Tumor Solid Stress: Assessment with MR Elastography under Compression of Patient-Derived Hepatocellular Carcinomas and Cholangiocarcinomas Xenografted in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
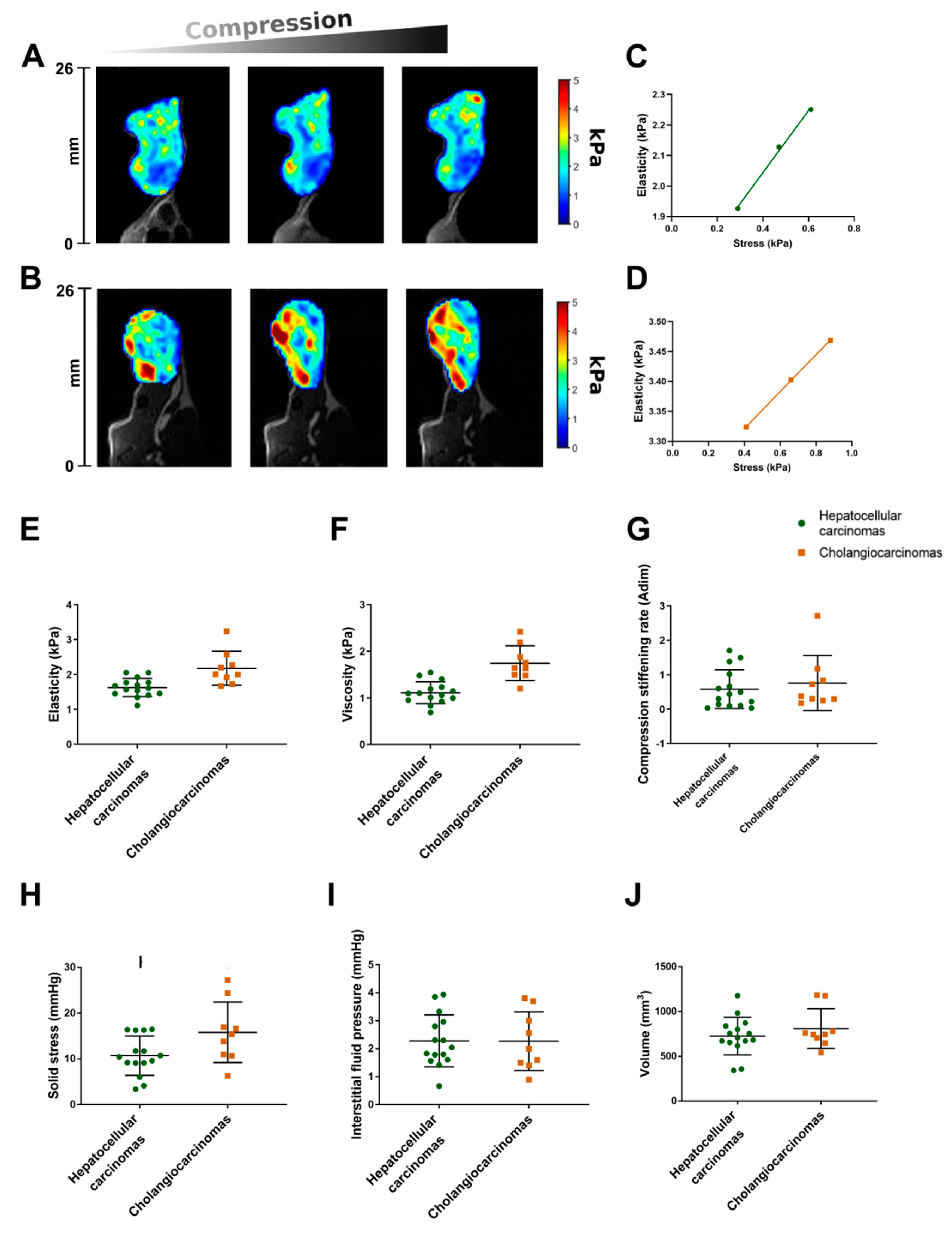
2.1. The Repeatability Index of the MR Elastography Measurements in Malignant Hepatic Tumors Is about 20%
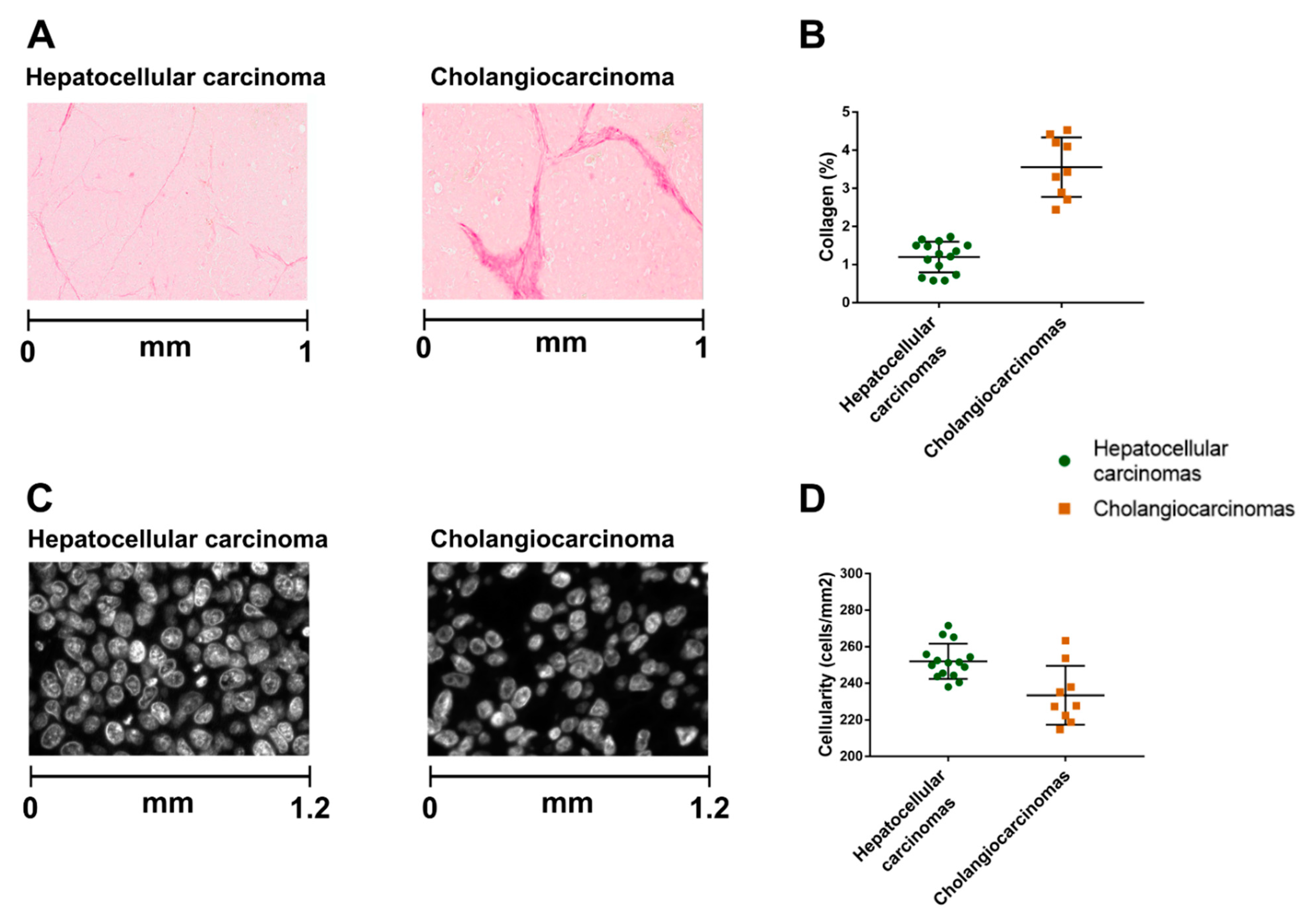
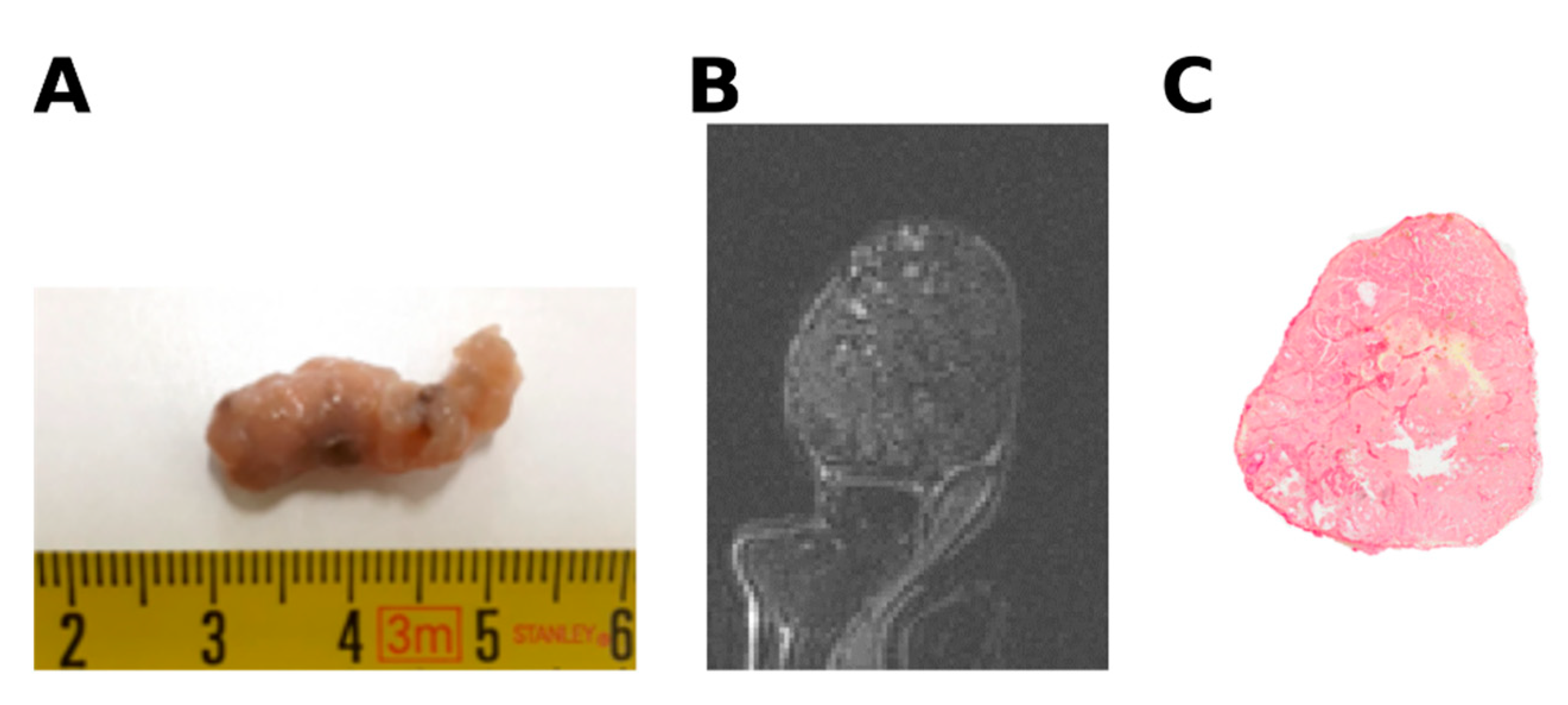
2.2. The Hepatocellular Carcinomas and Cholangiocarcinomas Have Different MR Elastography Pressure and Histological Characteristics
2.3. Interstitial Fluid Pressure Is an Independent Determinant of Basal Viscoelasticity, whereas Solid Stress Is an Independent Determinant of Compression Stiffening Rate
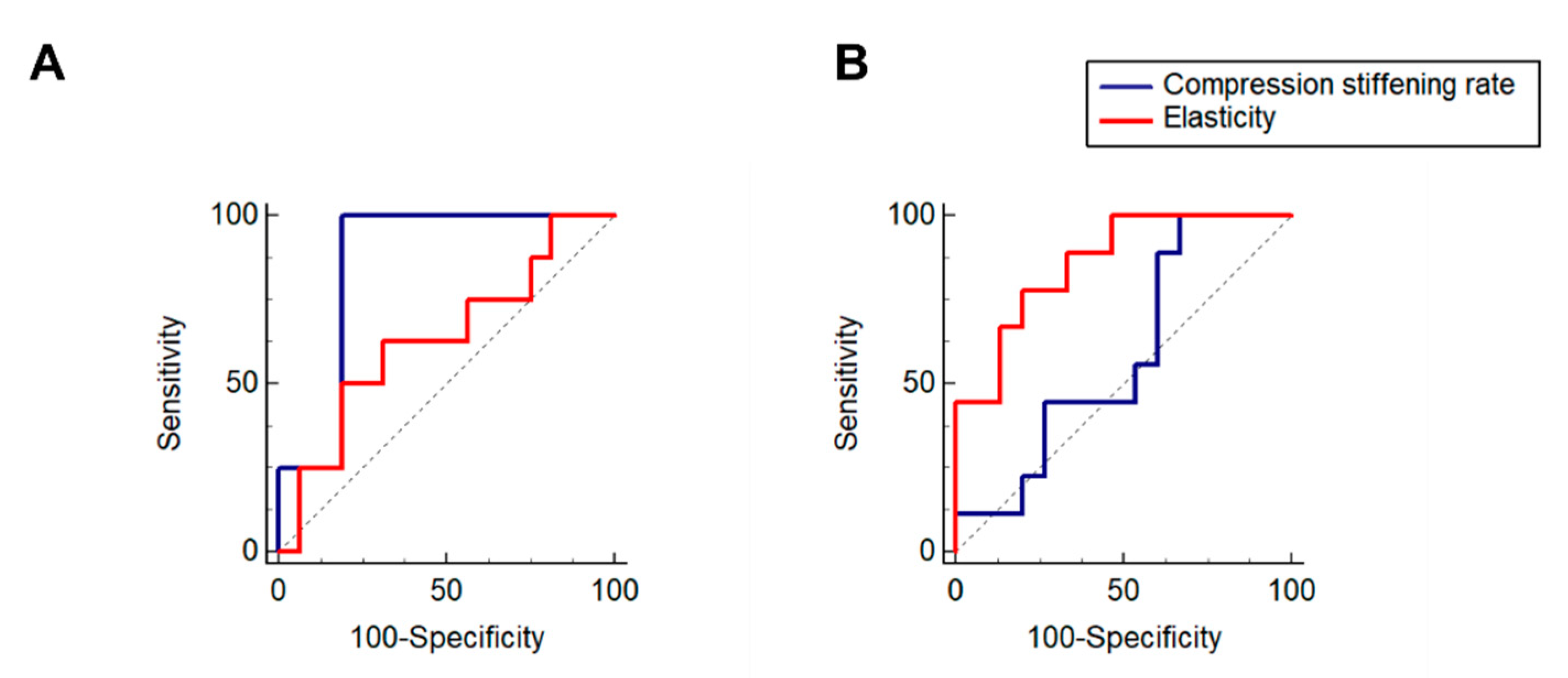
2.4. In MR Elastography the Compression Stiffening Rate Has High Performance in Diagnosing Elevated Solid Stress
3. Discussion
4. Materials and Methods
4.1. Patient-Derived Tumors Xenografted in Mice
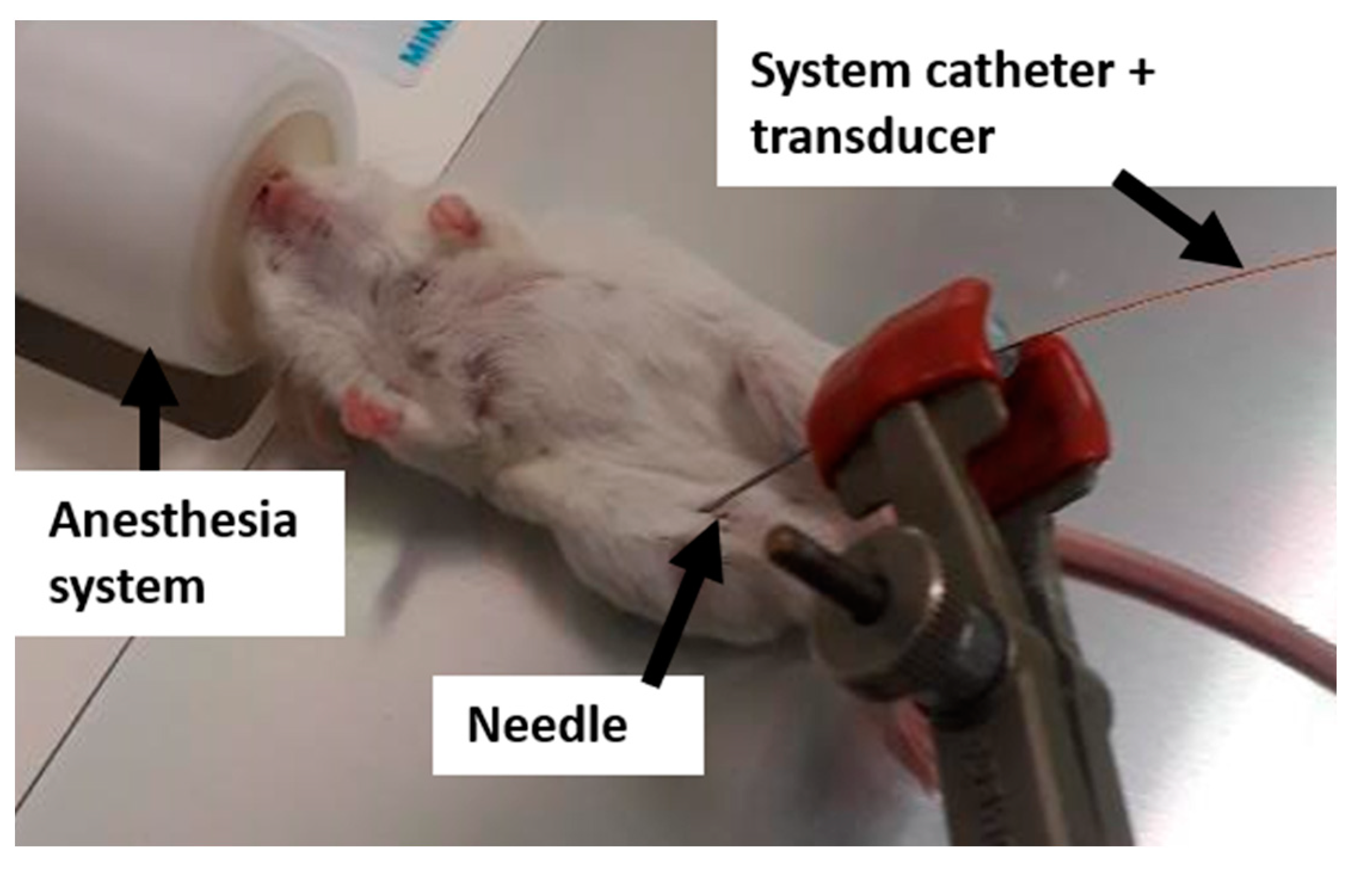
4.2. Compression Setup for MR Elastography
4.3. MR Imaging
4.4. MR Image Analysis
4.5. Tumor Pressure Measurements
4.6. Digital Histological Image Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Sánchez, M.E.; Barbier, S.; Whitehead, J.; Béalle, G.; Michel, A.; Latorre-Ossa, H.; Rey, C.; Fouassier, L.; Claperon, A.; Brullé, L.; et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 2015, 523, 92–95. [Google Scholar] [CrossRef]
- Nia, H.T.; Liu, H.; Seano, G.; Datta, M.; Jones, D.; Rahbari, N.; Incio, J.; Chauhan, V.P.; Jung, K.; Martin, J.D.; et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 2016, 1, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical traits of cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Shah, A.D.; Bouchard, M.J.; Shieh, A.C. Interstitial Fluid Flow Increases Hepatocellular Carcinoma Cell Invasion through CXCR4/CXCL12 and MEK/ERK Signaling. PLoS ONE 2015, 10, e0142337. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mislati, R.; Ahmed, R.; Vincent, P.; Nwabunwanne, S.F.; Gunn, J.R.; Pogue, B.W.; Doyley, M.M. Elastography Can Map the Local Inverse Relationship between Shear Modulus and Drug Delivery within the Pancreatic Ductal Adenocarcinoma Microenvironment. Clin. Cancer Res. 2019, 25, 2136–2143. [Google Scholar] [CrossRef] [Green Version]
- Laklai, H.; Miroshnikova, Y.A.; Pickup, M.W.; Collisson, E.A.; Kim, G.E.; Barrett, A.S.; Hill, R.C.; Lakins, J.N.; Schlaepfer, D.D.; Mouw, J.K.; et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 2016, 22, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, S.J.P.; Lee, R.M.; Martin, S.S. The Mechanical Microenvironment in Breast Cancer. Cancers 2020, 12, 1452. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zormpas-Petridis, K.; Boult, J.K.R.; Reeves, E.L.; Heindl, A.; Vinci, M.; Lopes, F.; Cummings, C.; Springer, C.J.; Chesler, L.; et al. Investigating the Contribution of Collagen to the Tumor Biomechanical Phenotype with Noninvasive Magnetic Resonance Elastography. Cancer Res. 2019, 79, 5874–5883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennisson, J.-L.; Grenier, N.; Combe, C.; Tanter, M. Supersonic shear wave elastography of in vivo pig kidney: Influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med. Biol. 2012, 38, 1559–1567. [Google Scholar] [CrossRef]
- Ronot, M.; Lambert, S.; Elkrief, L.; Doblas, S.; Rautou, P.-E.; Castera, L.; Vilgrain, V.; Sinkus, R.; Beers, B.E.V.; Garteiser, P. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur. Radiol. 2014, 24, 1394–1402. [Google Scholar] [CrossRef]
- Leitao, H.S.; Doblas, S.; Garteiser, P.; d’Assignies, G.; Paradis, V.; Mouri, F.; Geraldes, C.F.; Ronot, M.; Van Beers, B.E. Hepatic Fibrosis, Inflammation, and Steatosis: Influence on the MR Viscoelastic and Diffusion Parameters in Patients with Chronic Liver Disease. Radiology 2017, 283, 98–107. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Mapping Physical Tumor Microenvironment and Drug Delivery. Clin. Cancer Res. 2019, 25, 2024–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, V.P.; Boucher, Y.; Ferrone, C.R.; Roberge, S.; Martin, J.D.; Stylianopoulos, T.; Bardeesy, N.; DePinho, R.A.; Padera, T.P.; Munn, L.L.; et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 2014, 26, 14–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nia, H.T.; Datta, M.; Seano, G.; Huang, P.; Munn, L.L.; Jain, R.K. Quantifying solid stress and elastic energy from excised or in situ tumors. Nat. Protoc. 2018, 13, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- van Oosten, A.S.G.; Chen, X.; Chin, L.; Cruz, K.; Patteson, A.E.; Pogoda, K.; Shenoy, V.B.; Janmey, P.A. Emergence of tissue-like mechanics from fibrous networks confined by close-packed cells. Nature 2019, 573, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nieskoski, M.D.; Marra, K.; Gunn, J.R.; Kanick, S.C.; Doyley, M.M.; Hasan, T.; Pereira, S.P.; Stuart Trembly, B.; Pogue, B.W. Separation of Solid Stress From Interstitial Fluid Pressure in Pancreas Cancer Correlates With Collagen Area Fraction. J. Biomech. Eng. 2017, 139, 061002. [Google Scholar] [CrossRef] [PubMed]
- Huwart, L.; Sempoux, C.; Vicaut, E.; Salameh, N.; Annet, L.; Danse, E.; Peeters, F.; Beek, L.C.T.; Rahier, J.; Sinkus, R.; et al. Magnetic Resonance Elastography for the Noninvasive Staging of Liver Fibrosis. Gastroenterology 2008, 135, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Garteiser, P.; Doblas, S.; Daire, J.-L.; Wagner, M.; Leitao, H.; Vilgrain, V.; Sinkus, R.; Beers, B.E.V. MR elastography of liver tumours: Value of viscoelastic properties for tumour characterisation. Eur. Radiol. 2012, 22, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.C.; Cheng, S.; Green, M.; Sinkus, R.; Bilston, L.E. Using static preload with magnetic resonance elastography to estimate large strain viscoelastic properties of bovine liver. J. Biomech. 2011, 44, 2461–2465. [Google Scholar] [CrossRef]
- Perepelyuk, M.; Chin, L.; Cao, X.; Oosten, A.v.; Shenoy, V.B.; Janmey, P.A.; Wells, R.G. Normal and Fibrotic Rat Livers Demonstrate Shear Strain Softening and Compression Stiffening: A Model for Soft Tissue Mechanics. PLoS ONE 2016, 11, e0146588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, G.; Tardieu, M.; Besret, L.; Blot, L.; Lopes, J.; Sinkus, R.; Van Beers, B.E.; Garteiser, P. Assessing Tumor Mechanics by MR Elastography at Different Strain Levels. J. Magn. Reson. Imaging 2019, 50, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Ossa, H.; Gennisson, J.; Brosses, E.D.; Tanter, M. Quantitative imaging of nonlinear shear modulus by combining static elastography and shear wave elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Capilnasiu, A.; Hadjicharalambous, M.; Fovargue, D.; Patel, D.; Holub, O.; Bilston, L.; Screen, H.; Sinkus, R.; Nordsletten, D. Magnetic resonance elastography in nonlinear viscoelastic materials under load. Biomech. Model. Mechanobiol. 2019, 18, 111–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deptuła, P.; Łysik, D.; Pogoda, K.; Cieśluk, M.; Namiot, A.; Mystkowska, J.; Król, G.; Głuszek, S.; Janmey, P.A.; Bucki, R. Tissue Rheology as a Possible Complementary Procedure to Advance Histological Diagnosis of Colon Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5620–5631. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Tasciotti, E.; Righetti, R. Non-invasive imaging of normalized solid stress in cancers in vivo. IEEE J. Trans. Eng. Health Med. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Fovargue, D.; Fiorito, M.; Capilnasiu, A.; Nordsletten, D.; Lee, J.; Sinkus, R. Towards noninvasive estimation of tumour pressure by utilising MR elastography and nonlinear biomechanical models: A simulation and phantom study. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Piecha, F.; Mandorfer, M.; Peccerella, T.; Ozga, A.K.; Poth, T.; Vonbank, A.; Seitz, H.K.; Rausch, V.; Reiberger, T.; Mueller, S. Pharmacological decrease of liver stiffness is pressure-related and predicts long-term clinical outcome. Am. J. Physiol.-Gastr. Liver Physiol. 2018, 315, G484–G494. [Google Scholar] [CrossRef]
- Guo, J.; Büning, C.; Schott, E.; Kröncke, T.; Braun, J.; Sack, I.; Althoff, C. In vivo abdominal magnetic resonance elastography for the assessment of portal hypertension before and after transjugular intrahepatic portosystemic shunt implantation. Investig. Radiol. 2015, 50, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Bernal, M.; Chamming’s, F.; Couade, M.; Bercoff, J.; Tanter, M.; Gennisson, J.L. In Vivo Quantification of the Nonlinear Shear Modulus in Breast Lesions: Feasibility Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 101–109. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef] [Green Version]
- Nia, H.T.; Datta, M.; Seano, G.; Zhang, S.; Ho, W.W.; Roberge, S.; Huang, P.; Munn, L.L.; Jain, R.K. In vivo compression and imaging in mouse brain to measure the effects of solid stress. Nat. Protoc. 2020, 15, 2321–2340. [Google Scholar] [CrossRef]
- Fovargue, D.; Lee, J.; Fiorito, M.; Capilnasiu, A.; Svensson, S.; Kyrre, E.; Garteiser, P.; Manzi, A.P.; Page, G.; Vilgrain, V.; et al. Extending an MR elastography based method for inferring total tumour pressure to multiple organs. In Proceedings of the ISMRM Virtual Conference, Online. 8–14 August 2020; p. 4745. [Google Scholar]
- Gennisson, J.L.; Renier, M.; Catheline, S.; Barriere, C.; Bercoff, J.; Tanter, M.; Fink, M. Acoustoelasticity in soft solids: Assessment of the nonlinear shear modulus with the acoustic radiation force. J. Acoust. Soc. Am. 2007, 122, 3211–3219. [Google Scholar] [CrossRef]
- Jing, C.Y.; Fu, Y.P.; Huang, J.L.; Zhang, M.X.; Yi, Y.; Gan, W.; Xu, X.; Shen, H.J.; Lin, J.J.; Zheng, S.S.; et al. Prognostic Nomogram Based on Histological Characteristics of Fibrotic Tumor Stroma in Patients Who Underwent Curative Resection for Intrahepatic Cholangiocarcinoma. Oncologist 2018, 23, 1482–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kairaluoma, V.; Kemi, N.; Pohjanen, V.M.; Saarnio, J.; Helminen, O. Tumour budding and tumour-stroma ratio in hepatocellular carcinoma. Br. J. Cancer 2020, 123, 38–45. [Google Scholar] [CrossRef]
- Sala, M.; Ros, M.; Saltel, F. A Complex and Evolutive Character: Two Face Aspects of ECM in Tumor Progression. Front. Oncol. 2020, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Jain, R.K.; Duda, D.G. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021, 7, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Gnoni, A.; Licchetta, A.; Delcuratolo, S.; Memeo, R.; Solimando, A.G.; Argentiero, A. Emerging role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Medicina 2019, 55, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinter, M.; Jain, R.K. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 2017, 9, eaan5616. [Google Scholar] [CrossRef] [Green Version]
- Datta, M.; Coussens, L.M.; Nishikawa, H.; Hodi, F.S.; Jain, R.K. Reprogramming the tumor microenvironment to improve immunotherapy: Emerging strategies and combination therapies. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 165–174. [Google Scholar] [CrossRef]
- Keenan, B.P.; Fong, L.; Kelley, R.K. Immunotherapy in hepatocellular carcinoma: The complex interface between inflammation, fibrosis, and the immune response. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jugé, L.; Doan, B.-T.; Seguin, J.; Albuquerque, M.; Larrat, B.; Mignet, N.; Chabot, G.G.; Scherman, D.; Paradis, V.; Vilgrain, V.; et al. Colon Tumor Growth and Antivascular Treatment in Mice: Complementary Assessment with MR Elastography and Diffusion-weighted MR Imaging. Radiology 2012, 264, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Sinkus, R.; Tanter, M.; Xydeas, T.; Catheline, S.; Bercoff, J.; Fink, M. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn. Reson. Imaging 2005, 23, 159–165. [Google Scholar] [CrossRef]
- Sinkus, R.; Siegmann, K.; Xydeas, T.; Tanter, M.; Claussen, C.; Fink, M. MR elastography of breast lesions: Understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn. Reson. Med. 2007, 58, 1135–1144. [Google Scholar] [CrossRef]
- Bohte, A.E.; Garteiser, P.; De Niet, A.; Groot, P.F.; Sinkus, R.; Stoker, J.; Nederveen, A.J. MR elastography of the liver: Defining thresholds for detecting viscoelastic changes. Radiology 2013, 269, 768–776. [Google Scholar] [CrossRef]
- Yuan, Y.; Failmezger, H.; Rueda, O.M.; Ali, H.R.; Gräf, S.; Chin, S.F.; Schwarz, R.F.; Curtis, C.; Dunning, M.J.; Bardwell, H.; et al. Quantitative image analysis of cellular heterogeneity in breast tumors complements genomic profiling. Sci. Transl. Med. 2012, 4, 143–157. [Google Scholar] [CrossRef]
- Helmlinger, G.; Netti, P.A.; Lichtenbeld, H.C.; Melder, R.J.; Jain, R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 1997, 15, 778–783. [Google Scholar] [CrossRef]
- Sefidgar, M.; Soltani, M.; Raahemifar, K.; Bazmara, H.; Nayinian, S.M.; Bazargan, M. Effect of tumor shape, size, and tissue transport properties on drug delivery to solid tumors. J. Biol. Eng. 2014, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
| Biomechanical Parameter | Partial Correlation with Tumor Type as Covariate | Multiple Regression | ||||
|---|---|---|---|---|---|---|
| r | p | β Value | rpartial | p | R2 | |
| Basal elasticity (n = 24) | 0.82 | |||||
| Solid stress | −0.07 | 0.74 | NI | |||
| Interstitial fluid pressure | 0.70 | <0.001 | 0.17 | 0.63 | 0.001 | |
| Volume | 0.20 | 0.35 | NI | |||
| Collagen | 0.84 | <0.001 | 0.28 | 0.86 | <0.001 | |
| Cellularity | −0.27 | 0.22 | NI | |||
| Tumor type | - | - | NI | |||
| Basal viscosity (n = 24) | 0.87 | |||||
| Solid stress | −0.02 | 0.91 | NI | |||
| Interstitial fluid pressure | 0.76 | <0.001 | 0.13 | 0.62 | 0.002 | |
| Volume | −0.19 | 0.38 | NI | |||
| Collagen | 0.75 | <0.001 | 0.30 | 0.91 | <0.001 | |
| Cellularity | −0.26 | 0.24 | NI | |||
| Tumor type | - | - | NI | |||
| Compression stiffening rate (n = 24) | 0.75 | |||||
| Solid stress | −0.67 | <0.001 | −0.06 | −0.67 | <0.001 | |
| Interstitial fluid pressure | −0.07 | 0.75 | NI | |||
| Volume | −0.41 | 0.04 | NI | |||
| Collagen | −0.55 | 0.007 | −0.44 | −0.54 | 0.01 | |
| Cellularity | 0.59 | 0.003 | 0.02 | 0.58 | 0.006 | |
| Tumor type | - | - | −1.80 | 0.76 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagé, G.; Tardieu, M.; Gennisson, J.-L.; Besret, L.; Garteiser, P.; Van Beers, B.E. Tumor Solid Stress: Assessment with MR Elastography under Compression of Patient-Derived Hepatocellular Carcinomas and Cholangiocarcinomas Xenografted in Mice. Cancers 2021, 13, 1891. https://doi.org/10.3390/cancers13081891
Pagé G, Tardieu M, Gennisson J-L, Besret L, Garteiser P, Van Beers BE. Tumor Solid Stress: Assessment with MR Elastography under Compression of Patient-Derived Hepatocellular Carcinomas and Cholangiocarcinomas Xenografted in Mice. Cancers. 2021; 13(8):1891. https://doi.org/10.3390/cancers13081891
Chicago/Turabian StylePagé, Gwenaël, Marion Tardieu, Jean-Luc Gennisson, Laurent Besret, Philippe Garteiser, and Bernard E. Van Beers. 2021. "Tumor Solid Stress: Assessment with MR Elastography under Compression of Patient-Derived Hepatocellular Carcinomas and Cholangiocarcinomas Xenografted in Mice" Cancers 13, no. 8: 1891. https://doi.org/10.3390/cancers13081891







