Simultaneous Expression of Th1- and Treg-Associated Chemokine Genes and CD4+, CD8+, and Foxp3+ Cells in the Premalignant Lesions of 4NQO-Induced Mouse Tongue Tumorigenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Carcinogen Treatment
2.2. Pathologic Examination
2.3. Preparation of Total RNA and the Polymerase Chain Reaction (PCR) Array
2.4. Quantitative Reverse Transcription (qRT)-PCR
2.5. IHC Staining
2.6. Statistical Analyses
3. Results
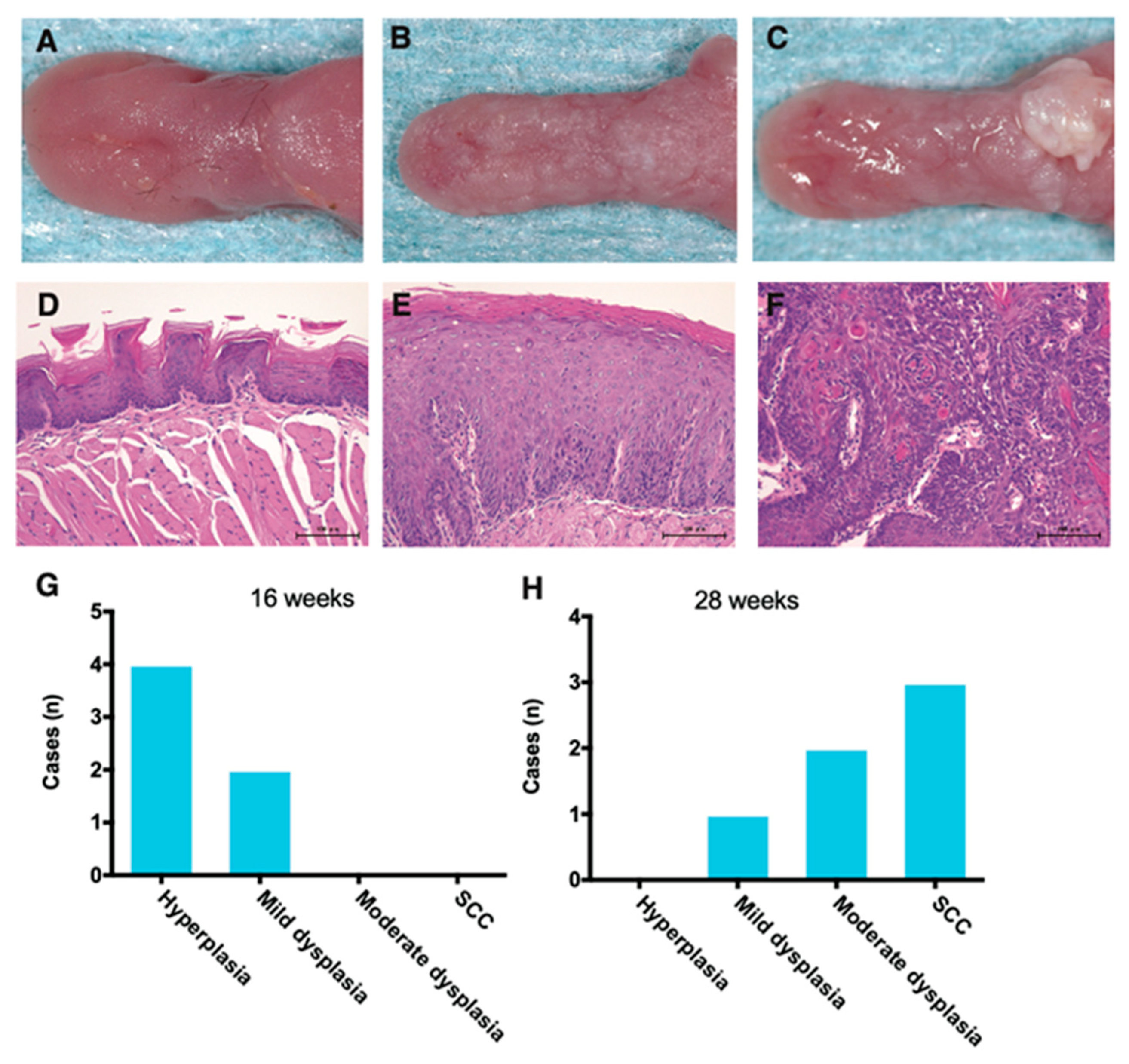
3.1. Development of Premalignant and Carcinoma Tongue Lesions in 4NQO-Treated Mice
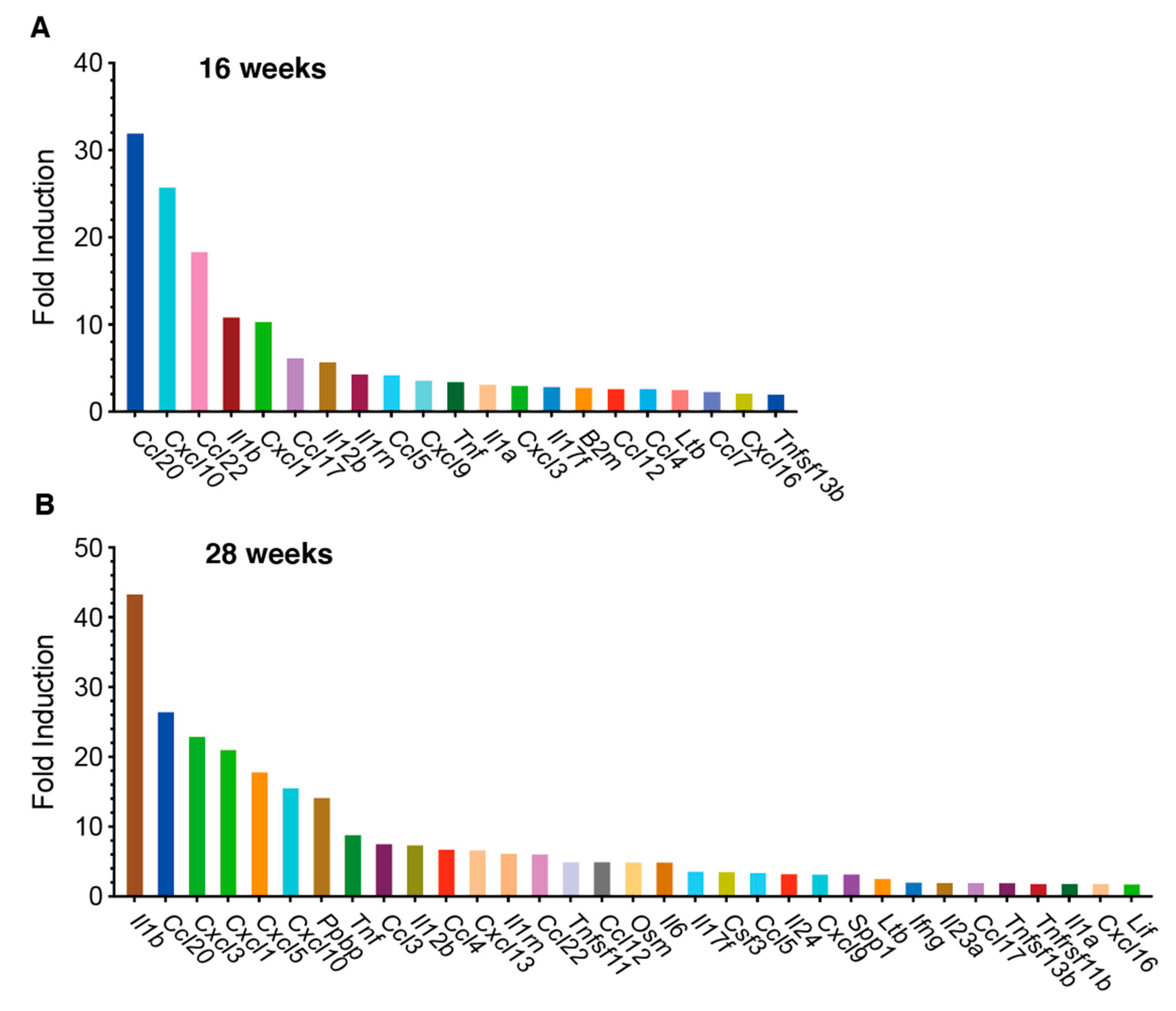
3.2. PCR Array Analysis of Mouse Chemokine/Cytokine Gene Expression in Tongue Tissues from 4NQO-Treated Mice
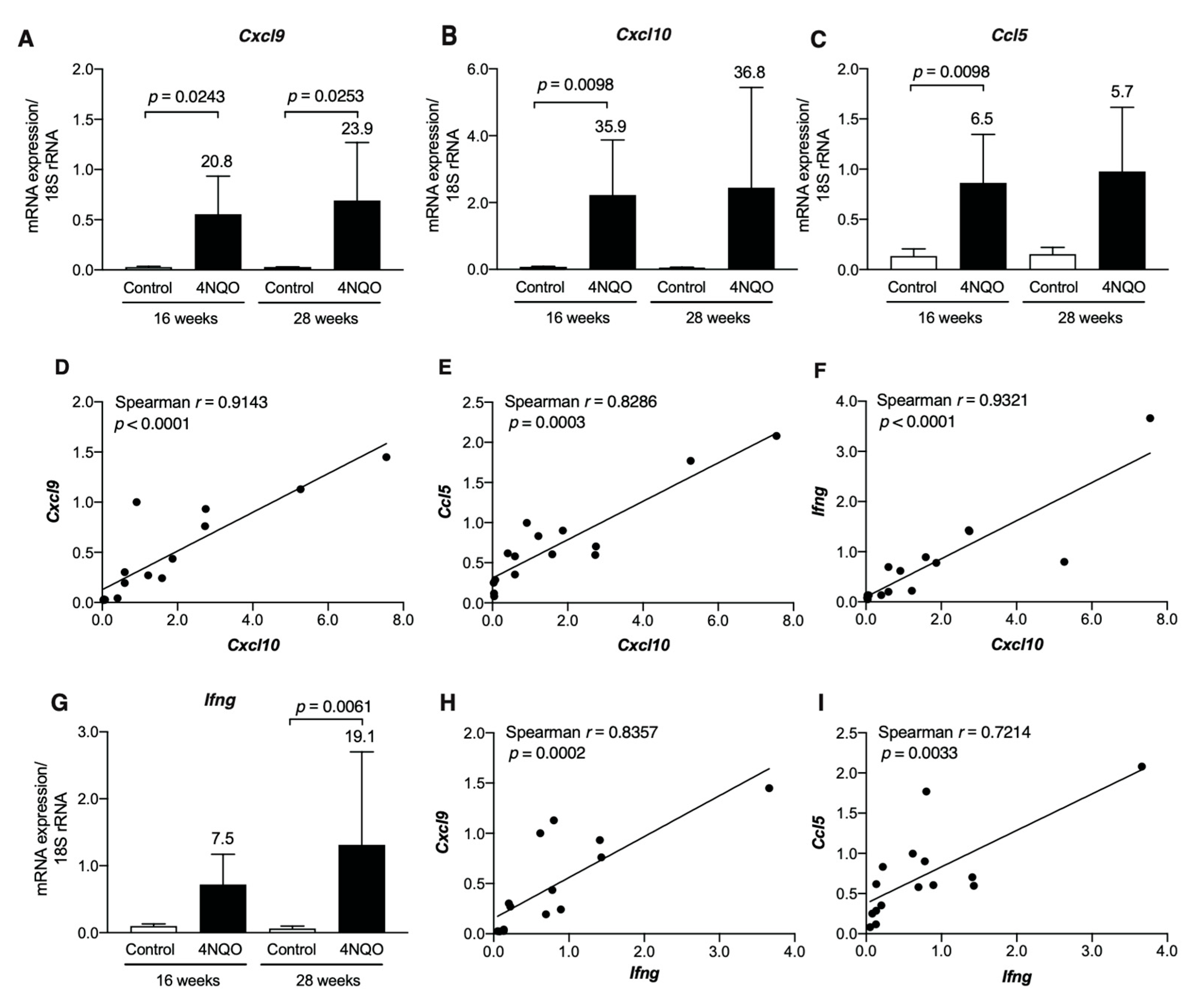
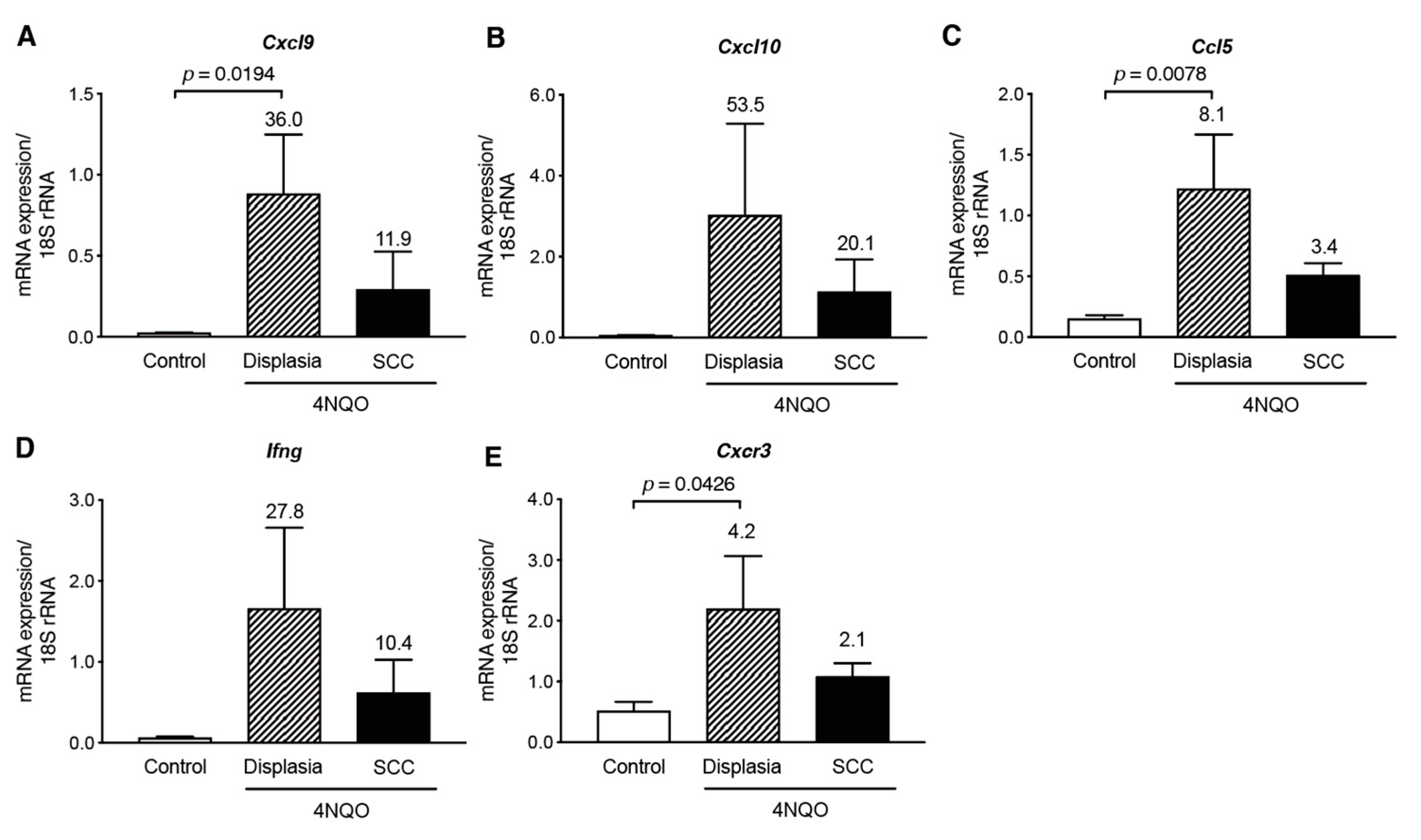
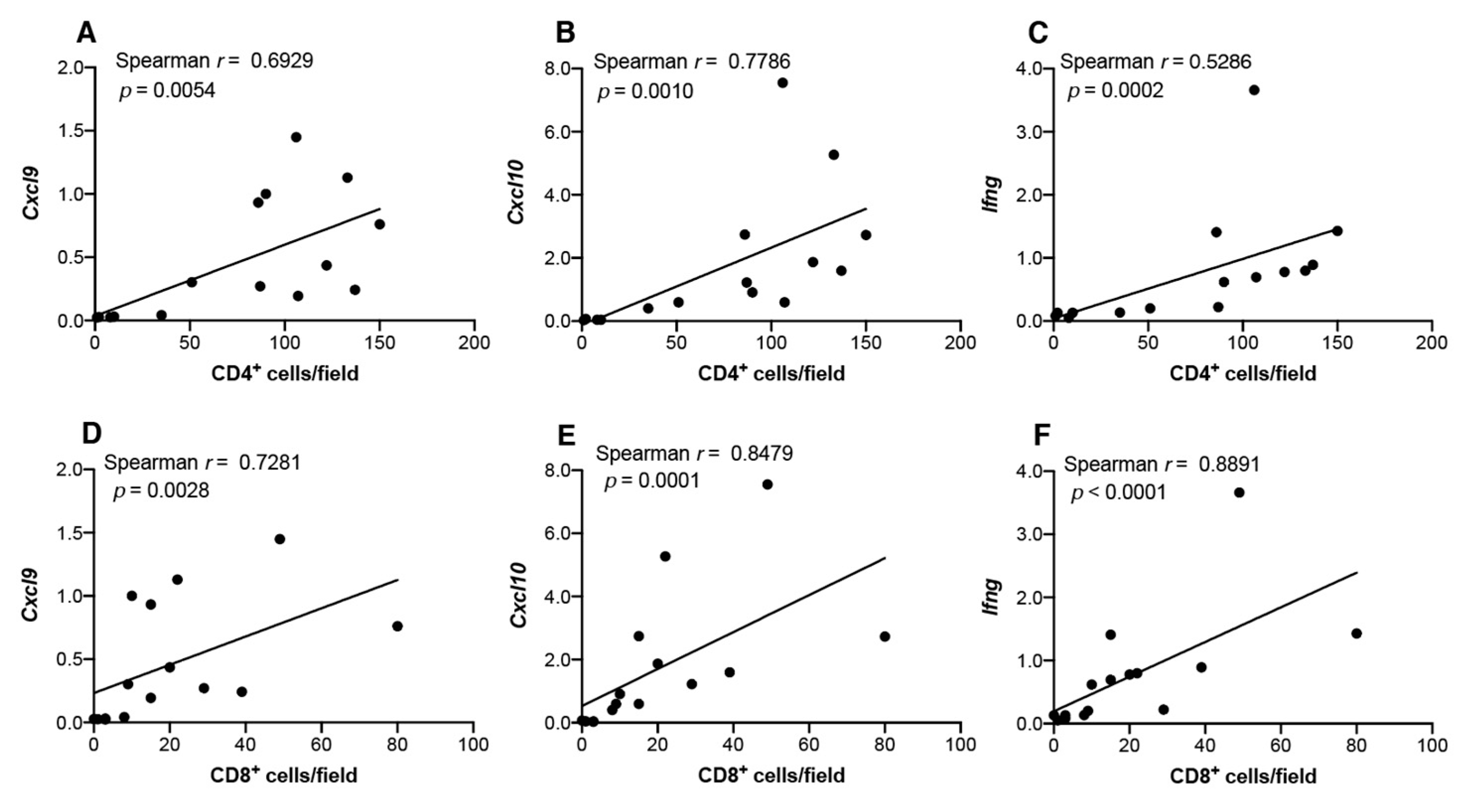
3.3. Th1-Associated Chemokine Gene Expression in 4NQO-Induced Mouse Tongue Tumorigenesis
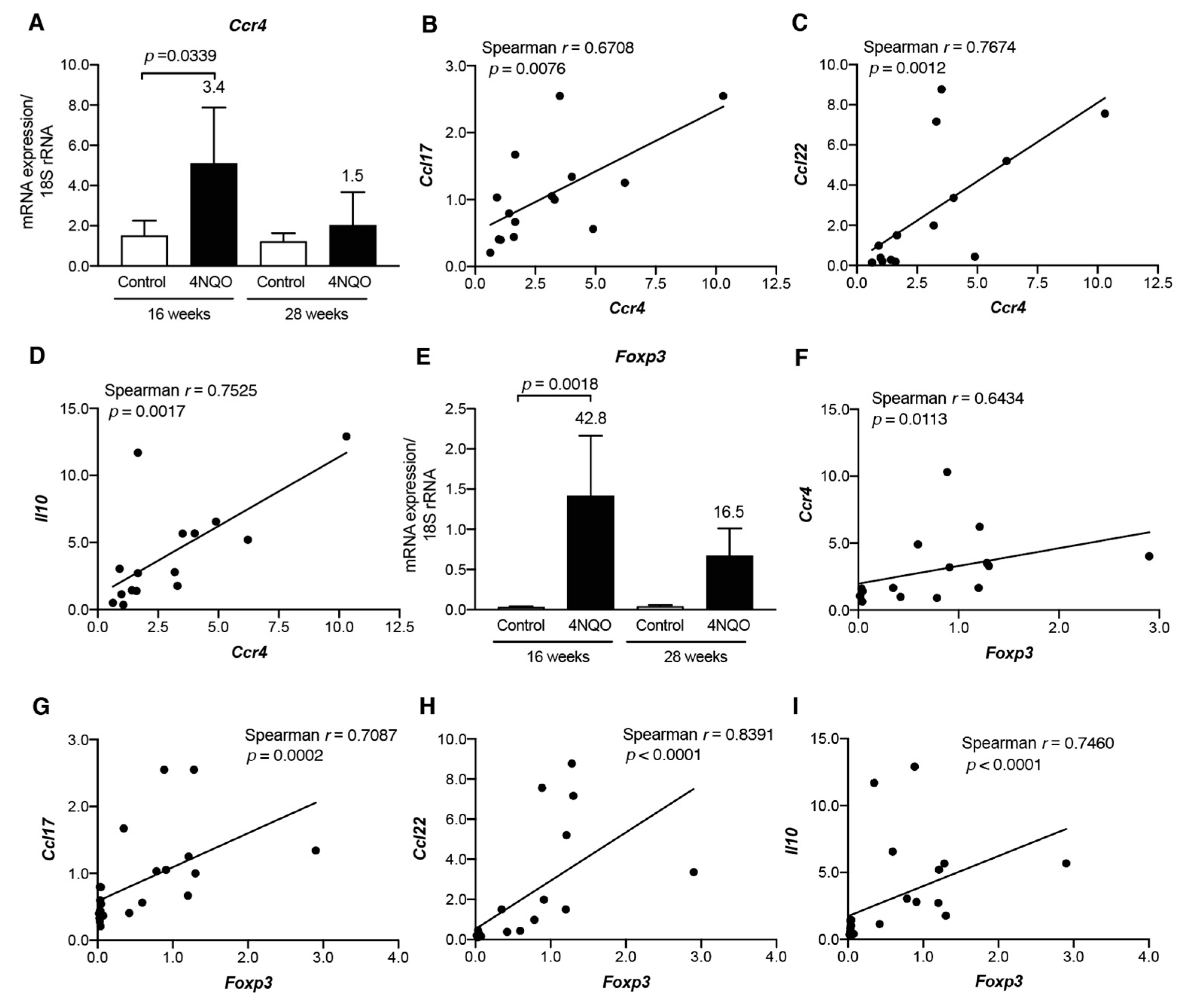
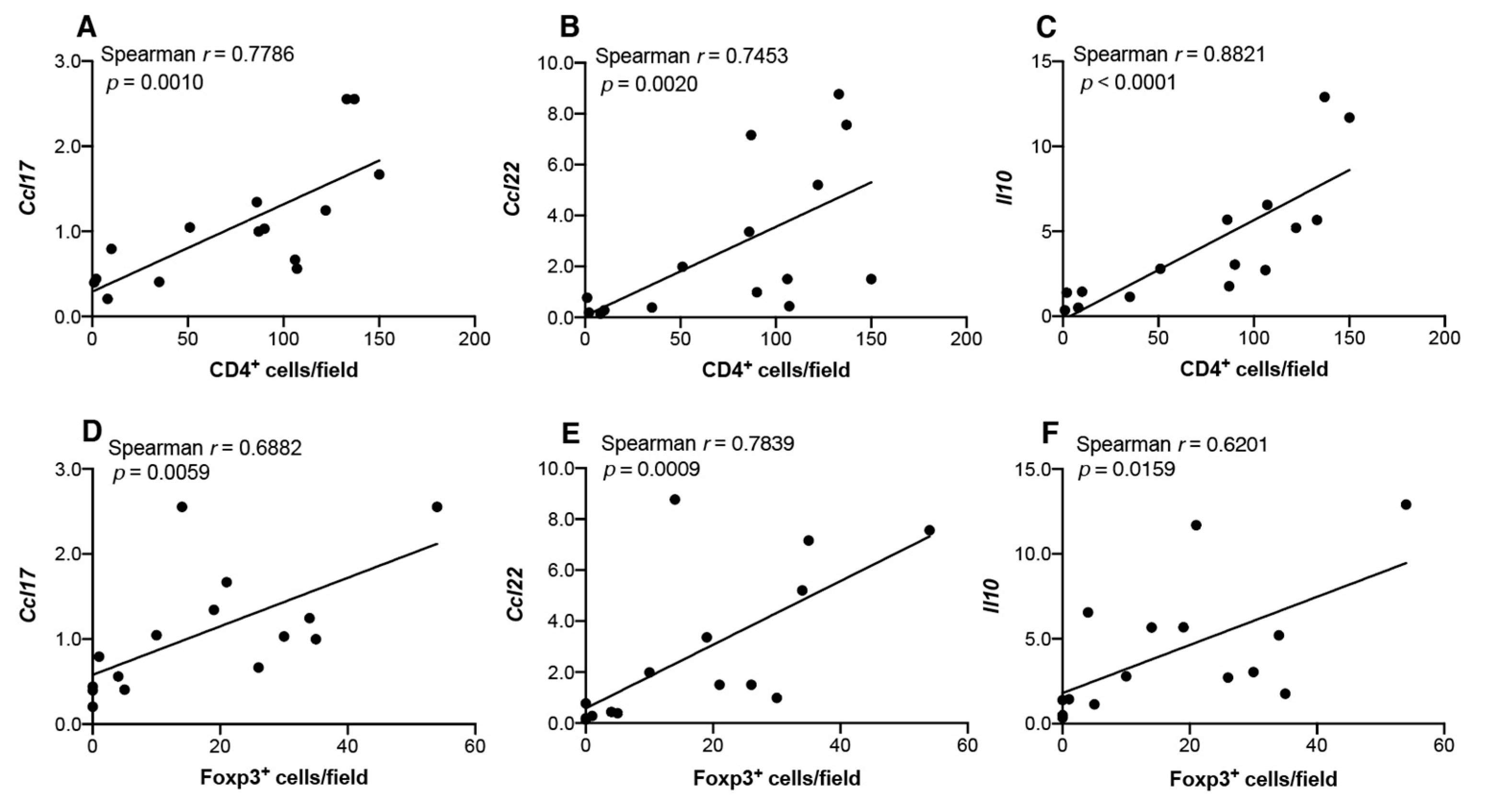
3.4 Th2/Treg-Associated Chemokine Gene Expression in 4NQO-Induced Mouse Tongue Tumorigenesis
3.5 Infiltration of CD4+, CD8+, and Foxp3+ Cells in Carcinogen-Induced Mouse Tongue Tumorigenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, L.D.R. Head and neck cancers. In Wold Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; International Agency for Research on Cancer: Lyon, France, 2014; pp. 422–431. [Google Scholar]
- Howlader, N.; Ries, L.A.G.; Mariotto, A.B.; Reichman, M.E.; Ruhl, J.; Cronin, K.A. Improved Estimates of Cancer-Specific Survival Rates From Population-Based Data. J. Natl. Cancer Inst. 2010, 102, 1584–1598. [Google Scholar] [CrossRef]
- Gale, N.; Pich, B.; Sidransky, D.; Westra, W.; Califano, J. Epithelial precusor lesions. In Pathology and Genetics of Head and Neck Tumors; Barnes, L., Eveson, J., Reichart, P., Sidransky, D., Eds.; IARCP Press: Lyon, France, 2005; pp. 140–143. [Google Scholar]
- Reibel, J. Prognosis of Oral Pre-malignant Lesions: Significance of Clinical, Histopathological, and Molecular Biological Characteristics. Crit. Rev. Oral Biol. Med. 2003, 14, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Öhman, J.; Mowjood, R.; Larsson, L.; Kovacs, A.; Magnusson, B.; Kjeller, G.; Jontell, M.; Hasseus, B. Presence of CD3-positive T-cells in oral premalignant leukoplakia indicates prevention of cancer transformation. Anticancer. Res. 2015, 35, 311–317. [Google Scholar] [PubMed]
- Chaves, A.L.F.; Silva, A.G.; Maia, F.M.; Lopes, G.F.M.; De Paulo, L.F.B.; Muniz, L.V.; Dos Santos, H.B.; Soares, J.M.A.; Souza, A.A.; Barbosa, L.A.D.O.; et al. Reduced CD8+ T cells infiltration can be associated to a malignant transformation in potentially malignant oral epithelial lesions. Clin. Oral Investig. 2018, 23, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Foy, J.-P.; Bertolus, C.; Ortiz-Cuaran, S.; Albaret, M.-A.; Williams, W.N.; Lang, W.; Destandau, S.; De Souza, G.; Sohier, E.; Kielbassa, J.; et al. Immunological and classical subtypes of oral premalignant lesions. OncoImmunology 2018, 7, e1496880. [Google Scholar] [CrossRef]
- Mori, K.; Haraguchi, S.; Hiori, M.; Shimada, J.; Ohmori, Y. Tumor-associated macrophages in oral premalignant lesions coexpress CD163 and STAT1 in a Th1-dominated microenvironment. BMC Cancer 2015, 15, 573. [Google Scholar] [CrossRef]
- Hawkins, B.L.; Heniford, B.W.; Ackermann, D.M.; Leonberger, M.; Martinez, S.A.; Hendler, F.J. 4NQO carcinogenesis: A mouse model of oral cavity squamous cell carcinoma. Head Neck 1994, 16, 424–432. [Google Scholar] [CrossRef]
- Kanojia, D.; Vaidya, M.M. 4-Nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006, 42, 655–667. [Google Scholar] [CrossRef]
- Schoop, R.A.L.; Noteborn, M.H.M.; De Jong, R.J.B. A mouse model for oral squamous cell carcinoma. J. Mol. Histol. 2009, 40, 177–181. [Google Scholar] [CrossRef]
- Tang, X.H.; Knudsen, B.; Bemis, D.; Tickoo, S.; Gudas, L.J. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin. Cancer Res. 2004, 10, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.I. Use of Carcinogen-induced Premalignant Oral Lesions in a Dendritic Cell-based Vaccine to Stimulate Immune Reactivity Against Both Premalignant Oral Lesions and Oral Cancer. J. Immunother. 2008, 31, 148–156. [Google Scholar] [CrossRef]
- Czerninski, R.; Amornphimoltham, P.; Patel, V.; Molinolo, A.A.; Gutkind, J.S. Targeting Mammalian Target of Rapamycin by Rapamycin Prevents Tumor Progression in an Oral-Specific Chemical Carcinogenesis Model. Cancer Prev. Res. 2009, 2, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Eveson, J.; Reichart, P.; Sidransky, D. Pathology and Genetics of Head and Neck Tumours; IARC Press: Lyon, France, 2005; pp. 163–175. [Google Scholar]
- Bromley, S.K.; Mempel, T.R.; Luster, A.D. Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat. Immunol. 2008, 9, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-P.; Flavell, R.A. The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef]
- Nakayama, T.; Hirahara, K.; Onodera, A.; Endo, Y.; Hosokawa, H.; Shinoda, K.; Tumes, D.J.; Okamoto, Y. Th2 Cells in Health and Disease. Annu. Rev. Immunol. 2017, 35, 53–84. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- De Costa, A.-M.A.; Schuyler, C.A.; Walker, D.D.; Young, M.R.I. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol. Immunother. 2012, 61, 927–939. [Google Scholar] [CrossRef]
- Woodford, D.; Johnson, S.D.; De Costa, A.M.; Young, M.R. An Inflammatory Cytokine Milieu is Prominent in Premalignant Oral Lesions, but Subsides when Lesions Progress to Squamous Cell Carcinoma. J. Clin. Cell. Immunol. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Han, J.; Qiu, X.; Pan, J.; Chen, J. Increased frequency of CD4+ CD25+ FOXP3+ cells correlates with the progression of 4-nitroquinoline1-oxide-induced rat tongue carcinogenesis. Clin. Oral Investig. 2013, 18, 1725–1730. [Google Scholar] [CrossRef]
- De Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. OncoImmunology 2017, 6, e1356148. [Google Scholar] [CrossRef]
- Johnson, S.D.; De Costa, A.-M.A.; Young, M.R.I.; De Costa, A.-M. Effect of the Premalignant and Tumor Microenvironment on Immune Cell Cytokine Production in Head and Neck Cancer. Cancers 2014, 6, 756–770. [Google Scholar] [CrossRef]
- Da Silva, J.M.; Soave, D.F.; Dos Santos, T.P.M.; Batista, A.C.; Russo, R.C.; Teixeira, M.M.; Da Silva, T.A. Significance of chemokine and chemokine receptors in head and neck squamous cell carcinoma: A critical review. Oral Oncol. 2016, 56, 8–16. [Google Scholar] [CrossRef]
- Miki, K.; Orita, Y.; Gion, Y.; Takao, S.; Ohno, K.; Takeuchi, M.; Ito, T.; Hanakawa, H.; Tachibana, T.; Marunaka, H.; et al. Regulatory T cells function at the early stage of tumor progression in a mouse model of tongue squamous cell carcinoma. Cancer Immunol. Immunother. 2016, 65, 1401–1410. [Google Scholar] [CrossRef]
- Da Silva, J.M.; Dos Santos, T.P.M.; Saraiva, A.M.; De Oliveira, A.L.F.; Garlet, G.P.; Batista, A.C.; De Mesquita, R.A.; Russo, R.C.; Da Silva, T.A. Role of atypical chemokine receptor ACKR2 in experimental oral squamous cell carcinogenesis. Cytokine 2019, 118, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Mumm, J.B.; Emmerich, J.; Zhang, X.; Chan, I.; Wu, L.; Mauze, S.; Blaisdell, S.; Basham, B.; Dai, J.; Grein, J.; et al. IL-10 Elicits IFNγ-Dependent Tumor Immune Surveillance. Cancer Cell 2011, 20, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Nunoshiba, T.; Demple, B. Potent intracellular oxidative stress exerted by the carcinogen 4-nitroquinoline-N-oxide. Cancer Res. 1993, 53, 3250–3252. [Google Scholar] [PubMed]
- Ba, X.; Aguilera-Aguirre, L.; Rashid, Q.T.A.N.; Bacsi, A.; Radak, Z.; Sur, S.; Hosoki, K.; Hegde, M.L.; Boldogh, I. The Role of 8-Oxoguanine DNA Glycosylase-1 in Inflammation. Int. J. Mol. Sci. 2014, 15, 16975–16997. [Google Scholar] [CrossRef]
- Boldogh, I.; Hajas, G.; Aguilera-Aguirre, L.; Hegde, M.L.; Radak, Z.; Bacsi, A.; Sur, S.; Hazra, T.K.; Mitra, S. Activation of Ras Signaling Pathway by 8-Oxoguanine DNA Glycosylase Bound to Its Excision Product, 8-Oxoguanine. J. Biol. Chem. 2012, 287, 20769–20773. [Google Scholar] [CrossRef]
- Wu, T.; Hong, Y.; Jia, L.; Wu, J.; Xia, J.; Wang, J.; Hu, Q.; Cheng, B. Modulation of IL-1β reprogrammes the tumor microenvironment to interrupt oral carcinogenesis. Sci. Rep. 2016, 6, 20208. [Google Scholar] [CrossRef]
- Solari, R.; Pease, J.E. Targeting chemokine receptors in disease—A case study of CCR4. Eur. J. Pharmacol. 2015, 763, 169–177. [Google Scholar] [CrossRef]
- Sun, W.; Li, W.-J.; Wei, F.-Q.; Wong, T.-S.; Lei, W.-B.; Zhu, X.-L.; Li, J.; Wen, W.-P. Blockade of MCP-1/CCR4 signaling-induced recruitment of activated regulatory cells evokes an antitumor immune response in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 37714–37727. [Google Scholar] [CrossRef]
- Chu, M.; Su, Y.-X.; Wang, L.; Zhang, T.-H.; Liang, Y.-J.; Liang, L.-Z.; Liao, G.-Q. Myeloid-derived suppressor cells contribute to oral cancer progression in 4NQO-treated mice. Oral Dis. 2011, 18, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.M.; Queiroz-Junior, C.M.; Batista, A.C.; Rachid, M.A.; Teixeira, M.M.; Da Silva, T.A. Eosinophil depletion protects mice from tongue squamous cell carcinoma induced by 4-nitroquinoline-1-oxide. Histol. Histopathol. 2014, 29, 387–396. [Google Scholar] [PubMed]
- Miki, K.; Orita, Y.; Gion, Y.; Takao, S.; Ohno, K.; Takeuchi, M.; Ito, T.; Minoura, A.; Tachibana, T.; Marunaka, H.; et al. Tumor-Associated Macrophages in the Development of 4-Nitroquinoline-1-Oxide-Induced Tongue Squamous Cell Carcinoma in a Mouse Model. Oncology 2017, 93, 204–212. [Google Scholar] [CrossRef]
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-Ad, V.; Pribitkin, E.; Tuluc, M. Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma. Semin. Oncol. 2014, 41, 217–234. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, H.; Hiroi, M.; Mori, K.; Ushio, R.; Matsumoto, A.; Yamamoto, N.; Shimada, J.; Ohmori, Y. Simultaneous Expression of Th1- and Treg-Associated Chemokine Genes and CD4+, CD8+, and Foxp3+ Cells in the Premalignant Lesions of 4NQO-Induced Mouse Tongue Tumorigenesis. Cancers 2021, 13, 1835. https://doi.org/10.3390/cancers13081835
Yamaguchi H, Hiroi M, Mori K, Ushio R, Matsumoto A, Yamamoto N, Shimada J, Ohmori Y. Simultaneous Expression of Th1- and Treg-Associated Chemokine Genes and CD4+, CD8+, and Foxp3+ Cells in the Premalignant Lesions of 4NQO-Induced Mouse Tongue Tumorigenesis. Cancers. 2021; 13(8):1835. https://doi.org/10.3390/cancers13081835
Chicago/Turabian StyleYamaguchi, Hana, Miki Hiroi, Kazumasa Mori, Ryosuke Ushio, Ari Matsumoto, Nobuharu Yamamoto, Jun Shimada, and Yoshihiro Ohmori. 2021. "Simultaneous Expression of Th1- and Treg-Associated Chemokine Genes and CD4+, CD8+, and Foxp3+ Cells in the Premalignant Lesions of 4NQO-Induced Mouse Tongue Tumorigenesis" Cancers 13, no. 8: 1835. https://doi.org/10.3390/cancers13081835
APA StyleYamaguchi, H., Hiroi, M., Mori, K., Ushio, R., Matsumoto, A., Yamamoto, N., Shimada, J., & Ohmori, Y. (2021). Simultaneous Expression of Th1- and Treg-Associated Chemokine Genes and CD4+, CD8+, and Foxp3+ Cells in the Premalignant Lesions of 4NQO-Induced Mouse Tongue Tumorigenesis. Cancers, 13(8), 1835. https://doi.org/10.3390/cancers13081835





