX15/2503 + nivolumab (anti-PD-1 mAb) + surgery | 32 | I | Recruiting (12/2022) |
| NCT04191421 | Siltuximab (anti-IL-6) + spartalizumab (anti-PD-1 mAb) | 42 | I, II | Recruiting (12/2022) |
| NCT04581343 | Canakinumab (anti-IL-1β mAb) + spartalizumab (anti-PD-1 mAb) + nab-paclitaxel + gemcitabine | 10 | I | Recruiting (03/2022) |
| NCT04116073 | INCMGA00012 (anti-PD-1 mAb) | 25 | II | Recruiting (08/2028) |
| NCT03983057 | mFOLFIRINOX + anti-PD-1 antibody vs. mFOLFIRINOX | 830 | III | Recruiting (04/2022) |
| NCT04498689 | Camrelizumab (anti-PD-1 mAb) + nab-paclitaxel + gemcitabine | 117 | II | Recruiting (12/2022) |
| NCT03989310 | Manganese primed anti-PD-1 antibody + nab-paclitaxel + gemcitabine | 20 | I, II | Recruiting (03/2021) |
| NCT04104672 | AB680 (CD73 inhibitor) + zimberelimab (anti-PD-1 mAb) + nab-paclitaxel + gemcitabine | 150 | I | Recruiting (01/2024) |
| NCT04181645 | SHR-1210 (anti-PD-1 mAb) + paclitaxel-albumin + gemcitabine | 20 | I | Recruiting (07/2022) |
| NCT04493060 | Niraparib + TSR-042 (anti-PD-1 mAb) | 20 | II | Not yet recruiting (12/2023) |
| NCT03816358 | Anetumab ravtansine (anti-mesothelin ADC) + nivolumab (anti-PD-1 mAb) vs. anetumab ravtansine + nivolumab + ipilimumab vs. anetumab ravtansine + nivolumab + gemcitabine | 64 | I, II | Recruiting (04/2021) |
| Anti-CLDN18.2 mAb | ||||
| NCT03816163 | Zolbetuximab (anti-CLDN18.2 mAb) + nab-paclitaxel + gemcitabine vs. nab-paclitaxel + gemcitabine | 141 | II | Recruiting (10/2022) |
| Anti-PDGFRα mAb | ||||
| NCT03086369 | Olaratumab (anti-PDGFRα mAb) + nab-paclitaxel + gemcitabine vs. placebo + nab-paclitaxel + gemcitabine | 186 | Ib, II | Active, not recruiting (01/2022) |
| Anti-CA19.9 mAb | ||||
| NCT03801915 | MVT-5873 (anti-Sialyl Lewis/CA19.9 mAb) | 105 | II | Recruiting (12/2023) |
| NCT03118349 | MVT-5873 (anti-Sialyl Lewis/CA19.9 mAb) + MVT-1075 | 7 | I | Active, not recruiting (12/2020) |
| NCT02672917 | MVT-5873 (HuMab-5B1) | 108 | I | Recruiting (12/2020) |
| Anti-OX40 mAb | ||||
| NCT04387071 | CMP-001 (TLR9 agonist) + INCAGN01949 (anti-OX40 mAb) | 42 | I, II | Not yet recruiting (07/2023) |
| Anti-HGF mAb | ||||
| NCT03316599 | Ficlatuzumab (anti-HGF mAb) + gemcitabine + nab-paclitaxel | 26 | Ib | Active, not recruiting (11/2023) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Pinilla, G.A.; Modjtahedi, H. Therapeutic Application of Monoclonal Antibodies in Pancreatic Cancer: Advances, Challenges and Future Opportunities. Cancers 2021, 13, 1781. https://doi.org/10.3390/cancers13081781
Arias-Pinilla GA, Modjtahedi H. Therapeutic Application of Monoclonal Antibodies in Pancreatic Cancer: Advances, Challenges and Future Opportunities. Cancers. 2021; 13(8):1781. https://doi.org/10.3390/cancers13081781
Chicago/Turabian StyleArias-Pinilla, Gustavo A., and Helmout Modjtahedi. 2021. "Therapeutic Application of Monoclonal Antibodies in Pancreatic Cancer: Advances, Challenges and Future Opportunities" Cancers 13, no. 8: 1781. https://doi.org/10.3390/cancers13081781
APA StyleArias-Pinilla, G. A., & Modjtahedi, H. (2021). Therapeutic Application of Monoclonal Antibodies in Pancreatic Cancer: Advances, Challenges and Future Opportunities. Cancers, 13(8), 1781. https://doi.org/10.3390/cancers13081781